Introduction
Insulin-like growth factor (IGF)-1 plays a crucial
role in human growth and is related to the action of growth hormone
(GH). Moreover, IGF-1 regulates growth, glucose uptake and protein
metabolism (1). IGF-1 is released
by the liver by the action of GH (2). IGF-1 promotes cell proliferation by
mediating the effects of GH and, through a negative feedback
mechanism, inhibits GH release by suppressing GH-releasing hormone
(3). IGF-1 is particularly abundant
during adolescence. The growth-promoting effects of GH are mediated
by IGF-1 through activation of downstream signaling pathways via
the IGF-1 receptor (IGF-1R) (4).
Although rare, a lack of the IGF-1 gene or GH results in muscle
development disorder and impaired growth (5). Thus, IGF-1 and GH have important
functions as growth factors.
STAT proteins function as upstream transcriptional
regulators of IGF-1 in-response to GH stimulation, while also
serving as downstream effectors in IGF-1R-mediated signaling, and
STAT is activated via phosphorylation by various factors and
cytokines (6). This protein
translocates to the nucleus and binds to its promoter site
(7). Phosphorylated (p)STAT
proteins bind in a dimeric form to the IGF-1 promoter, thereby
regulating the expression of IGF-1 mRNA (8). In addition to STAT1, STAT2 and STAT5B
bind and influence GH (9). STAT5B
serves a pivotal role in the growth-promoting effects of GH,
particularly in GH signaling, osteoblast differentiation and the
maintenance of bone homeostasis (10,11).
Hemp seed (HS) was first cultivated in China and are
nutritious, containing ~25% protein and 35% oil-based antioxidants
(12,13). Previously, HS was crushed or
consumed whole, as well as being used as an important grain in
traditional food and medicines (12). In addition, the nutritional benefits
of HS have long been recognized in Asia and Eastern Europe, where
they have been used as food for both humans and livestock (12). Hemp is more commonly consumed as
marijuana rather than as HS, and this has an association with
addiction, which leads to negative perceptions (14,15).
However, HS is increasingly recognized for benefits (16-18).
HS is rich in fatty acids, minerals, vitamins and essential amino
acids (12). Several studies have
reported their positive effects on cardiovascular disease, cancer,
inflammatory conditions, atopic dermatitis and antimicrobial
activity (19-22).
To the best of our knowledge, however, there are no studies on the
effects of HS on GH or growth outcomes.
C2C12 cells were selected as they are a
well-established in vitro model for skeletal muscle biology,
capable of differentiating into myotubes and widely used for
investigating the GH-IGF-1 axis, muscle hypertrophy and
regeneration (23,24). C3H10T1/2 cells were selected for
their multipotent differentiation potential into myogenic,
osteogenic and chondrogenic lineages (25). These cells are used to assess
early-stage proliferation and growth factor responsiveness prior to
lineage commitment (26). HS
extracts and their bioactive compounds promote myoblast
differentiation (27). Recent
studies have demonstrated that hemp extracts improve muscle health
by regulating muscle protein degradation pathways (27,28).
Hemp-derived compounds have been shown to promote the proliferation
of hair follicle dermal papilla stem cells through genetic
regulation, cell differentiation and modulation of the immune
system (29). Based on this, it was
hypothesized the active components in HS extract promote cell
proliferation via cellular signaling pathways.
Materials and methods
Antibodies and reagents
Edible HS (origin: Canada) were purchased from a
NongHyup market in the Republic of Korea and extracted with 50%
ethanol for 48 h at room temperature to obtain the HS extract.
DMEM, penicillin-streptomycin, trypsin-EDTA (0.05%) and fetal
bovine serum (FBS; cat. no. A5670801) were purchased from Gibco
(Thermo Fisher Scientific, Inc.). Primary antibodies against pSTAT5
(cat. no. #9351), JAK2 (cat. no. #3230), pJAK2 (cat. no. #3776),
pIGF-1Rβ (cat. no. #3012s) and cleaved caspase3 (cat. no. #9661s)
were obtained from Cell Signaling Technology, Inc. IGF-1 antibody
(cat. no. ab9572) was purchased from Abcam. Antibodies against
β-actin (cat. no. sc-47778), GHR (cat. no. sc-137185), IGF-1Rβ
(cat. no. sc-713) and STAT5b (cat. no. sc-1656), as well as
HRP-conjugated secondary antibodies (goat anti-mouse and rabbit
IgG; cat. nos. sc-516102, sc-2357), were obtained from Santa Cruz
Biotechnology, Inc. JAK2 inhibitor AG490 (cat. no. 658411;
Sigma-Aldrich, Merck KGaA) was used at 25 µM for 24 h at 37˚C in a
humidified 5% CO2 incubator during the western blot
analysis to confirm the involvement of the JAK2/STAT5b pathway.
Cell viability assay
The mouse muscle and embryo cell lines C2C12 and
C3H10T1/2 (cat. nos. CRL-1772 and KCLB 10226, respectively; both
American Type Culture Collection) were seeded in 96-well plates
(5x10³ cells/well) and incubated for 24 h at 37˚C. HS extract
(10-600 µg/ml) was added for another 24, 48 and 72 h at 37˚C in 5%
CO2. MTT reagent was applied and incubated for 4 h. The
purple formazan was dissolved in dimethyl sulfoxide. Absorbance at
560 nm was measured to assess cell viability. All experiments were
performed in triplicate.
Cell culture
The cells were cultured in DMEM with 10% FBS and 1%
penicillin at 37˚C in 5% CO2.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was conducted using the
Imprint® ChIP kit (Sigma-Aldrich; Merck KGaA cat. no.
CHP1) following the manufacturer's protocol at 4˚C. C2C12 and
C3H10T1/2 cells were cross-linked with 1% formaldehyde and quenched
with 1.25 M glycine for 5 min. Following lysis, chromatin was
sonicated (25% amplitude, 30 sec on/off for 20 min on ice) and
centrifuged at 21,000 x g for 10 min at 4˚C. The supernatant was
diluted with the dilution buffer (1:1) and 5 µl aliquots diluted
samples were used as internal controls. The supernatant was
immunoprecipitated with 5 µl anti-STAT5b antibody (cat. no.
sc-1656, Santa Cruz Biotechnology, Inc.), while controls used 1 µl
normal goat IgG and 1 µl anti-RNA polymerase II. The amount of
lysate used per immunoprecipitation reaction was ~100 µl, Bound
DNA–protein complexes were treated with 1 µl Proteinase K for
cross-link reversal and DNA release. Washing was performed with the
wash buffers provided in the kit. Bound DNA was purified and
analyzed by quantitative PCR using 10 µl TB Green Advantage Premix
(Takara Bio, cat. no. 639676) using IGF-1 primers (forward:
5'-TGCTCACAAACCCACATCAA-3' and reverse:
3'-GCTAGGTTCTTCACAGCTCC-5'). Thermocycling was performed with 40
cycles at 95˚C (30 sec), 60˚C (30 sec) and 72˚C (40 sec), followed
by final extension at 72˚C for 5 min. The calculations were
performed using the 2-ΔΔCq values obtained (30).
Comet assay
C2C12 and C3H10T1/2 cells were seeded in 6-well
plates at a density of 1x105 cells/well and cultured for
24 h at 37˚C in 5% CO2. Control cells were cultured
under the same conditions without HS treatment. DNA damage was
assessed using the Comet Assay kit (Abcam; cat. no. ab238544)
according to the manufacturer's protocol. Cell morphology was
analyzed using a fluorescence microscope (Olympus Corporation
IX71/DP72).
DAPI staining
C2C12 and C3H10T1/2 cells were seeded in 6-well
plates (1x105 cells/well) and cultured for 24 h at 37˚C.
The cells were then washed with PBS and fixed with 100% methanol
for 10 min at room temperature. After fixation, the cells were
washed twice with PBS and incubated with 1 ml of 5 µM DAPI staining
solution for 15 min at room temperature. Cells were then washed
twice with PBS and images were captured using a fluorescence
microscope (Olympus Corporation IX71/DP72)
ELISA
C3H10T1/2 cells were cultured (1x106
cells/ml) for 24 h at 37˚C in DMEM containing 100 or 200 µg/ml HS
extract. IGF-1 levels in the culture supernatant were measured
using a mouse IGF-1 ELISA kit (Abcam, cat. no. ab100695) according
to the manufacturer's instructions.
Total cell lysis and western
blotting
Whole cell lysates were prepared from untreated
cells or cells treated for 24 h at 37˚C with 100 or 200 µg/ml HS
using RIPA buffer (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with protease and phosphatase inhibitors. Cell lysates
were incubated for 30 min on ice and total protein concentration
was measured using the Bradford assay (Thermo Fisher Scientific,
Inc.). Equal amounts of protein (30 µg/lane) were separated by
6-15% SDS-PAGE and transferred to nitrocellulose membranes. The
membranes were blocked with TBS-T buffer (0.1% Tween-20) and
blocked with 5% skimmed milk (BD Biosciences; cat. no. 90002-594)
prepared in TBS-T buffer for 1 h at room temperature. The membrane
was incubated overnight at 4˚C with primary antibody diluted in 5%
skimmed milk, followed by incubation with HRP-conjugated secondary
antibody for 1 h at room temperature. Immunoreactive bands were
visualized using an enhanced chemiluminescence detection kit (Femto
Clean ECL, GenDEPOT, LLC; cat. no. 77449) and captured with a
LAS-4000 imaging system (FUJIFILM Corporation). Densitometric
analysis was performed using ImageJ software (version 1.53;
National Institutes of Health).
Statistical analysis
All data are presented as the mean ± SEM. All
experiments were performed in triplicate and analyzed using one-way
ANOVA followed by post hoc Tukey's test was used to evaluate
differences among groups. Prior to analysis, data were tested for
normality using the Shapiro-Wilk test and for homogeneity of
variances using Levene's test. All statistical analyses were
performed using SAS software (version 9.3, SAS Institute Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
HS enhances C2C12 and C3H10T1/2 cell
viability in a concentration-dependent manner
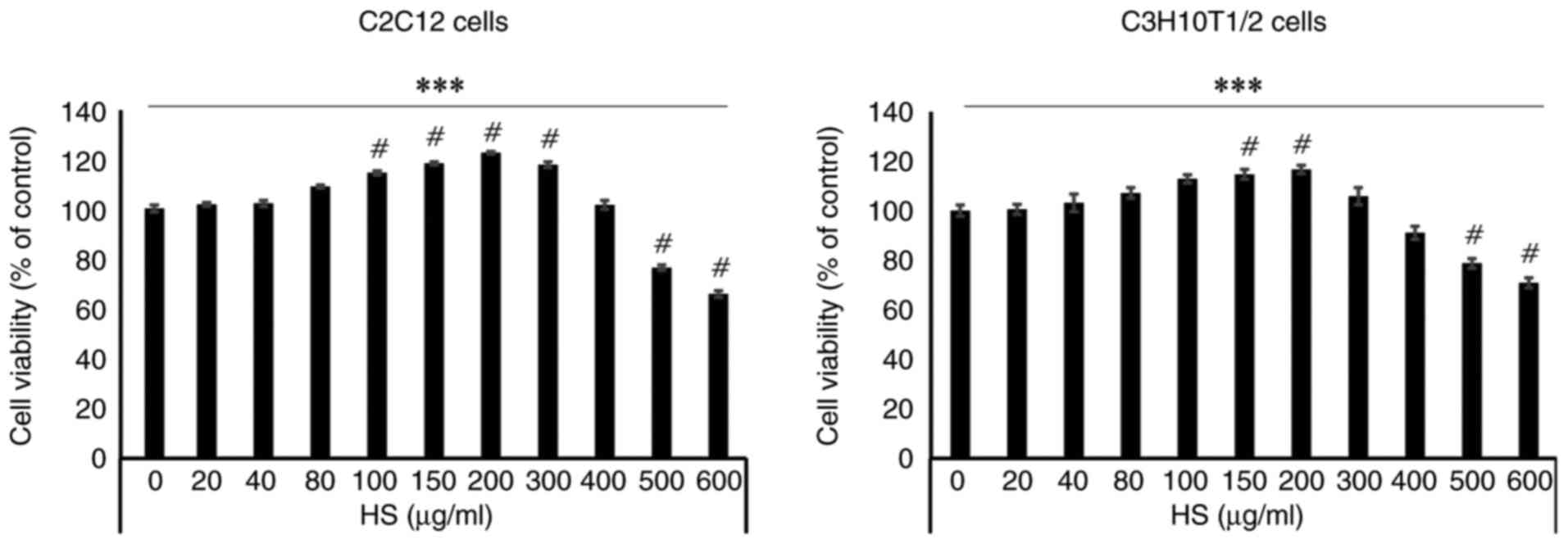
HS was extracted in 50% ethanol and allowed to react
for 48 h. Following filtration, only the pure liquid was used. To
evaluate the effect of HS on cells, MTT assay was performed using
C2C12 cells. There were significant differences between cells
treated with HS concentrations ranging from 100 to 600 µg/ml. HS
exhibited no cytotoxicity up to a concentration of 500 µg/ml.
However, at concentrations >500 µg/ml, cell viability decreased,
indicating cytotoxicity. Therefore, the optimal concentration for
subsequent cell experiments was set at 200 µg/ml (Figs. 1 and S1).
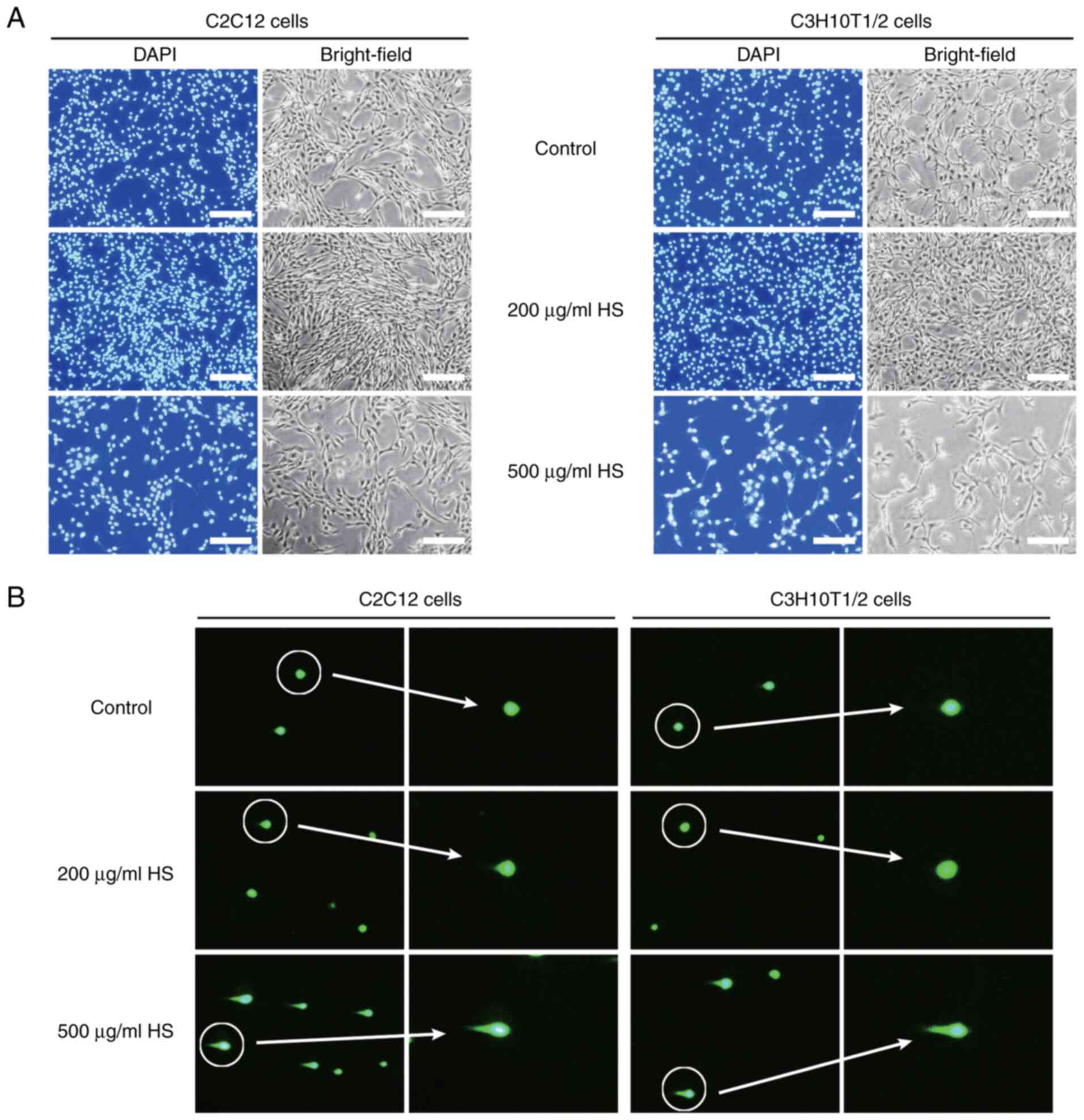
HS (200 µg/ml) exhibits no
cytotoxicity
When cells were treated with HS at a concentration
of 200 µg/ml, there was a marked increase in the number of stained
nuclei, indicating enhanced cell proliferation. By contrast, when
cells were treated with HS at 500 µg/ml, the number of nuclei
notably decreased, confirming cytotoxicity (Fig. 2A). Treatment with high
concentrations of HS may lead to DNA damage (DNA unwinding)
(31); therefore, comet assay was
performed to determine whether HS induces DNA damage. Fluorescence
microscopy revealed that treatment with 500 µg/ml HS increased the
length of comet tails and the number of comet-positive cells,
whereas no comet tails were formed in the untreated control and 200
µg/ml HS groups (Fig. 2B),
consistent with the absence of detectable DNA damage at the optimal
concentration. High concentrations of HS extract (500 µg/ml)
increased cleaved caspase-3 levels, indicative of apoptosis
induction (Fig. S2). These results
indicated that HS at 100-300 µg/ml was not cytotoxic and promoted
cell viability.
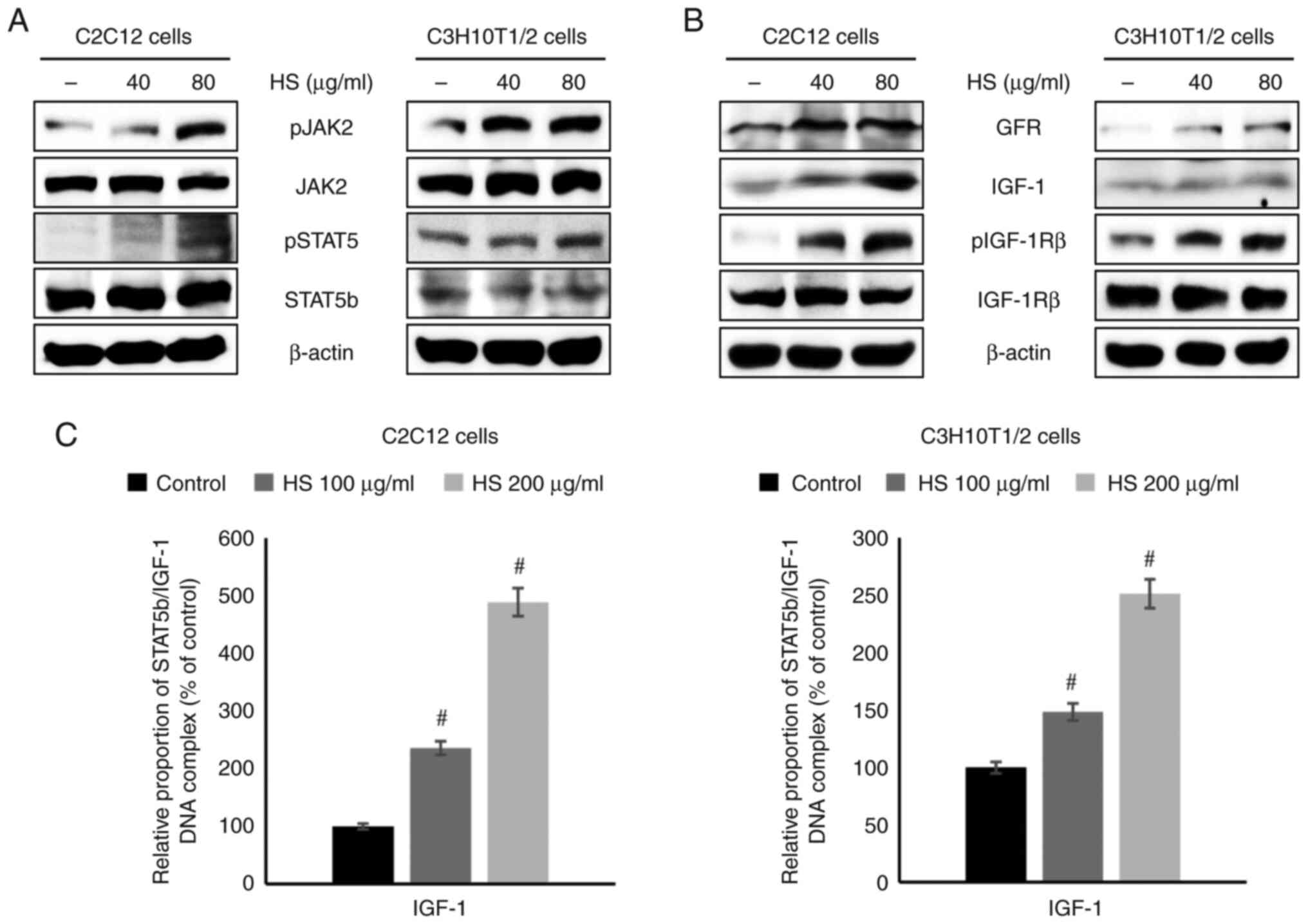
HS increases the expression of GHR and
IGF-1 via the JAK2/STAT5b pathway
Western blotting was performed to investigate the
cell signaling mechanisms. The protein levels of pJAK2 and pSTAT5
in C2C12 and C3H10T1/2 cells were upregulated by HS treatment in a
concentration-dependent manner. Furthermore, the phosphorylation of
JAK2 and STAT5b was induced, thereby activating the cellular
proliferation signaling pathway (Figs.
3A and S3A and B). Furthermore, the protein levels of
GHR, IGF-1, and pIGF-1Rβ were also increased by HS treatment in a
concentration-dependent manner (Fig.
3B). The present study investigated the effect of HS on the
binding of STAT5b to the IGF-1 promoter region. ChIP analysis using
a STAT5b antibody revealed that HS at 100-200 µg/ml increased the
binding of STAT5b to the IGF-1 promoter. STAT5b/IGF-1 complex
formation was induced, resulting in the activation of downstream
transcription (Fig. 3C). These
findings suggest that pSTAT5 and IGF-1 are key targets of HS, with
their expression enhanced to upregulate IGF-1 transcription. To
validate that STAT5b activation is mediated via JAK2, cells were
treated with JAK2 inhibitor. The inhibition of JAK2 markedly
reduced STAT5 phosphorylation and downstream IGF-1 expression in
C3H10T1/2 cells (Fig. S4),
confirming that HS-induced STAT5b activation was dependent on JAK2
signaling. Overall, the results indicated that HS may function as a
stimulator of key GH-associated cell signaling. ELISA further
confirmed that HS treatment at 200 µg/ml significantly increased
IGF-1 secretion in C3H10T1/2 cells (Fig. S5), supporting the upregulation of
IGF-1 expression observed in the western blot analysis.
Discussion
Cell proliferation studies have primarily focused on
GH (32,33). One of the key upstream regulators of
GH is IGF-1, which binds IGF-1R to enhance GH activity, thereby
influencing growth and development (34). This phenomenon occurs in numerous
types of tissue and involves the continuous secretion of these
factors to maintain homeostasis, although differences arise
depending on the specific receptors involved (35,36).
The benefits of IGF-1 include building muscle and bone mass,
preventing muscle wasting, helping with growth, regulating blood
sugar levels and protecting against neurological disorders. IGF-1
increases skeletal muscle protein synthesis via the PI3K/Akt/mTOR
and PI3K/Akt/glycogen synthase kinase 3 β pathways (37). The dangers of IGF-1 include
potentially increasing the risk of developing certain types of
cancer (including breast, prostate, colorectal and thyroid cancer)
and reducing life span (38).
The present study used C2C12 cells as a committed
myogenic model responsive to GH/IGF-1 via the JAK2/STAT5b/IGF-1
pathway (35). C3H10T1/2 cells, as
multipotent mesenchymal progenitors capable of differentiating into
osteogenic, chondrogenic or, under certain conditions, myogenic
lineages, were used to examine early proliferative and signaling
effects prior to full myogenic differentiation. This dual-cell line
approach enabled investigation of distinct stages of
muscle-associated cellular responses and provided a robust platform
for evaluating the effects of HS extract.
GH levels peak during the pubertal growth period,
serving a crucial role in adolescent growth spurts, and decline
progressively with aging. Because GH serves a crucial role in
muscle and bone metabolism, maintaining its secretion is important
in old age (39). In older adults
aged ≥70 years, muscle loss, or sarcopenia, occurs because of
decreased levels of GH and IGF-1, which notably affects muscle mass
and strength (40). Increasing and
activating GH and IGF-1 levels, as well as muscle-strengthening
exercises, are key to mitigate the development of sarcopenia. In
addition, as digestive efficiency declines with age, nutrient-dense
foods such as HS may help support muscle health by enhancing the
action of IGF-1 or mimicking its effects (41,42).
HS shows promise as a potential dietary strategy to delay the onset
and progression of sarcopenia.
Recent studies have demonstrated that plant-derived
compounds regulate the GH-IGF-1 signaling axis and modulate
downstream STAT pathways (43,44).
Clinical investigations have identified GH and IGF-1 as key
regulators of metabolic activity, immune response and hepatic
stellate cell function in non-alcoholic fatty liver disease
(45,46). In pediatric populations, researchers
have described the key role of GH and its hepatic effector IGF-1 in
normal growth processes and have emphasized the regulatory
involvement of SIRT1 in GH secretion and IGF-1 expression (47). Daucosterol, a phytosteroid derived
from walnut meat, is a plant-based compound that promotes IGF-1
protein expression and enhances cell proliferation (48). Similarly, researchers have evaluated
the effects of Epimedium extract, particularly icariin, and
have shown that it increases IGF-1 expression in skeletal muscle
cells (23). In C2C12 myotubes,
icariin has been reported to activate the IGF-1/PI3K/Akt pathway,
induce muscle hypertrophy and suppress catabolic atrophy markers
(49). Furthermore, studies have
demonstrated that the targeted deletion of STAT5a/b in skeletal
muscle decreases local IGF-1 expression by ~60%, thereby impairing
muscle growth and function (50,51).
Consistent with previous studies (45-47,49,50),
the present results demonstrated that STAT5b served as a key
transcriptional mediator of IGF-1 in peripheral and muscle-related
cells. Previous studies have examined the effects of individual
plant-derived compounds (daucosterol, icariin) or employed genetic
knockout models to demonstrate the role of STAT5a/b in muscle
growth (45-47,49).
The aforementioned studies established that STAT5 activation is key
for transcriptional regulation of IGF-1 target genes. The present
study demonstrated that HS extract, a commonly consumed nutritional
source, pharmacologically enhances IGF-1/STAT5b signaling in
muscle-associated cells, demonstrating a dietary approach to
modulate this pathway. Targeted deletion of STAT5a/b decreases
IGF-1 expression and impairs muscle growth (50). In agreement, the present data showed
that increased STAT5b activation was associated with upregulated
IGF-1 signaling, supporting the functional relevance of STAT5b in
muscle regeneration. Thus, the present findings not only
corroborate the established role of STAT5b in muscle biology but
also demonstrated that a nutritional extract can modulate this
pathway pharmacologically.
HS is a highly nutritious food source rich in
protein, essential amino acids, polyunsaturated fatty acids
(including linoleic acid and α- and γ-linolenic acid), tocopherols
such as vitamin E and essential minerals (phosphorus, potassium,
magnesium, iron and zinc), which collectively contribute to
cardiovascular health, blood pressure regulation, antioxidant
defense and immune function (12).
However, despite these benefits, little is known about their
effects on cell proliferation, and the underlying mechanisms remain
unclear.
The present study evaluated the cytotoxicity of HS
extract on mouse muscle (C2C12) and embryonic (C3H10T1/2) cells and
determined the optimal treatment concentration (200 µg/ml). The
primary objective was to assess whether HS extract exerts any
biological effects before conducting detailed phytochemical
analyses. At 200 µg/ml, the protein levels of growth factors were
elevated, and there was increased phosphorylation of the downstream
Jak2/STAT5b signaling pathway members. Furthermore, HS extract did
not notably affect the levels of damaged DNA, and enhanced the
binding of the transcription factor pSTAT5b protein, which acts on
IGF-1 DNA. Therefore, HS may support cell proliferation and aid in
muscle recovery by stimulating IGF-1 and GH via the JAK2/STAT5b
signaling pathway.
Effective muscle regeneration requires not only
activation of signaling pathways but also viability and
proliferative capacity of muscle cells. The present data
demonstrated that HS promoted viability of C2C12 and C3H10T1/2
cells, providing a cellular basis for regenerative effects. While
PI3K/AKT and MAPK/ERK pathways are recognized regulators of
IGF-1-mediated muscle hypertrophy, their primary roles are
typically associated with downstream events such as protein
synthesis and cell proliferation. By contrast, STAT5b serves as a
direct transcriptional mediator of IGF-1 target genes (51). In the present study, HS extract
consistently activated the JAK2/STAT5b pathway, providing a
mechanistic link to IGF-1 transcriptional regulation. However, the
present study did not exclude the possibility of crosstalk with
PI3K/AKT or MAPK/ERK.
In addition to STAT5b, the IGF-1 signaling axis also
engages the PI3K/Akt and MAPK pathways, which are major downstream
effectors regulating muscle cell function. The PI3K/Akt pathway is
key for normal muscle growth, survival and hypertrophy (35,52).
Following IGF-1 stimulation, PI3K activation leads to Akt
phosphorylation, which promotes muscle hypertrophy, supports cell
proliferation and differentiation and conveys anti-apoptotic
signals that preserve muscle integrity (53,54).
The MAPK pathway is critical, particularly in controlling myoblast
proliferation and differentiation. Activation through Ras triggers
the ERK cascade, which drives myoblast proliferation but can
transiently suppress differentiation by inhibiting key regulators
such as MyoD. Conversely, other MAPK branches, including p38 MAPK
and myocyte enhancer factor 2C, are key for initiating and
maintaining myogenic differentiation (55,56).
Together, these pathways complement STAT5b by regulating the
balance between proliferation and differentiation.
Beyond the GH/JAK2/STAT5b/IGF-1 signaling pathway,
HS contains bioactive compounds that may influence muscle cells
more generally. The ω-3 fatty acid α-linolenic acid and the ω-6
fatty acid γ-linolenic acid decrease muscle inflammation and
protect against cell injury, while L-arginine contributes to
anti-inflammatory effects and supports cell repair processes
(57-59).
Together, these components maintain muscle cell membrane integrity,
attenuate inflammatory responses and provide essential substrates
for recovery. These actions suggest that HS extract supports
overall muscle function through diverse mechanisms, rather than
acting solely on a single growth-associated pathway.
Future work should identify and characterize the
active components responsible for these effects. Based on previous
reports, essential amino acids such as arginine and lysine,
together with storage proteins such as edestin, may contribute to
the increased cell proliferation (60-62).
In addition, polyunsaturated fatty acids (γ- and α-linolenic acid
and linoleic acid) and minerals such as methylsulfonylmethane
modulate GH signaling and underlie the enhanced JAK2/STAT5b
activation and IGF-1 production (16,63-65).
Although further experimental validation is needed, these bioactive
components provide a mechanistic basis for the present findings.
Due to potential cytotoxic effects at lower concentrations, the
clinical application of HS extract may be limited, underscoring the
need for further research to evaluate tissue-specific safety in
vivo.
In summary, HS induces expression of IGF-1 via the
Jak2/STAT5b signaling pathway to promote proliferation in C2C12 and
C3H/10T1/2 mouse cells. Moreover, pSTAT5b bound to IGF-1 DNA as a
direct transcription factor, serving a key role in enhancing cell
signaling. Therefore, HS may deliver specific growth signals for
cell proliferation and muscle recovery.
Supplementary Material
Effect of HS extract on the viability
of C3H10T1/2 cells at 24, 48, and 72 h. viability was determined
using the MTT assay. ***P<0.001;
#P<0.001 vs. Con. HS, hemp seed; con, control.
Western blotting of the protein
expression of c-caspase3 and caspase3 following treatment of
C3H10T1/2 cells with 100, 200 and 500 μg/ml HS extracts for
24 h. c, cleaved; HS, hemp seed; con, control.
Quantitative analysis of protein
expression levels in C2C12 and C3H10T1/2 cells treated with hemp
seed extract. (A) GHR, IGF-1, pIGF-1Rβ, and IGF-1Rβ protein levels
in cells treated with varying concentrations of HS extract. (B)
pJAK2, JAK2, pSTAT5, and STAT5b protein levels in cells treated
with varying concentrations of HS extract. Protein expression
levels were normalized to β-actin. ***P<0.001;
#P<0.001 vs. Control; GHR, growth hormone receptor;
IGF-1R, insulin-like growth factor-1receptor; p, phospho; NS,
non-significant; HS, hemp seed.
Western blot analysis of pJAK2, total
JAK2, pSTAT5, total STAT5b and IGF-1 protein expression in
C3H10T1/2 cells treated with HS extract. Cells were incubated with
200 μg/ml HS extract and 25 μM AG490 (JAK2 inhibitor)
for 24 h, followed by protein extraction and immunoblotting. p,
phosphorylated; IGF, insulin-like growth factor; HS, hemp
seed.
ELISA showing increased IGF-1
production in C3H10T1/2 cells treated with hemp seed extract.
#P<0.001 vs. control. IGF, insulin-like growth
factor.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National
Research Foundation of Korea grant funded by the Korea government
(Ministry of Science and ICT grant no. RS-2024-00450676) and Korea
Basic Science Institute (National Research Facilities and Equipment
Center) grant funded by the Ministry of Education (grant no.
2023R1A6C101A045) and The Technology development Program (grant no.
S3452042) funded by the Ministry of Small and Medium-sized
Enterprises and Startups.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JC and KJJ designed the experiments. DYK, HTK and YK
performed experiments. DYK and KJJ analyzed the data. DYK wrote the
manuscript. JC and KJJ edited and reviewed the manuscript. DYK and
KJJ confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625.
2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cheung LYM, George AS, McGee SR, Daly AZ,
Brinkmeier ML, Ellsworth BS and Camper SA: Single-cell RNA
sequencing reveals novel markers of male pituitary stem cells and
hormone-producing cell types. Endocrinology. 159:3910–3924.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ashpole NM, Sanders JE, Hodges EL, Yan H
and Sonntag WE: Growth hormone, insulin-like growth factor-1 and
the aging brain. Exp Gerontol. 68:76–81. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Darvin P, Joung YH and Yang YM:
JAK2-STAT5B pathway and osteoblast differentiation. JAK-STAT.
2(e24931)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Laron Z: Insulin-like growth factor 1
(IGF-1): A growth hormone. Mol Pathol. 54:311–316. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Uematsu S and Akira S: The role of
Toll-like receptors in immune disorders. Expert Opin Biol Th.
6:203–214. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kumar H, Kawai T and Akira S: Toll-like
receptors and innate immunity. Biochem Bioph Res Co. 388:621–625.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oberbauer A: The Regulation of IGF-1 gene
transcription and splicing during development and aging. Front
Endocrinol (Lausanne). 4(39)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Varco-Merth B and Rotwein P: Differential
effects of STAT proteins on growth hormone-mediated IGF-I gene
expression. Am J Physiol Endocrinol Metab. 307:E847–E855.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hu X, Li J, Fu M, Zhao X and Wang W: The
JAK/STAT signaling pathway: From bench to clinic. Sig Transduct
Target Ther. 6(402)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu T, Goh ELK, Graichen R, Ling L and
Lobie PE: Signal transduction via the growth hormone receptor. Cell
Signal. 13:599–616. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Callaway JC: Hempseed as a nutritional
resource: An overview. Euphytica. 140:65–72. 2004.
|
|
13
|
Barbara F, Romina M, Nicolò LC and
Merendino N: The seed of industrial hemp (Cannabis sativa
L.): Nutritional Quality and Potential Functionality for Human
Health and Nutrition. Nutrients. 12(1935)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Salehi A, Puchalski K, Shokoohinia Y,
Zolfaghari B and Asgary S: Differentiating Cannabis products:
Drugs, food, and supplements. Front Pharmacol.
13(906038)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pellegrino C, CB 00A0 and Jacopo GC: A
review of hemp as food and nutritional supplement. Cannabis
Cannabinoid Res. 6:19–27. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sirangelo TM, Diretto G, Fiore A, Felletti
S, Chenet T, Catani M and Spadafora ND: Nutrients and bioactive
compounds from cannabis sativa seeds: A review focused on
Omics-based investigations. Int J Mol Sci. 26(5219)2025.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tănase Apetroaei V, Pricop EM, Istrati DI
and Vizireanu C: Hemp seeds (Cannabis sativa L.) as a
valuable source of natural ingredients for functional foods.
Molecules. 29(2097)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hossain L, Whitney K and Simsek S: Hemp
seed as an emerging source of nutritious functional ingredients.
Crit Rev Food Sci Nutr: 1-17, 2025 doi:
10.1080/10408398.2025.2534839 (Epub ahead of print).
|
|
19
|
Chen T, He J, Zhang J, Zhang H, Qian P,
Hao J and Li L: Analytical characterization of Hempseed (seed of
Cannabis sativa L.) oil from eight regions in China. J Diet
Suppl. 7:117–129. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Roche HM: Unsaturated fatty acids. Proc
Nutr Soc. 58:397–401. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Saini RK and Keum YS: Omega-3 and omega-6
polyunsaturated fatty acids: Dietary sources, metabolism, and
significance-A review. Life Sci. 203:255–267. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jin S and Lee MY: The ameliorative effect
of hemp seed hexane extracts on the Propionibacterium acnes-induced
inflammation and lipogenesis in sebocytes. PLoS One.
13(202933)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lin YA, Li YR, Chang YC, Hsu MC and Chen
ST: Activation of IGF-1 pathway and suppression of atrophy related
genes are involved in Epimedium extract (icariin) promoted C2C12
myotube hypertrophy. Sci Rep. 11(10790)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sadowski CL, Wheeler TT, Wang LH and
Sadowski HB: GH Regulation of IGF-I and suppressor of cytokine
signaling gene expression in C2C12 skeletal muscle cells.
Endocrinology. 142:3890–3900. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ker DF, Sharma R, Wang ET and Yang YP:
Development of mRuby2-transfected C3H10T1/2 fibroblasts for
musculoskeletal tissue engineering. PLoS One.
10(e0139054)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Milasincic DJ, Calera MR, Farmer SR and
Pilch PF: Stimulation of C2C12 myoblast growth by basic fibroblast
growth factor and insulin-like growth factor 1 can occur via
mitogen-activated protein kinase-dependent and -independent
pathways. Mol Cell Biol. 16:5964–5973. 1996.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hwangbo Y and Kim JH, Kim T and Kim JH:
Effects of hemp seed protein hydrolysates on the differentiation of
C2C12 cells and muscle atrophy. J Korean Soc Food Sci Nutr.
52:1225–1232. 2023.
|
|
28
|
Hwangbo Y, Pan JH, Lee JJ, Kim T and Kim
JH: Production of protein hydrolysates from hemp (Cannabis
sativa L.) seed and its protective effects against
dexamethasone-induced muscle atrophy. Food Biosci.
59(104046)2024.
|
|
29
|
Kim D, Kim NP and Kim B: Effects of
biomaterials derived from germinated hemp seeds on stressed hair
stem cells and immune cells. Int J Mol Sci. 25(7823)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Azqueta A, Stopper H, Zegura B, Dusinska M
and Møller P: Do cytotoxicity and cell death cause false positive
results in the in vitro comet assay? Mutat Res Genet Toxicol
Environ Mutagen. 881(503520)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kassem M, Blum W, Ristelli J, Mosekilde L
and Eriksen EF: Growth hormone stimulates proliferation and
differentiation of normal human Osteoblast-like cells in vitro.
Calcif Tissue Int. 52:222–226. 1993.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Waters MJ and Brooks AJ: Growth hormone
and cell growth. Endocr Dev. 23:86–95. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Caron JM, Bannon M, Rosshirt L, Luis J,
Monteagudo L, Caron JM and Sternstein GM: Methyl sulfone induces
loss of metastatic properties and reemergence of normal phenotypes
in a metastatic cloudman S-91 (M3) murine melanoma cell line. PLoS
One. 5(e11788)2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kang DY, Sp N, Jo ES, Kim HD, Kim IH, Bae
SW, Jang KJ and Yang YM: Non-toxic sulfur enhances growth hormone
signaling through the JAK2/STAT5b/IGF-1 pathway in C2C12 cells. Int
J Mol Med. 45:931–938. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
van den Beld AW, Kaufman JM, Zillikens MC,
Lamberts SWJ, Egan JM and van der Lely AJ: The physiology of
endocrine systems with ageing. Lancet Diabetes Endocrinol.
6:647–658. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tadashi Y and Delafontaine P: Mechanisms
of IGF-1-Mediated regulation of skeletal muscle hypertrophy and
atrophy. Cells. 9(1970)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mukama T, Srour B, Johnson T, Katzke V and
Kaaks R: IGF-1 and risk of morbidity and mortality from cancer,
cardiovascular diseases, and all causes in EPIC-Heidelberg. J Clin
Endocrinol Metab. 108:e1092–e1105. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ricci Bitti S, Franco M, Albertelli M,
Gatto F, Vera L, Ferone D and Boschetti M: GH Replacement in the
elderly: Is it worth it? Front Endocrinol (Lausanne).
12(680579)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Silvia G, Emanuele M, Stephen E and
Christiaan L: Modulation of GH/IGF-1 axis: Potential strategies to
counteract sarcopenia in older adults. Mech Ageing Dev.
129:593–601. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Maggio M, De Vita F, Lauretani F, Buttò V,
Bondi G, Cattabiani C, Nouvenne A, Meschi T, Dall'Aglio E and Ceda
GP: IGF-1, the crossroad of the nutritional, inflammatory and
hormonal pathways to frailty. Nutrients. 5:4184–4205.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Barclay RD, Burd NA, Tyler C, Tillin NA
and Mackenzie RW: The role of the IGF-1 signaling cascade in muscle
protein synthesis and anabolic resistance in aging skeletal muscle.
Front Nutr. 6(146)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yue X, Hao W, Wang M and Fu Y: Astragalus
polysaccharide ameliorates insulin resistance in HepG2 cells
through activating the STAT5/IGF-1 pathway. Immun Inflamm Dis.
11(e1071)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li Z, Dai X, Yang F, Zhao W, Xiong Z, Wan
W, Wu G, Xu T and Cao H: Compound probiotics promote the growth of
piglets through activating the JAK2/STAT5 signaling pathway. Front
Microbiol. 16(1480077)2025.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ma IL and Stanley TL: Growth hormone and
nonalcoholic fatty liver disease. Immunometabolism.
5(e00030)2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Dichtel LE, Cordoba-Chacon J and Kineman
RD: Growth hormone and insulin-like growth factor 1 regulation of
nonalcoholic fatty liver disease. J Clin Endocrinol Metab.
107:1812–1824. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fedorczak A, Lewiński A and Stawerska R:
Involvement of sirtuin 1 in the growth hormone/insulin-like growth
factor 1 signal transduction and its impact on growth processes in
children. Int J Mol Sci. 24(15406)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jiang LH, Yang NY, Yuan XL, Zou YJ, Zhao
FM, Chen JP, Wang MY and Lu DX: Daucosterol promotes the
proliferation of neural stem cells. J Steroid Biochem Mol Biol.
140:90–99. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kim HK, Kim MG and Leem KH: Icariin
stimulates myogenic differentiation and enhances muscle
regeneration in injured skeletal muscle. Biol Pharm Bull.
39:455–462. 2016.
|
|
50
|
Klover P and Hennighausen L: Postnatal
body growth is dependent on the transcription factors signal
transducers and activators of transcription 5a/b in muscle: a role
for autocrine/paracrine insulin-like growth factor I.
Endocrinology. 148:1489–1497. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Klover P, Chen W, Zhu BM and Hennighausen
L: Skeletal muscle growth and fiber composition in mice are
regulated through the transcription factors STAT5a/b: Linking
growth hormone to the androgen receptor. FASEB J. 23:3140–3148.
2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Joshi AS, da Silva MT, Kumar S and Kumar
A: Signaling networks governing skeletal muscle growth, atrophy,
and cachexia: By. Skeletal Muscle. 15(29)2025.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Schiaffino S and Mammucari C: Regulation
of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights
from genetic models. Skelet Muscle. 1(4)2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rommel C, Bodine SC, Clarke BA, Rossman R,
Nunez L, Stitt TN, Yancopoulos GD and Glass DJ: Mediation of
IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and
PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 3:1009–1013.
2001.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sandri M, Sandri C, Gilbert A, Skurk C,
Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH and Goldberg
AL: Foxo transcription factors induce the atrophy-related ubiquitin
ligase atrogin-1 and cause skeletal muscle atrophy. Cell.
117:399–412. 2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wu Z, Woodring PJ, Bhakta KS, Tamura K,
Wen F, Feramisco JR, Karin M, Wang JY and Puri PL: p38 and
extracellular signal-regulated kinases regulate the myogenic
program at multiple steps. Mol Cell Biol. 20:3951–3964.
2000.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zetser A, Gredinger E and Bengal E: p38
mitogen-activated protein kinase pathway promotes skeletal muscle
differentiation. Participation of the Mef2c transcription factor. J
Biol Chem. 274:5193–5200. 1999.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Chauhan A: Nutrition and health benefits
of hemp-seed protein (Cannabis sativa L.). Pharma
Innovation. 10:16–19. 2021.
|
|
59
|
Jeromson S, Gallagher IJ, Galloway SDR and
Hamilton DL: Omega-3 fatty acids and skeletal muscle health. Mar
Drugs. 13:6977–7004. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hnia K, Gayraud J, Hugon G, Ramonatxo M,
De La Porte S, Matecki S and Mornet D: L-arginine decreases
inflammation and modulates the nuclear factor-kappaB/matrix
metalloproteinase cascade in mdx muscle fibers Am J. Pathol.
172:1509–1519. 2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Greene JM, Feugang JM, Pfeiffer KE, Stokes
JV, Bowers SD and Ryan PL: L-arginine enhances cell proliferation
and reduces apoptosis in human endometrial RL95-2 cells. Reprod
Biol Endocrinol. 11(15)2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Jin CL, Ye JL, Yang J, Gao CQ, Yan HC, Li
HC and Wang XQ: mTORC1 mediates lysine-induced satellite cell
activation to promote skeletal muscle growth. Cells.
8(1549)2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Sun X, Sun Y, Li Y, Wu Q and Wang L:
Identification and characterization of the seed storage proteins
and related genes of Cannabis sativa L. Front Nutr.
8(678421)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Barakat H and Aljutaily T: Hemp-based meat
analogs: An updated review on nutritional and functional
properties. Foods. 14(2835)2025.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Joung YH, Lim EJ, Darvin P, Chung SC, Jang
JW, Park KD, Lee HK, Kim HS, Park T and Yang YM: MSM enhances GH
signaling via the Jak2/STAT5b pathway in osteoblast-like cells and
osteoblast differentiation through the activation of STAT5b in
MSCs. PLoS One. 7(e47477)2012.PubMed/NCBI View Article : Google Scholar
|