Introduction
Heterotopic ossification (HO) is the formation of
mature bone tissues in non-skeletal tissues (1). HO is classified into genetic and
non-genetic forms. The non-genetic form is usually associated with
trauma or tissue injury and is most frequently observed in
atherosclerotic plaques and soft tissues surrounding the joint
(1,2). Moreover, intra- or peritumoral HO has
been reported in various types of benign and malignant lesions,
including meningiomas, melanocytic nevi (osteonevus of Nanta),
epidermal cysts, thyroid tumors and lung tumors (2-9).
The frequency of HO may depend on the origin or histology of the
tumor (6,7,10). HO
is relatively common in papillary thyroid cancer (13%), lung
carcinoid (up to 10%), osteonevus of Nanta (1.56%), and meningiomas
(1%) and rare in others. Although the mechanism of HO formation
remains unclear, it may be related to the tumor microenvironment
and influence tumor behavior (10).
In the digestive system, HO is most frequently
observed in colorectal tumors (10-13).
The most frequent benign polyps with HO were juvenile polyps,
followed by tubulovillous adenomas and traditional serrated
adenomas. By contrast, the most common histological subtype of
colorectal carcinoma with HO was conventional adenocarcinoma,
followed by mucinous adenocarcinoma and serrated adenocarcinoma
(10). The presence of HO in
pancreatic neoplasms is rare, with only 16 patients with pancreatic
neoplasms accompanying HO reported in the English-language
literature (14-25).
The most common histological type of pancreatic neoplasm associated
with HO is the solid pseudopapillary neoplasm (SPN), followed by
intraductal papillary mucinous neoplasm (IPMN). Pancreatic ductal
adenocarcinoma (PDAC) and intrapapillary tubulopapillary neoplasms
have rarely been associated with HO. In the present study, three
patients with pancreatic neuroendocrine tumor (NET), mucinous
cystic neoplasm (MCN), and IPMN with HO were described, and the
clinicopathological features of pancreatic neoplasms with HO were
discussed, as well as the current status of the diagnosis and
significance of tumor-associated HO.
Case report
Patient 1
A 64-year-old Japanese female underwent plain chest
radiography during a medical checkup and was found to have a
nodular shadow in her left pulmonary hilum. Her medical history
included a total hysterectomy for uterine leiomyoma 31 years prior
to presentation. For a close examination, the patient presented to
Osaka Medical College Health and Science Clinic (Takatsuki, Japan)
in July 2020 (as a supplement, the name of this college was changed
to Osaka Medical and Pharmaceutical University in April 2021). The
patient underwent computed tomography (CT), which suggested that
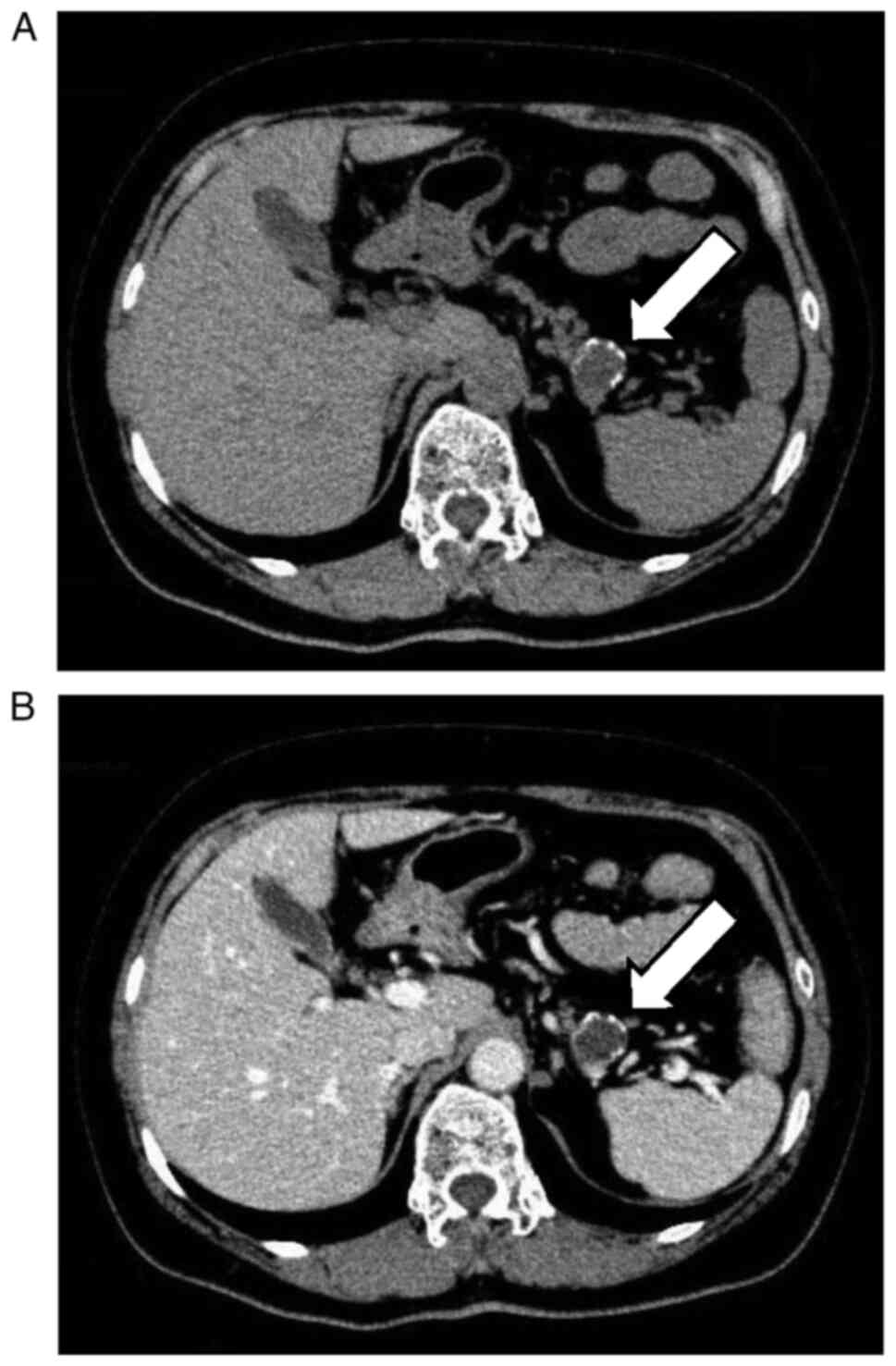
the shadow was of vascular origin and coincidentally detected a
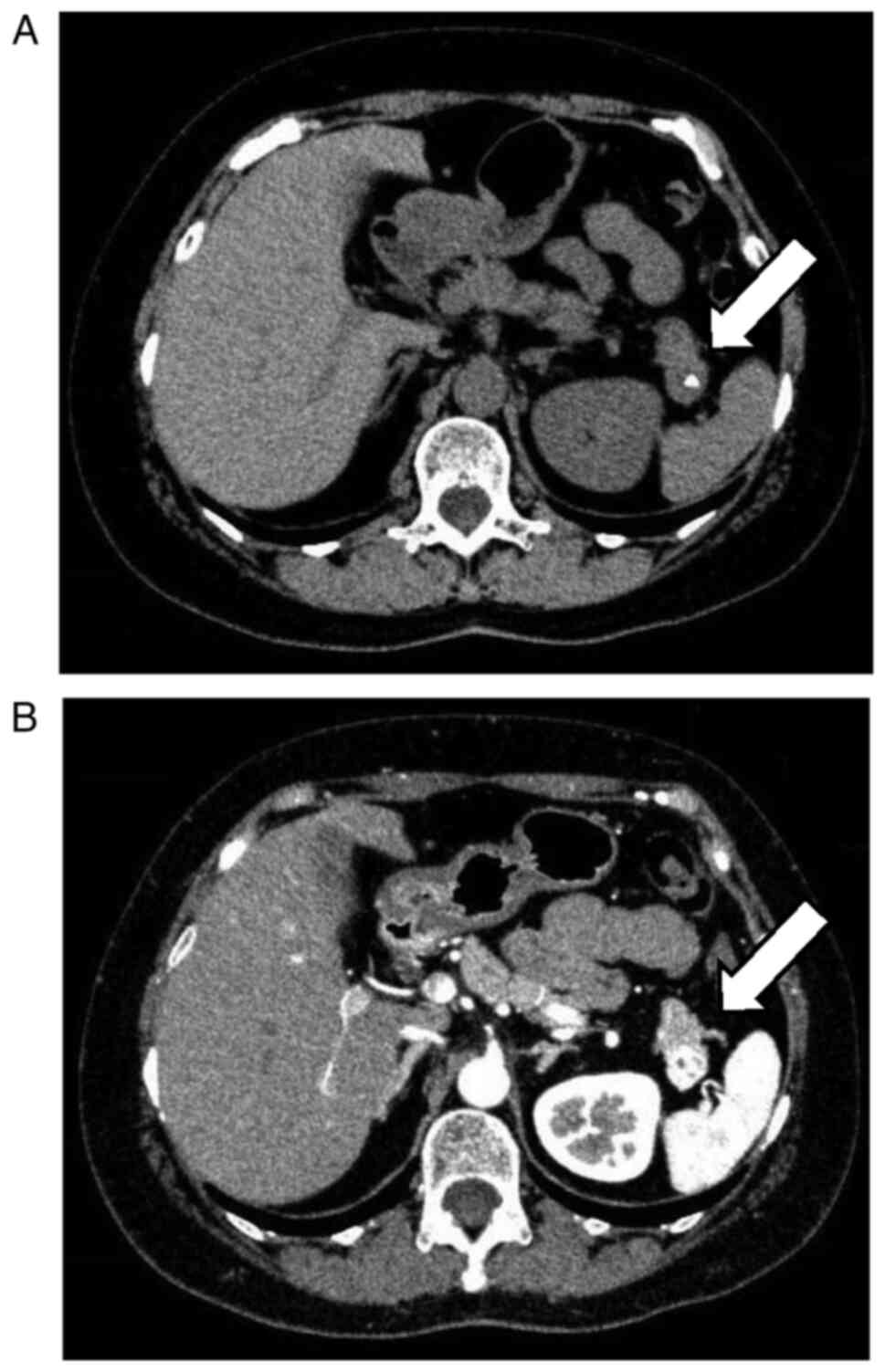
mass measuring 1.5 cm in diameter with calcification in the
pancreatic tail (Fig. 1A and
B). The patient was referred to the
Department of Gastroenterology at Osaka Medical College Hospital
(Takatsuki, Japan) for further examination. As the mass was small
and showed no tendency to enlarge, the patient was followed up for
17 months starting in July 2020. Although tumor size did not
increase during this period, repeat CT showed that the tumor had
solidified, suggesting possible malignancy. Thus, the patient
underwent endoscopic ultrasonography-guided fine-needle aspiration,
and pathological examination of the specimen revealed loosely
cohesive clusters of small round cells, suggesting a pancreatic
NET. The patient underwent a laparoscopic distal pancreatectomy in
February 2022.
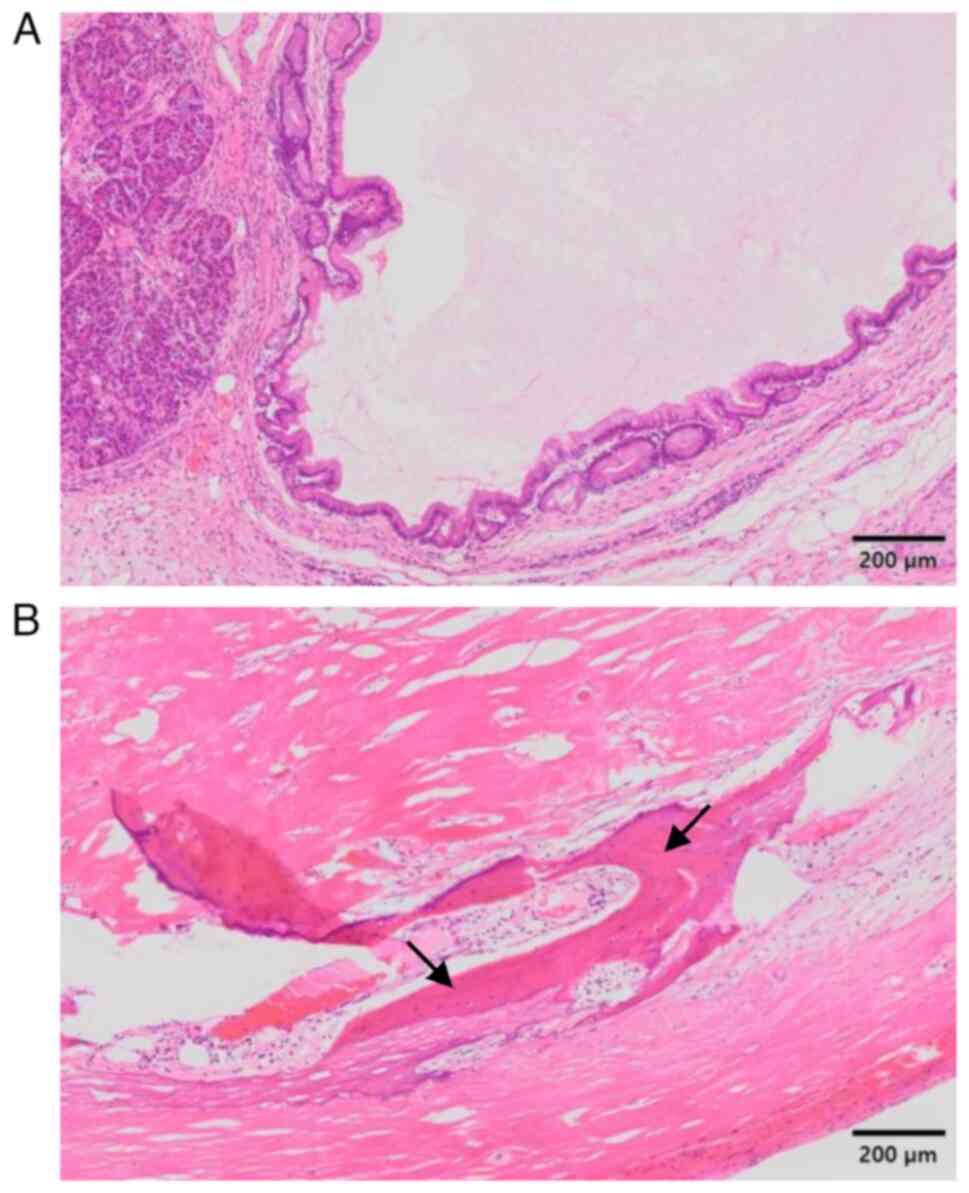
Histopathological examination of the surgically
resected specimens demonstrated that the tumor was covered by a
fibrous capsule and was well-circumscribed from the surrounding
pancreatic parenchyma. Neoplastic cells showing trabecular or
ribbon-like growth had small round nuclei and inconspicuous
nucleoli (Fig. 2A and B). Necrotic or mitotic cells were not
observed. Immunohistochemical analysis demonstrated that the
neoplastic cells expressed chromogranin A and synaptophysin with a
Ki-67 labelling index of 1%. Based on these findings, NET G1 was
diagnosed according to the diagnostic criteria of the World Health
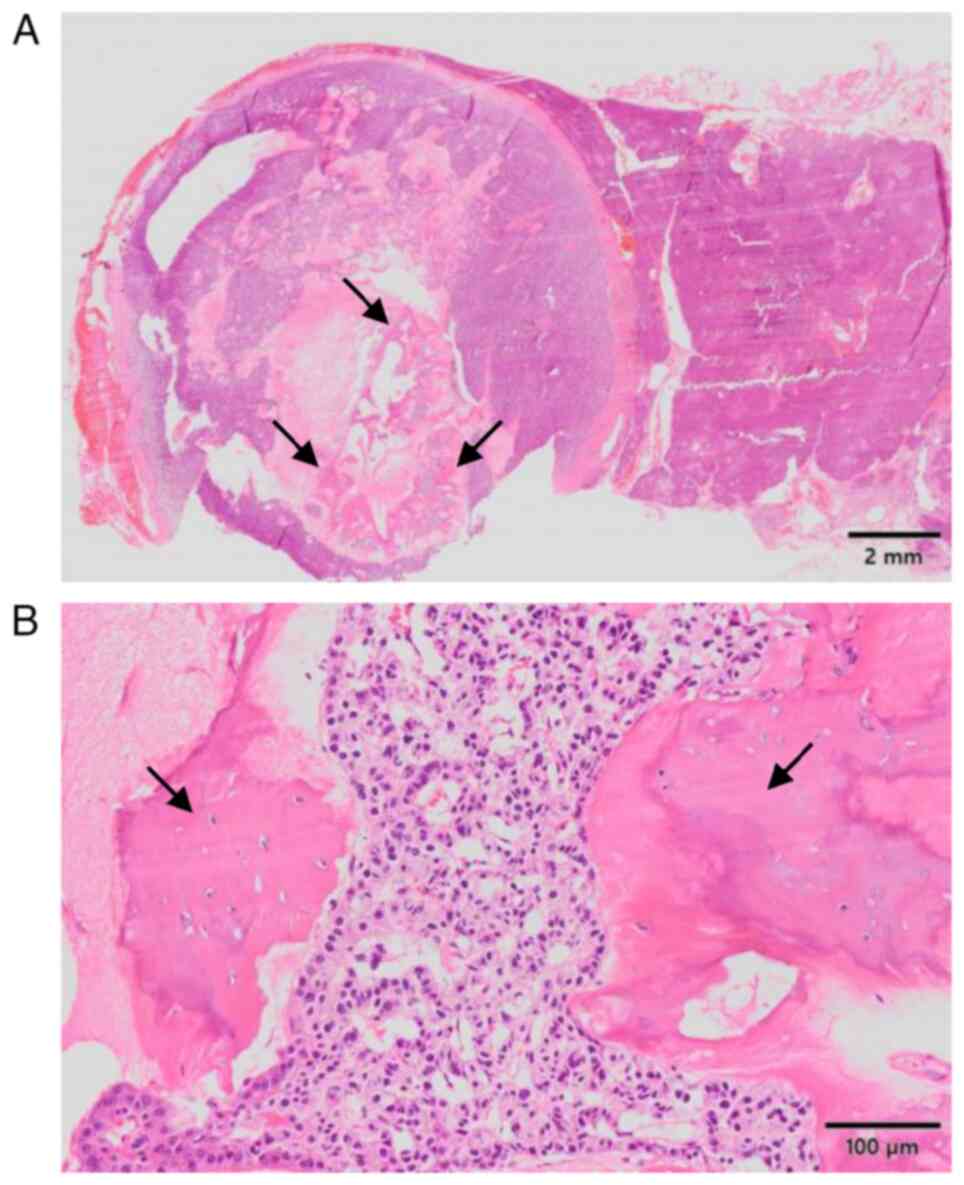
Organization Classification 2019(26). A peculiar finding of the present
tumor was the presence of mature bone tissue, which was composed of
lamellar bone and osteocytes without nuclear atypia within the
fibrosclerotic stroma and with no cartilaginous components
(Fig. 2A and B). The postoperative course was
uneventful, and the patient was free from tumor recurrence for 3
years after surgery without any treatment.
Patient 2
A 62-year-old Japanese female visited Hokusetsu
General Hospital (Takatsuki, Japan) in May 2022 with a complaint of
epigastric pain and was hospitalized for 10 days with a diagnosis
of acute pancreatitis. Her medical history included cholecystectomy
for cholecystolithiasis ~20 years prior to presentation,
hyperlipidemia and Helicobacter pylori (H. pylori)
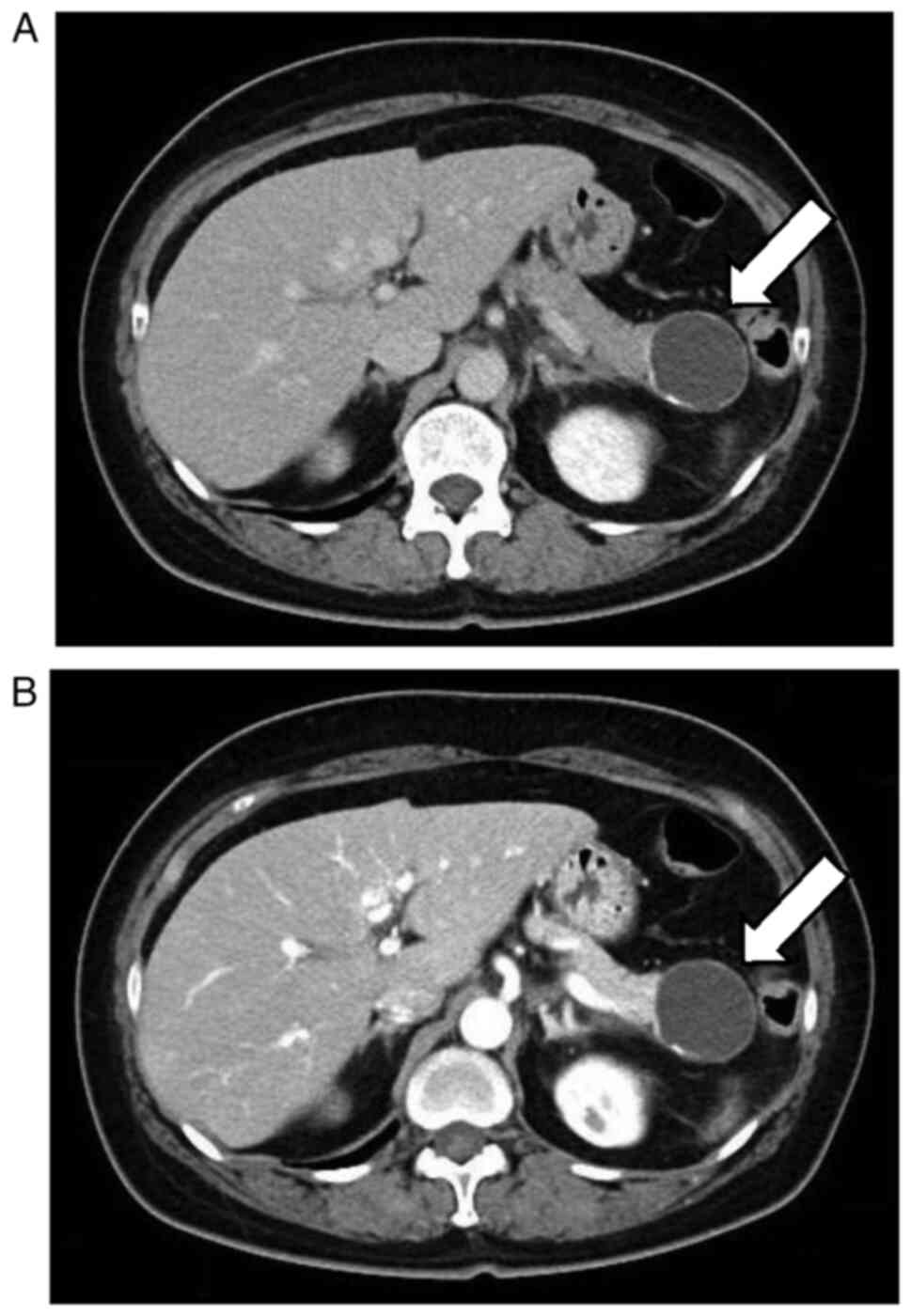
infection. CT revealed a cystic lesion measuring 4 cm in diameter
with calcification in the pancreatic tail (Fig. 3A and B), which was suggested to be MCN. The
patient was referred to the Department of Gastrointestinal Surgery
at Osaka Medical and Pharmaceutical University Hospital (Takatsuki,
Japan). Laparoscopic distal pancreatectomy was performed in
September 2022.
Histopathological examination of the surgically
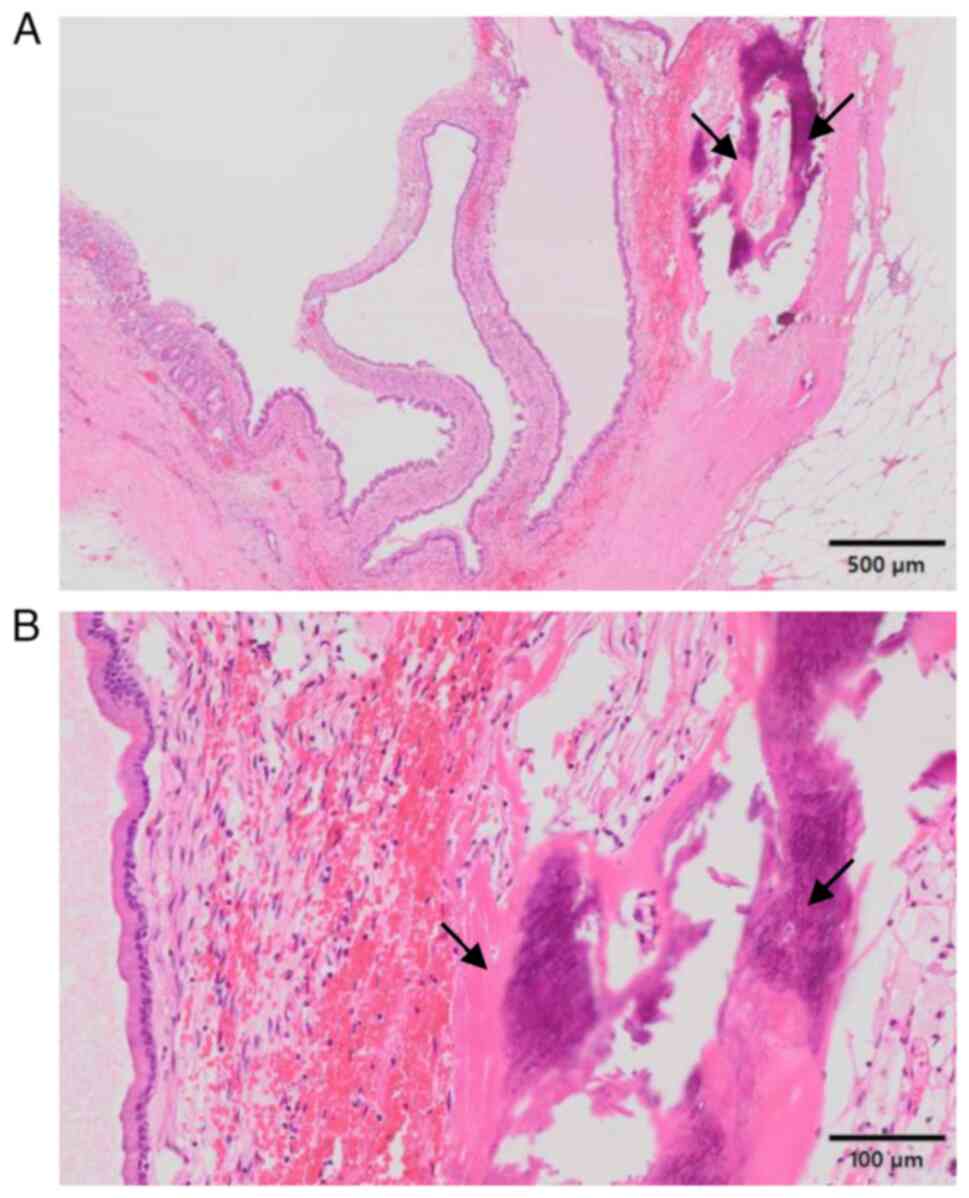
resected specimens revealed a well-circumscribed cystic lesion
covered by mucus-containing columnar cells without nuclear atypia
or invasive growth (Fig. 4A and
B). Characteristically,
ovarian-like stroma was observed around the lesion (Fig. 4B). Moreover, mature bone formation
with osteocytes was noted within the cystic wall, with no
cartilaginous components (Fig. 4A
and B). Accordingly, MCN with
low-grade dysplasia accompanied by HO was diagnosed. The
postoperative course was uneventful, and the patient was free of
tumor recurrence 27 months after surgery without any treatment.
Patient 3
A 71-year-old Japanese female was treated for
diabetes mellitus by her primary care physician for 7 years. For
further evaluation of elevated levels of serum amylase (274 U/l;
range 44-132) and hemoglobin A1c (9.5%; range 4.9-6.0), the patient
was referred to the Department of Diabetes, Metabolism and
Endocrinology at Osaka Medical and Pharmaceutical University
Hospital in November 2022. In addition to diabetes, her medical
history included postoperative cataract, hypertension,
hyperlipidemia, and H. pylori infection. Coincidentally, a
CT scan revealed multiple cystic changes from the pancreatic head
to the tail, and the cystic lesion in the tail was 1.7 cm in
diameter with scattered calcifications (Fig. 5A and B). It was strongly suspected to be an IPMN
with high-grade dysplasia or pancreatic NET, and laparoscopic
distal pancreatectomy was performed in April 2023.
Histopathological examination of the surgically
resected specimens revealed that the main and branch pancreatic
ducts were cystically dilated, and the proliferation of
mucus-containing columnar cells without nuclear atypia or invasive
growth was noted (Fig. 6A).
Ossification composed of lamellar bone and osteocytes without
nuclear atypia was present, with sclerotic fibrosis but no
cartilaginous components around the cyst (Fig. 6B). Accordingly, IPMN with low-grade
dysplasia containing HO was diagnosed. The postoperative course was
uneventful. Multiple cystic lesions in the remnant pancreas were
followed up with periodic CT, and no tumor progression was noted 20
months after surgery.
Discussion
In the present study, the cases of three patients
with pancreatic neoplasms and HO were reported. Only 16 patients
with pancreatic neoplasms accompanying HO have been reported in the
English-language literature (14-25),
which is considered rare. The clinicopathological features of the
previously reported cases as well as those of the three present
cases are summarized in Table I.
There was a slight female predominance (13 females and 6 males),
with the tail region being the most common site of occurrence (10
cases in the tail and 9 in the head and body). Regarding its
histology, HO of pancreatic neoplasms can be observed in benign and
malignant forms, as observed in colorectal tumors (10). HO is observed in benign tumors, such
as IPMN with low-grade dysplasia and MCN with low-grade dysplasia;
low-grade malignant tumors, including SPN and NET G1; and malignant
tumors, such as IPMN with high-grade dysplasia and PDAC. The most
frequent histological subtype of pancreatic neoplasms with HO is
SPN (10 of 19 patients), followed by IPMN (4 patients) and PDAC (2
patients). To the best of the authors' knowledge, this is the first
report of a pancreatic NET and MCN with HO. The reasons for the
differences in the frequency of HO among histological types of
pancreatic neoplasms remain unclear. The reported cases of SPN
suggest that the presence of HO in the abundant fibrous stroma
reflects a reactive or metaplastic process (14,17).
In our current cases of IPMN, MCN and NET, HO was also observed
adjacent to fibrous components, which may further support the
involvement of the aforementioned process. Alternatively, HO has
been reported in two patients with PDAC (15,24),
which might lead to the hypothesis that HO is associated with
proliferative potential. However, HO also occurs in tumors with low
proliferative activity, such as IPMN with low-grade dysplasia, MCN
with low-grade dysplasia, and NET G1, indicating that the causal
relationship between proliferative capacity and HO remains unclear.
Regarding the relationship with prognosis, Table I may suggest that the presence of HO
is not associated with poor prognosis or is not a prognostic
factor; however, there are limitations to drawing further
conclusions.
 | Table IPancreatic neoplasms with heterotopic
ossification in English-language literature. |
Table I
Pancreatic neoplasms with heterotopic
ossification in English-language literature.
| Case no. | Age/Sex | Location | Histological
type | Reason for
detection | Diagnostic
imaging | Postoperative
outcome | Follow-up
duration | (Refs.) |
|---|
| 1 | 44/M | Tail | SPN | Abdominal pain | Calcification/PR and
CT | Alive without
recurrence (AWR) | 4 years and 3
months | (14) |
| 2 | 39/M | Body | SPN | Incidental | Calcification/PR and
CT | AWR | 3 years and 2
months | (14) |
| 3 | 51/F | Tail | SPN | Incidental | Calcification/PR and
CT | AWR | 3 years | (14) |
| 4 | 44/F | Body | SPN | Abdominal pain | Calcification/PR and
CT | AWR | 2 years and 9
months | (14) |
| 5 | 34/F | Tail | SPN | Incidental | Calcification/PR, CT,
and US | AWR | 8 months | (16) |
| 6 | 36/F | Tail | SPN | Dyspepsia and chest
pain | Calcification/CT | AWR | 10 months | (17) |
| 7 | 34/F | Tail | SPN | Incidental | Calcification/CT | AWR | 3 months | (17) |
| 8 | 25/F | Body | SPN | Incidental | Ossified lesion
(ossific focus)/CT, US, EUS, MRI, and ERP | AWR | 2 years | (18) |
| 9 | 38/F | Head | SPN | Abdominal
discomfort | Calcification/CT, US,
and EUS | AWR | 6 months | (20) |
| 10 | 66/F | Body | SPN | Abnormal genital
bleeding | Calcification/CT,
EUS, and MRI | Died of concurrent
PDAC | 2 months | (23) |
| 11 | 71/F | Body | PDAC | Epigastric
fullness, discomfort and pain in the upper abdomen and back, and
loss of weight |
Calcification/CT | Not surgically
removed; diagnosed at autopsy | | (15) |
| 12 | 39/M | Body | PDAC | Epigastric
pain | Calcification/CT
and EUS | Not available
(NA) | NA | (24) |
| 13 | 66/M | Tail | ITPN | Epigastric
pain |
Calcification/CT | AWR | 15 months | (19) |
| 14 | 56/M | Head | IPMN high | Incidental | High-density
area/CT, high signal lesion/MRI T1-weighted | AWR | 2 years and 9
months | (21) |
| 15 | 70/M | Tail | IPMN low | Abdominal
discomfort and pain |
Calcification/CT | NA | NA | (22) |
| 16 | 58/F | Body | IPMN | Upper abdominal
discomfort | Mural
nodule/multidetector CT | Died of other
causes | 2 years and 4
months | (25) |
| 17 | 71/F | Tail | IPMN low | Incidental | Calcification/CT
and EUS | AWR | 1 years and 8
months | Present case |
| 18 | 62/F | Tail | MCN low | Epigastric
pain | Calcification/CT
and EUS | AWR | 2 years and 3
months | Present case |
| 19 | 64/F | Tail | NET | Incidental | Calcification/CT,
EUS, and MRI | AWR | 2 years and 10
months | Present case |
In daily practice, HO is most likely to be
recognized as a simple calcification on radiographs, as is the case
in all three presentations here, and is therefore underestimated.
The frequency of calcification depends on the histological type;
acinar cell carcinoma (6-50%), NET (30%), pancreatoblastoma (30%),
serous cystic neoplasm (30%), SPN (22%) and MCN (15%) are
relatively common, whereas other types, such as IPMN and pancreatic
ductal carcinoma, are rare. Comparing the frequency of
calcification by histological type of pancreatic tumor to that of
HO, the two are somewhat discrepant and thus do not appear to be
directly related. From a mechanistic perspective, although not yet
fully understood, the activation of bone morphogenetic protein
signaling pathways has been implicated in HO in some pancreatic
tumors, again supporting the distinction between HO and dystrophic
calcification (18,21). The involvement of BMP in HO
formation associated with tumors has been reported not only in
pancreatic tumors but also in colorectal cancer, pulmonary
carcinoid and papillary thyroid carcinoma (7,9,10).
Does it make sense to distinguish HO from calcifications? One
possible significance of HO is its potential as a prognostic marker
for pancreatic tumors. Matsunou et al (14) reported that SPN cases with
ossification and calcification had fibrotic areas in their centers
and showed infiltrative growth patterns and pleomorphic atypia,
indicating that they are aggressive. Nakamura et al
(16) also reported a case of SPN
with HO, arguing that this may have a high malignant potential
because of its infiltrating growth pattern. However, the prognostic
significance of HO in SPN has not been established, as this finding
is rare, and recurrence or metastasis in such cases has not been
reported (Table I). HO may also
provide clues for the differential diagnosis of pancreatic tumors,
which may require advances in diagnostic modalities to identify HO
accurately.
In conclusion, the present study describes three
patients with pancreatic neoplasms and HO. Although rare,
clinicians should recognize that several types of pancreatic
neoplasms including NET and MCN can accompany HO. Additional
studies, which are based on increased awareness and interest in HO
and improved accuracy of its identification in diagnostic imaging,
are required to clarify the prognostic significance and underlying
mechanisms of it in pancreatic neoplasms.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
YF, MI and YH drafted the manuscript and figures and
contributed to conception, design, data collection and data
analysis. EY, KN, SK, AT and MA contributed to data collection and
data analysis. YF and YH confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
principles of the Declaration of Helsinki. All data were
anonymized. Written consent for these case reports was obtained
from all patients.
Patient consent for publication
Written consent for publication of patient data and
associated images was obtained from the patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meyers C, Lisiecki J, Miller S, Levin A,
Fayad L, Ding C, Sono T, McCarthy E, Levi B and James AW:
Heterotopic ossification: A comprehensive review. JBMR Plus.
3(e10172)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu K, Tripp S and Layfield LJ:
Heterotopic ossification: Review of histologic findings and tissue
distribution in a 10-year experience. Pathol Res Pract.
203:633–640. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Caffo M, Caruso G, Barresi V and Tomasello
F: Ossified intracranial meningiomas: Description of the first
series of cases and review of the literature. World Neurosurg.
94:458–464. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bezić J, Karaman I, Zekić Tomaš S,
Živković PM and Božić J: Osteonevus of nanta revisited:
Clinicopathological features of 33 cases. Am J Dermatopathol.
38:859–861. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ishida M, Iwai M, Kagotani A, Iwamoto N
and Okabe H: Epidermal cyst of the skin with ossification: report
of two cases. Int J Clin Exp Pathol. 7:1823–1825. 2014.PubMed/NCBI
|
|
6
|
Bai Y, Zhou G, Nakamura M, Ozaki T, Mori
I, Taniguchi E, Miyauchi A, Ito Y and Kakudo K: Survival impact of
psammoma body, stromal calcification, and bone formation in
papillary thyroid carcinoma. Mod Pathol. 22:887–894.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Takeda M, Mikami T, Numata Y, Okamoto M
and Okayasu I: Papillary thyroid carcinoma with heterotopic
ossification is a special subtype with extensive progression. Am J
Clin Pathol. 139:587–598. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim GY, Kim J, Kim TS and Han J: Pulmonary
adenocarcinoma with heterotopic ossification. J Korean Med Sci.
24:504–510. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tsubochi H, Endo S, Oda Y and Dobashi Y:
Carcinoid tumor of the lung with massive ossification: Report of a
case showing the evidence of osteomimicry and review of the
literature. Int J Clin Exp Pathol. 6:957–961. 2013.PubMed/NCBI
|
|
10
|
Vos AM, Pijnenborg L, van Vliet S, Kodach
LL, Ciompi F, van der Post RS, Simmer F and Nagtegaal ID:
Biological background of colorectal polyps and carcinomas with
heterotopic ossification: A national study and literature review.
Hum Pathol. 145:34–41. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nagano H, Togawa T, Watanabe T, Ohnishi K,
Kimura T, Iida A, Noriki S, Imamura Y, Sato Y and Goi T:
Heterotopic ossification in lymph node metastasis after rectal
cancer resection: A case report and literature review. World J Surg
Oncol. 19(2)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shimazaki J, Takemura A, Nishida K,
Kajiyama H, Shimoda M and Suzuki S: Heterotopic ossification in
rectal carcinoma: report of a case and review of the literature.
Case Rep Oncol. 9:698–704. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Haque S, Eisen RN and West AB: Heterotopic
bone formation in the gastrointestinal tract. Arch Pathol Lab Med.
120:666–670. 1996.PubMed/NCBI
|
|
14
|
Matsunou H, Konishi F, Yamamichi N,
Takayanagi N and Mukai M: Solid, infiltrating variety of papillary
cystic neoplasm of the pancreas. Cancer. 65:2747–2757.
1990.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kimura W, Shimada H and Akabane H: An
autopsy case of pancreatic duct cell carcinoma associated with
ossification. Hepatogastroenterology. 38:396–399. 1991.PubMed/NCBI
|
|
16
|
Nakamura S, Okayama Y, Imai H, Aoki S,
Kobayashi S, Hattori T, Shiraki S, Goto K, Sano H, Ohara H, et al:
A solid cystic tumor of the pancreas with ossification and possible
malignancy, coexisting nonfusion of the pancreatic ducts. J Clin
Gastroenterol. 33:333–336. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim NR, Han J, Chung DH and Ha SY: Solid
and papillary epithelial neoplasm of the pancreas with
ossification: A report of two cases. Histopathology. 46:355–357.
2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tajima K, Kawaguchi Y, Ito H, Ogawa M,
Toriumi K, Hirabayashi K, Takekoshi S and Mine T: A case of
pancreatic solid-pseudopapillary neoplasm with marked ossification.
Clin J Gastroenterol. 4:112–117. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jokoji R, Tsuji H, Tsujimoto M, Shinno N
and Tori M: Intraductal tubulopapillary neoplasm of pancreas with
stromal osseous and cartilaginous metaplasia; A case report. Pathol
Int. 62:339–343. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Carrara S, Spaggiari P and Zerbi A: A bone
in the pancreas. Gastroenterology. 150:320–321. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hadano A, Hirabayashi K, Yamamuro H,
Takanashi Y, Yamada M, Kawanishi A, Kawaguchi Y, Furukawa D,
Nakagohri T, Imai Y, et al: Bone morphogenetic protein-2 expression
in an intraductal papillary mucinous neoplasm with marked
ossification: A case report. Pathol Int. 66:343–347.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chetty R, Kalimuthu SN and Khalili K:
Intraductal papillary mucinous neoplasm with extensive mural
osseous metaplasia and calcification. J Pancreas. 19:43–47.
2018.
|
|
23
|
Tsutsumi C, Abe T, Sawatsubashi Y, Tamiya
S, Kakihara D, Nishihara K and Nakano T: Synchronous solid
pseudopapillary neoplasm and invasive ductal carcinoma of the
pancreas: A case report. Surg Case Rep. 6(202)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sugiura R, Sasaki K, Nakamura H, Horita S,
Meguro T, Kagaya H, Yoshida T, Aoki H, Morita T, Fujita M, et al: A
rare case of pancreatic ductal adenocarcinoma with ossification
mimicking a pancreatic stone impaction. Endosc Ultrasound.
12:290–291. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tezuka K, Yamakawa M, Murakami R, Hirai I,
Toya R, Suzuki A, Kawamura H, Miyano Y, Sato H and Motoi F:
Familial intraductal papillary mucinous neoplasm associated with
the germline MSH6 missense variant and progression of pancreatic
cancer. Pancreas. 53:e476–e486. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Klimstra DS, Klöppel G, La Rosa S and
Rindi G: Classification of neuroendocrine neoplasms of the
digestive system. In WHO Classification of Tumors 5th Edition.
Digestive system tumors. IARC, Lyon, pp14-19, 2019.
|