Introduction
Iron deficiency anemia (IDA) is a prevalent health
concern, particularly in children, women, and elderly populations.
IDA is the most common type of anemia and has a multifactorial
etiology, including insufficient iron intake, impaired absorption,
increased demand, and excessive loss (1,2). Oral
iron supplementation is a standard treatment; however, its low
absorption rate and gastrointestinal side effects often pose
challenges. Intravenous iron administration is an alternative;
however, its long-term use raises concerns regarding adverse
effects, such as atherosclerosis and an increased risk of
infections (3,4). Therefore, identifying molecular
regulators of intestinal iron absorption could reduce reliance on
high-dose supplementation and lower adverse risks.
Iron absorption primarily occurs in the duodenum,
where iron transporters, such as solute carrier family 11 member 2
(SLC11A2; also known as divalent metal transporter 1), solute
carrier family 40 member 1 (SLC40A1; also known as ferroportin),
and solute carrier family 46 member 1 (SLC46A1; also known as heme
carrier protein 1), play key roles (5-7).
Understanding the regulatory mechanisms of these transporters is
essential to improving IDA treatment strategies.
Serotonin is widely recognized as a
neurotransmitter; however, studies conducted in the 1960s have
suggested its potential involvement in iron absorption (8). Recently, abnormal peripheral serotonin
levels have been linked to dysregulation of iron homeostasis in
patients with myelodysplastic syndromes (9). Serotonin has also been demonstrated to
regulate erythropoiesis (10) and
may influence iron metabolism via adenosine monophosphate-activated
protein kinase (AMPK)-dependent pathways (11). Elevated peripheral serotonin levels
have been reported in pediatric patients with IDA, suggesting that
serotonin may contribute to its pathophysiology (12). In addition, serotonin is secreted by
enterochromaffin cells in the gut, where it regulates intestinal
motility, secretion, and epithelial cell function (13,14).
This suggests that serotonin influences iron transport in the
intestine. However, no studies have directly examined how serotonin
affects specific intestinal iron transporters such as SLC11A2.
Addressing this gap may reveal new regulatory pathways for
optimizing iron supplementation.
In this study, we investigated the effects of
serotonin on the expression and function of iron transporters.
Specifically, we used Caco-2 cells, a widely used intestinal
epithelial cell model, to analyze the effect of serotonin on
iron-ion transport mediated by SLC11A2. We hypothesize that
serotonin modulates intestinal iron absorption by regulating
SLC11A2 expression and function.
Materials and methods
Chemicals and reagents
Serotonin and receptor ligands were obtained from
Nacalai Tesque Inc. (Kyoto, Japan), Cayman Chemical Co. (Ann Arbor,
MI, USA) and Abcam (Cambridge, UK). Antibodies were obtained from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), Proteintech
Group, Inc. (IL, USA) and R&D Systems Inc. (MN, USA). Other
general reagents used in the biochemical experiments were obtained
from Wako (Osaka, Japan). All antibodies and reagents used were of
analytical grade. All primers were obtained from FASMAC (Kanagawa,
Japan). The primer sequences are listed in Table I.
 | Table IPrimers designed for RT-PCR and
RT-qPCR. |
Table I
Primers designed for RT-PCR and
RT-qPCR.
| Gene | Forward, 5'-3' | Reverse, 5'-3' |
|---|
| GAPDH |
GCGAGATCCCTCCAAAATCA |
ATGGTTCACACCCATGACGA |
| SLC11A2 |
AGTCATCCTGTGGCTGAT |
GTATCTGCAATGGTGATGAG |
| 5-HT1A receptor |
ACAACACCACATCACCACC |
AGCAGCAGAGAGGTGATCA |
| 5-HT1B receptor |
ATGGCGCAGAGAAACCTT |
TGTGGCTTGACAATCGCT |
| 5-HT2A receptor |
TCAGCTCTTGCATGCAGT |
TTCTGTGACTCGCTGCAT |
| 5-HT2B receptor |
TGCTGGATGGTTCTCGAA |
CGTTGGAAATGGTCTGCA |
| 5-HT2C receptor |
ACCGCTGACGATTATGGT |
TCTTGCAGCACTTCAGGA |
| 5-HT3 receptor |
AACATCTCTTTGTGGCGC |
TGCTGAACTCCCGAAAGT |
| 5-HT4 receptor |
TTCACCTGAGGCTTTCCGTC |
AGGAGGCCATTATGTCCCCT |
| 5-HT5 receptor |
ACCTCCTTTTCCCTCTCCA |
ATAAGCATCCGAAGACCG |
| 5-HT6 receptor |
TACCTGCAGGGATCATAGC |
CAACATCCAGAGTCCTCCA |
| 5-HT7 receptor |
AGGGTCTCTGTGATTTCCC |
TCTCAAGACCCTTCAGAGC |
Cell culture
Human colon carcinoma Caco-2 cells were maintained
in Dulbecco's modified Eagle's medium (Wako) supplemented with 10%
heat-inactivated fetal bovine serum (Equitech-Bio, Inc., TX, USA)
and 1X MEM non-essential amino acid solution, with 5%
CO2 at 37˚C. For assays, cells were cultured for 21 days
after reaching confluence to allow differentiation into
enterocyte-like monolayers. The cells were used between passages 10
and 20 and seeded at 2x105 cells/dish in a 35 mm dish
for all experiments. Caco-2 cells were chosen as they are a
validated intestinal epithelial model for iron transport
studies.
cDNA synthesis from total RNA
Caco-2 cells were treated with 100, 200, 300, 400 or
500 µg/ml serotonin for 6 h prior to RNA extraction. In another
experiment, Caco-2 cells were treated with serotonin (500 µg/ml)
for 1, 3, 6, 9 or 24 h. Untreated cells, which received culture
medium without serotonin, were included as controls. Total RNA was
extracted from cultured Caco-2 cells using ISOGEN II (Nippon Gene,
Tokyo, Japan), and cDNA was synthesized using the ReverTra
Ace® qPCR RT Master Mix (TOYOBO, Osaka, Japan). In
brief, RNA (0.5 µg) was combined with 2 µl of 5X RT Master Mix and
incubated at 37˚C for 15 min. The reverse transcription reaction
was terminated by heating at 98˚C for 5 min to inactivate the
enzyme.
Reverse transcription (RT)-PCR and
RT-quantitative PCR (RT-qPCR)
RT-PCR was carried out using serotonin
receptor-specific forward and reverse primers (Table I) with Takara Ex Taq reagent
(Takara, Shiga, Japan). The thermal cycling conditions consisted of
an initial denaturation at 94˚C for 30 sec, followed by 30 cycles
of denaturation at 98˚C for 10 sec, annealing at 55˚C for 30 sec,
and extension at 72˚C for 1 min. The PCR products were separated
via electrophoresis on a 2% agarose gel, stained with ethidium
bromide, and visualized under UV light.
RT-qPCR was performed using SLC11A2-specific forward
and reverse primers (Table I) and
THUNDERBIRD® Next SYBR™ qPCR Mix (TOYOBO). The reaction
protocol involved an initial denaturation at 95˚C for 30 sec,
followed by 40 cycles of 95˚C for 5 sec and 60˚C for 34 sec.
Expression levels of SLC11A2 mRNA were quantified using a standard
curve and normalized to GAPDH expression within the same
sample.
Western blotting
Caco-2 cells were treated with serotonin (500 µg/ml)
for 6 or 24 h prior to protein extraction. Untreated cells, which
received culture medium without serotonin, were included as
controls. Total proteins extracted from Caco-2 cells (5 µg per
sample) were separated via SDS-PAGE (4-15% gradient gel, 200 V, 30
min) and then blotted onto a PVDF membrane at 0.35 A for 1 h. The
membrane was blocked with 1% non-fat dry milk in Tris-buffered
saline containing 0.05% Tween-20 (TBS-T) for 1 h and then incubated
overnight at 4˚C with an anti-NRAMP2 (another name of SLC11A2)
polyclonal antibody (1:5,000). After washing with TBS-T, the
membrane was incubated with HRP-conjugated anti-mouse IgG
polyclonal antibody (1:5,000) for 2 h at room temperature.
Detection was carried out using Clarity Western ECL Substrate
(Bio-Rad, Hercules, CA, USA), and signal intensities were
quantified using the Fusion-FX imaging system (Vilber,
Marne-la-Vallée, France). Protein expression levels of SLC11A2 were
normalized to GAPDH levels in the same sample.
Measurement of intracellular iron
concentration (Ferrozine assay)
Caco-2 cells treated with serotonin (500 µg/ml) for
6 or 24 h were administered 10 mM ammonium iron (II) sulfate
hexahydrate as a soluble iron salt. After 30 min of incubation,
cells were washed with phosphate-buffered saline (-), and lysates
were prepared using RIPA buffer. Untreated cells, which received
culture medium without serotonin and a soluble iron salt, were
included as controls. Protein content (50 µg) from each lysate was
mixed with an acidic solution (pH 2.5) of HCl. Intracellular
Fe2+ levels were quantified using a ferrozine-based iron
assay kit (Metallogenics, Chiba, Japan), according to the
manufacturer's instructions (15).
Ferrozine specifically forms a magenta complex with ferrous iron,
exhibiting a characteristic absorbance peak at approximately 562
nm, which enables selective spectrophotometric detection of
Fe²+ (16). Therefore,
absorbance at 562 nm, corresponding to the
Fe2+-ferrozine complex, was measured to determine iron
concentration.
Statistical analysis
All experimental data were obtained from four
independent replicates and are expressed as means ± standard error
(SE). Normality of the data was assessed using the Shapiro-Wilk
test in JMP Pro 17 (SAS Institute). Comparisons between groups were
conducted using one-way ANOVA followed by Tukey-Kramer post hoc
test. Effect sizes (Cohen's d) and 95% confidence intervals were
calculated where appropriate. Statistical analyses were performed
using JMP Pro 17, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of serotonin on SLC11A2 mRNA
and protein expression
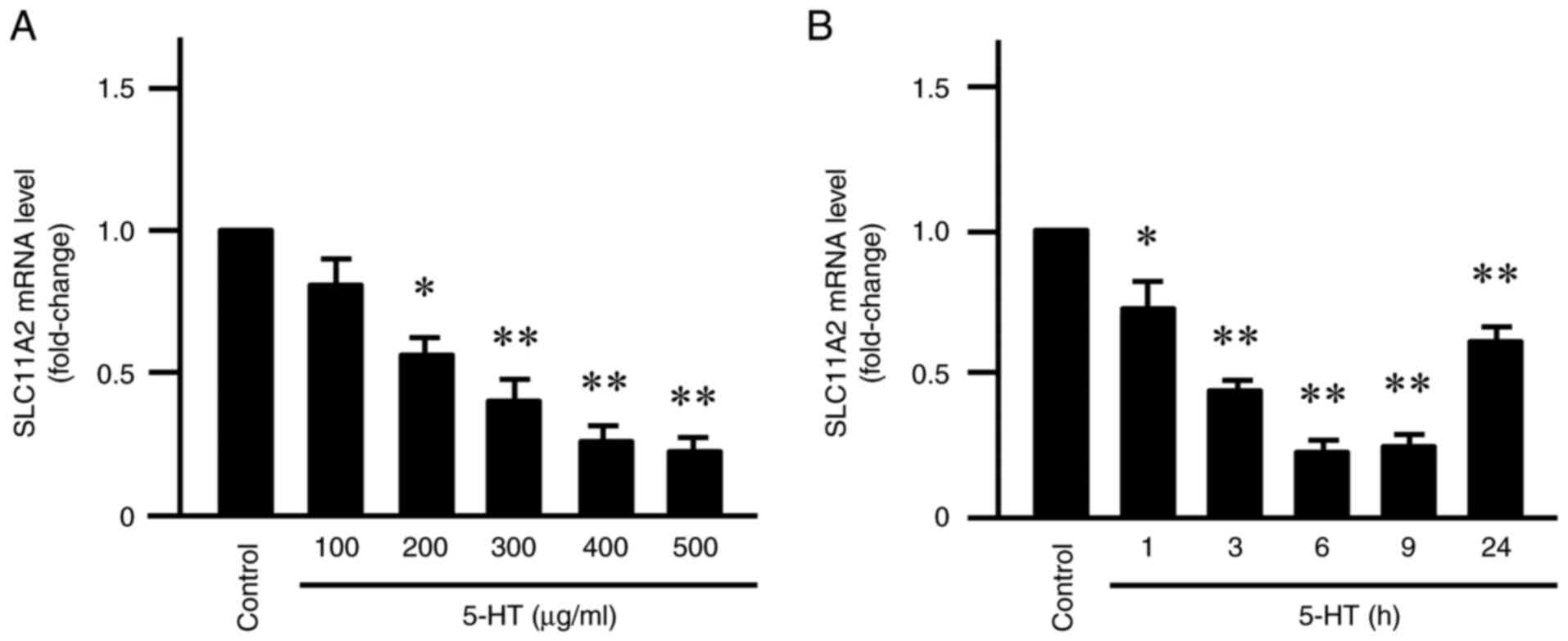
First, the effect of serotonin treatment on SLC11A2
mRNA expression in Caco-2 cells was examined via RT-qPCR. As shown
in Fig. 1A, when Caco-2 cells were
treated with serotonin (100-500 µg/ml) for 6 h, SLC11A2 mRNA levels
decreased in a dose-dependent manner. The most significant
reduction was observed at 500 µg/ml, where mRNA levels were
~0.25-fold lower compared to those in the control group (P<0.01;
Fig. 1A). Furthermore, time-course
analysis showed that SLC11A2 mRNA levels remained low for up to 24
h after serotonin (500 µg/ml) treatment, showing a significant
decrease of ~0.25-fold at 6 and 9 h (P<0.01; Fig. 1B). In addition, serotonin treatment
reduced the mRNA levels of SLC40A1 and SLC46A1 (Fig. S1).
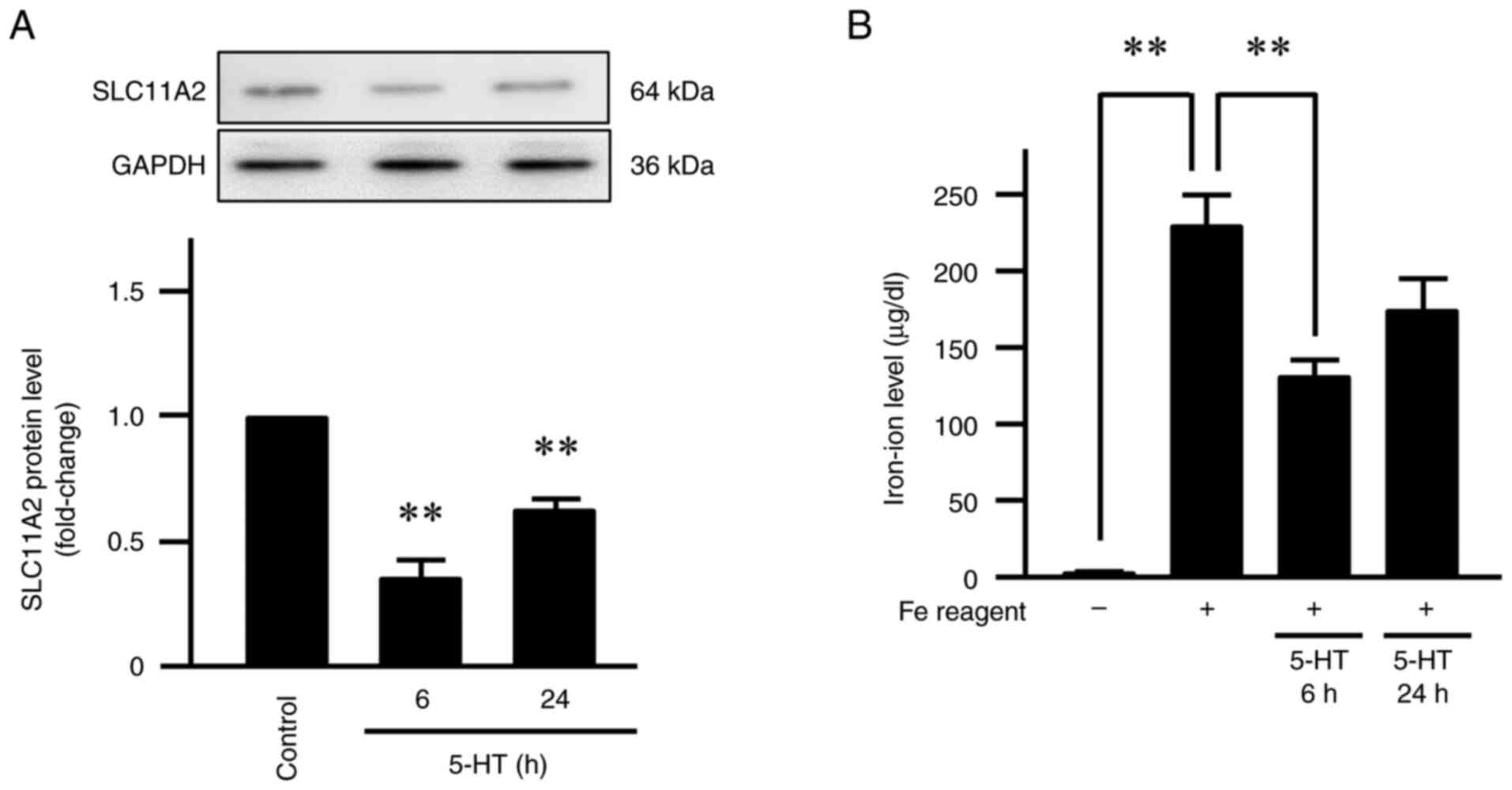
SLC11A2 protein expression levels were evaluated via
western blotting. SLC11A2 protein expression decreased
significantly, by ~0.35-fold at 6 h and ~0.62-fold at 24 h after
serotonin treatment (P<0.01; Fig.
2A). The image in Fig. 2A
presents a typical result from biological replicates obtained from
four independent experiments.
Effect of serotonin on iron-ion
transport into Caco-2 cells
Intracellular iron concentrations were measured
using the ferrozine assay. Cells treated with ammonium iron (II)
sulfate hexahydrate (hereafter, ‘Fe reagent’) was 230 µg/dl,
approximately 80 times higher than that in control cells (2.96
µg/dl). When serotonin-treated Caco-2 cells were subsequently
exposed to Fe reagent, intracellular iron concentrations decreased
to 132 µg/dl after 6 h and 176 µg/dl after 24 h of the treatment
(P<0.01; Fig. 2B). Fig. 2B shows intracellular iron
concentrations in control and serotonin-treated cells, with error
bars representing SE of four independent biological replicates.
Expression of serotonin receptors in
Caco-2 cells
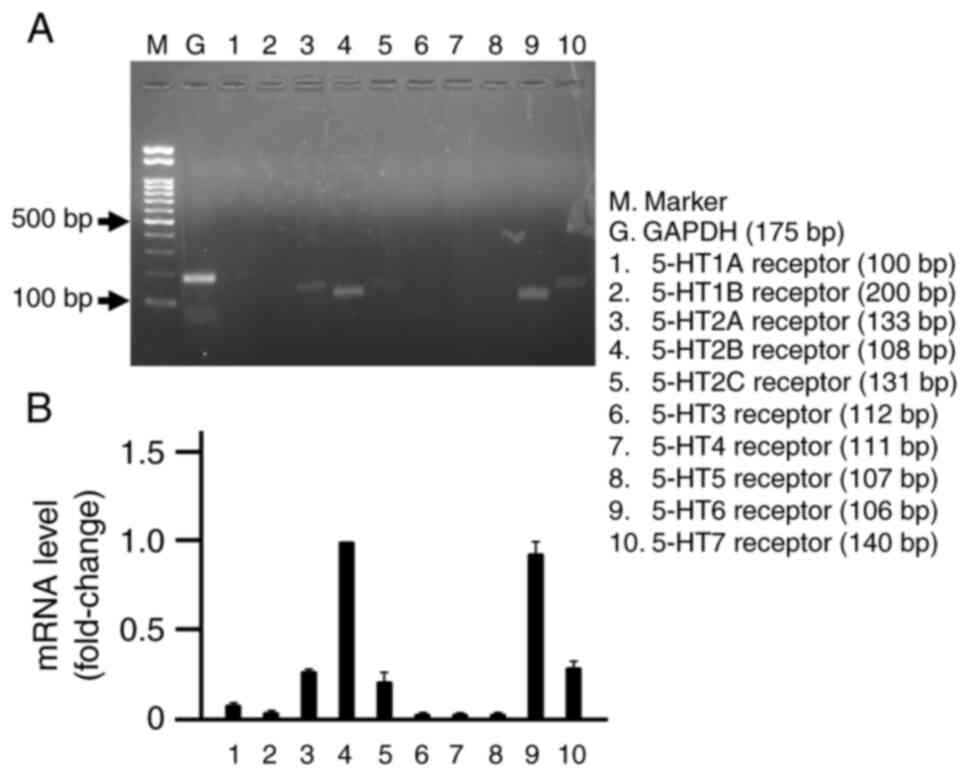
The mRNA expression of serotonin receptor subtypes
in Caco-2 cells was analyzed using RT-PCR. Fig. 3 shows the presence or absence of
each receptor subtype. The results showed mRNA expression of
5-HT2A, 5-HT2B, 5-HT2C, 5-HT6, and 5-HT7 receptors; however,
5-HT1A, 5-HT1B, 5-HT3, 5-HT4, and 5-HT5 receptors were not
expressed (Fig. 3).
Involvement of 5-HT2B receptor in
serotonin actions
5-HT2B receptor is widely expressed in peripheral
tissues such as vascular and gastrointestinal smooth muscles
(17,18), making it the most likely candidate
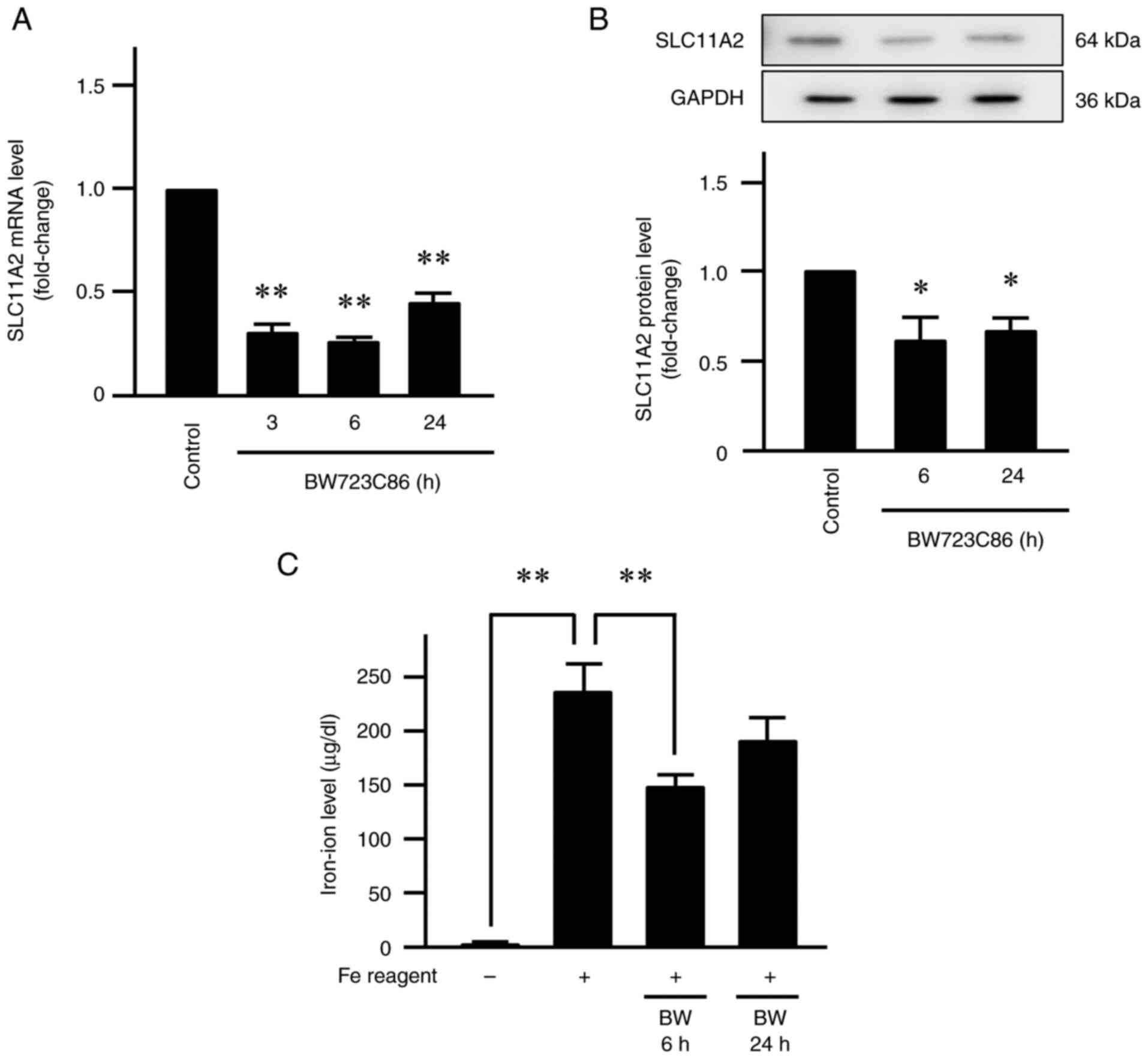
for regulating SLC11A2 expression in Caco-2 cells. Therefore, we
treated Caco-2 cells with BW723C86 (100 µM). This resulted in a
significant reduction in SLC11A2 mRNA expression by ~0.31-0.45-fold
(P<0.01; Fig. 4A), as well as a
decrease in protein expression by ~0.65-fold (P<0.05; Fig. 4B). Intracellular iron concentration
also decreased from 237 µg/dl (cells treated only with Fe reagent)
to 149 µg/dl after 6 h and to 192 µg/dl after 24 h of BW723C86
treatment (P<0.01; Fig. 4C).
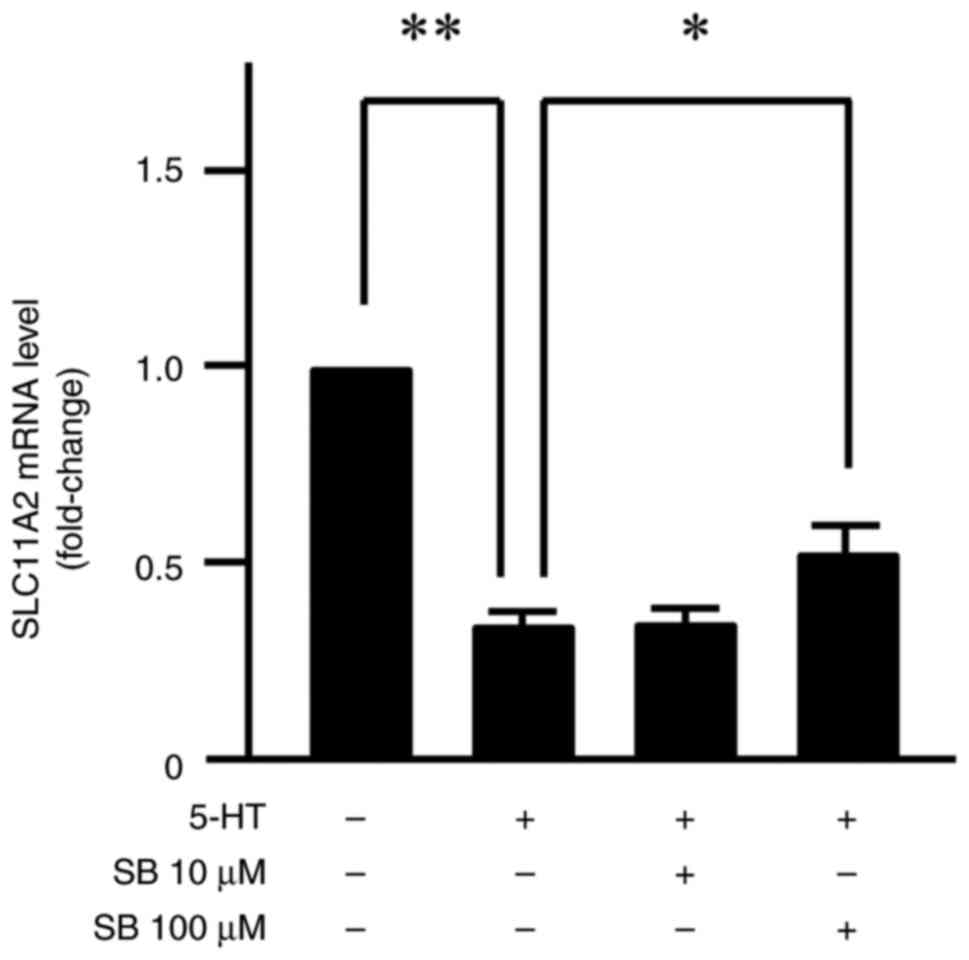
Furthermore, treatment with SB204741 (100 µM) inhibited the
serotonin-induced reduction in SLC11A2 mRNA expression (P<0.05;
Fig. 5). Figs. 4 and 5 summarize representative results from
four independent biological replicates, with error bars indicating
SE and asterisks denoting statistically significant differences
(P<0.05).
Additionally, to examine whether other serotonin
receptor subtypes might also be involved, we tested TCB-2, a
selective 5-HT2A receptor agonist, and MK-212, a selective 5-HT2C
receptor agonist. TCB-2 reduced the mRNA levels of SLC11A2
(Fig. S2).
Discussion
Iron ion absorption primarily occurs in the small
intestine. Therefore, this study required a cultured cell line that
closely resembled human intestinal epithelial cells. Consequently,
we used Caco-2 cells, a human colorectal cancer-derived cell line
with a morphology and structure similar to those of human
intestinal epithelial cells (19,20).
Previous studies have reported that Caco-2 cells can be used to
evaluate the cellular uptake of drugs and ions, and that they
possess sufficient amounts of various iron transport-related
factors, including iron transporters (21,22).
Based on these characteristics, we determined that Caco-2 cells are
the most suitable model for this study.
In this study, we selected SLC11A2 as the target
iron transporter. SLC11A2 is primarily responsible for divalent
cation transport, and its expression in the brush border membrane
of intestinal epithelial cells fluctuates depending on iron
deficiency in the body, such as in IDA (23,24).
Therefore, it is reasonable to hypothesize that SLC11A2 plays a
crucial role in the response to changes in systemic iron levels.
Identifying the factors that influence SLC11A2 expression and
function could elucidate the mechanisms of iron absorption and
provide insights into new therapeutic targets for anemia caused by
iron deficiency.
Based on these considerations, we examined the
effects of serotonin on the expression of SLC11A2 and iron-influx
in Caco-2 cells. As a result, serotonin consistently reduced
SLC11A2 expression at both the mRNA and protein levels, accompanied
by decreased intracellular iron accumulation. These results suggest
that serotonin suppresses SLC11A2 expression, thereby affecting
iron transport capacity. Although measurement methods using this
study do not fully replicate the polarized intestinal epithelium,
previous studies have validated Caco-2 cells as a reliable in vitro
model for investigating intestinal iron absorption and transporter
function (25).
Serotonin receptors are classified into seven
subtypes, each containing multiple variants (26,27).
We found that 5-HT2A, 2B, 2C, 6, and 7 receptor mRNAs were involved
in the serotonin-mediated effects. Among these, 5-HT2A, 2C, 6, and
7 receptors are primarily expressed in the central nervous system,
whereas 5-HT2B receptors are widely expressed in peripheral
tissues, including gastrointestinal smooth muscle (17,26).
Therefore, we investigated the involvement of 5-HT2B receptors in
mediating the effects of serotonin.
BW723C86 consistently reduced SLC11A2 expression at
both the mRNA and protein levels, and decreased intracellular iron
accumulation. Moreover, SB204741 suppressed serotonin-induced
reduction in SLC11A2 mRNA levels. These results suggest that
serotonin suppresses SLC11A2 expression and iron influx through the
5-HT2B receptors. However, partial inhibition by SB204741 suggests
the involvement of additional receptor subtypes such as 5-HT2A,
5-HT2C, 5-HT6, or 5-HT7, which were also expressed in Caco-2 cells.
TCB-2 also reduced the mRNA levels of SLC11A2. Thus, further
investigations are warranted to elucidate their potential
involvement in iron absorption.
This work focused on SLC11A2 as a primary iron
transporter. However, serotonin treatment reduced the mRNA levels
of SLC40A1 and SLC46A1 in Caco-2 cells. These results suggest that
serotonin may influence SLC11A2 as well as other iron transporters,
regulating both intracellular iron influx and extracellular iron
export. Detailed analyses of these transporters are necessary to
gain a comprehensive understanding of the effects of serotonin on
iron metabolism.
Serotonin is abundant in the gastrointestinal tract,
where it regulates motility, secretion, and epithelial renewal.
Previous clinical reports have shown altered serotonin levels in
patients with iron deficiency anemia and chronic inflammatory
disorders such as inflammatory bowel disease, which are frequently
associated with disturbed iron absorption (12,28,29).
Our findings therefore provide a potential mechanistic link between
peripheral serotonin signaling and intestinal iron metabolism.
Whereas, this study is limited by the use of supraphysiological
serotonin concentrations, reliance on an in vitro model, and lack
of in vivo validation. Future work should address whether
physiological serotonin levels exert comparable effects in animal
models and humans.
The findings of this study contribute to the
understanding of the pathophysiology of IDA. Notably, we
demonstrated that 5-HT2B regulates iron influx by modulating
SLC11A2 expression, suggesting that this receptor may be a novel
therapeutic target for IDA. However, therapeutic targeting of this
receptor requires caution. Both 5-HT2B agonists and antagonists
have been implicated in cardiac valvulopathy and other
cardiovascular risks (30,31). Further validation using animal
models is essential to determine whether 5-HT2B receptor ligands
can serve as novel therapeutic strategies for enhancing iron
absorption.
In summary, our findings support 5-HT2B-mediated
regulation of intestinal iron transport, pointing to serotonin
signaling as a previously underrecognized modulator of iron
homeostasis. Future in vivo studies will be necessary to clarify
its physiological relevance and therapeutic potential in IDA.
Supplementary Material
Effect of 5-HT on the mRNA expression
of (A and B) SLC40A1 and (C and D) SLC46A1 in Caco-2 cells. Data
represent mean ± standard error (n=4). Statistical significance was
determined using one-way ANOVA followed by the Tukey-Kramer post
hoc test. *P<0.05; **P<0.01 vs.
control. Exact P-values: (A) 0.009; (B) 0.020, 0.021, 0.007; (C)
<0.001, <0.001, <0.001, <0.001, <0.001; (D) 0.005,
<0.001, <0.001, <0.001. 5-HT, serotonin; SLC40A1, solute
carrier family 40 member 1; SLC46A1, solute carrier family 46
member 1.
Effects of (A) TCB-2 and (B) MK-212 on
the mRNA expression of SLC11A2 in Caco-2 cells. Data represent mean
± standard error (n=3). Statistical significance was determined by
one-way ANOVA followed by the Tukey-Kramer post hoc test.
*P<0.05 vs. control. Exact P-value: 0.048. SLC11A2,
solute carrier family 11 member 2.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by Hyogo Medical University
Grant for Research Promotion, 2024.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YS conceptualized the study, designed the
methodology, performed formal data analysis, managed the overall
progress of the project (project administration), prepared figures
and graphical representations (visualization), and wrote the
original draft of the manuscript. MA contributed to formal data
analysis and experimental investigations, and assisted in the
preparation of figures (visualization). NK contributed to formal
data analysis and experimental investigations, and assisted in
figure preparation (visualization). SN conceptualized the study,
supervised the research activities, managed the project (project
administration), and revised and edited the manuscript. YS and SN
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leung AKC, Lam JM, Wong AHC, Hon KL and Li
X: Iron deficiency anemia: An updated review. Curr Pediatr Rev.
20:339–356. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cappellini MD, Santini V, Braxs C and
Shander A: Iron metabolism and iron deficiency anemia in women.
Fertil Steril. 118:607–614. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Johnson-Wimbley TD and Graham DY:
Diagnosis and management of iron deficiency anemia in the 21st
century. Therap Adv Gastroenterol. 4:177–184. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
De Franceschi L, Iolascon A, Taher A and
Cappellini MD: Clinical management of iron deficiency anemia in
adults: Systemic review on advances in diagnosis and treatment. Eur
J Intern Med. 42:16–23. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yanatori I and Kishi F: DMT1 and iron
transport. Free Radic Biol Med. 33:55–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Donovan A, Lima CA, Pinkus JL, Pinkus GS,
Zon LI, Robine S and Andrews NC: The iron exporter
ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab.
1:191–200. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Le Blanc S, Garrick MD and Arredondo M:
Heme carrier protein 1 transports heme and is involved in heme-Fe
metabolism. Am J Physiol Cell Physiol. 302:C1780–C1785.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Thompson JH: Serotonin
(5-hydroxytryptamine) and iron absorption. Ir J Med Sci. 6:383–388.
1965.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sibon D, Coman T, Rossignol J, Lamarque M,
Kosmider O, Bayard E, Fouquet G, Rignault R, Topçu S, Bonneau P, et
al: Enhanced renewal of erythroid progenitors in myelodysplastic
anemia by peripheral serotonin. Cell Rep. 26:3246–3256.e4.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Amireault P, Hatia S, Bayard E, Bernex F,
Collet C, Callebert J, Launay JM, Hermine O, Schneider E, Mallet J,
et al: Ineffective erythropoiesis with reduced red blood cell
survival in serotonin-deficient mice. Proc Natl Acad Sci U S A.
108:13141–13146. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Merrill JF, Thomson DM, Hardman SE,
Hepworth SD, Willie S and Hancock CR: Iron deficiency causes a
shift in AMP-activated protein kinase (AMPK) subunit composition in
rat skeletal muscle. Nutr Metab (Lond). 9(104)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bani-Ahmad M, Ahmad M, Obeidat M and
Barqawi M: The modulation of plasma levels of dopamine, serotonin,
and Brain-derived neurotrophic factor in response to variation in
iron availability. Acta Biomed. 93(e2022293)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu X, Chen R, Zhan G, Wang D, Tan X and Xu
H: Enterochromaffin cells: Sentinels to gut microbiota in
hyperalgesia? Front Cell Infect Microbiol.
11(760076)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wei L, Singh R and Ghoshal UC:
Enterochromaffin cells-gut microbiota crosstalk: Underpinning the
symptoms, pathogenesis, and pharmacotherapy in disorders of
gut-brain interaction. J Neurogastroenterol Motil. 28:357–375.
2022.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Yoshinaga M, Nakatsuka Y, Vandenbon A, Ori
D, Uehata T, Tsujimura T, Suzuki Y, Mino T and Takeuchi O:
Regnase-1 maintains iron homeostasis via the degradation of
transferrin receptor 1 and prolyl-hydroxylase-domain-containing
protein 3 mRNAs. Cell Rep. 19:1614–1630. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abramson R, Wilson H, Natile MM and
Natrajan LS: Development of an Fe2+ sensing system based
on the inner filter effect between upconverting nanoparticles and
ferrozine. RSC Adv. 13:26313–26322. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bonhaus DW, Bach C, DeSouza A, Salazar FH,
Matsuoka BD, Zuppan P, Chan HW and Eglen RM: The pharmacology and
distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene
products: Comparison with 5-HT2A and 5-HT2C receptors. Br J
Pharmacol. 115:622–628. 1995.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Naito K, Moteki H, Kimura M, Natsume H and
Ogihara M: Serotonin 5-HT2B receptor-stimulated DNA synthesis and
proliferation are mediated by autocrine secretion of transforming
growth factor-α in primary cultures of adult rat hepatocytes. Biol
Pharm Bull. 39:570–577. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Meunier V, Bourrié M, Berger Y and Fabre
G: The human intestinal epithelial cell line Caco-2;
pharmacological and pharmacokinetic applications. Cell Biol
Toxicol. 11:187–194. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hidalgo IJ, Raub TJ and Borchardt RT:
Characterization of the human colon carcinoma cell line (Caco-2) as
a model system for intestinal epithelial permeability.
Gastroenterology. 96:736–749. 1989.PubMed/NCBI
|
|
21
|
Tsuji Y: Transmembrane protein western
blotting: Impact of sample preparation on detection of SLC11A2
(DMT1) and SLC40A1 (ferroportin). PLoS One.
15(e0235563)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li X, Xie J, Lu L, Zhang L, Zhang L, Zou
Y, Wang Q, Luo X and Li S: Kinetics of manganese transport and gene
expressions of manganese transport carriers in Caco-2 cell
monolayers. Biometals. 26:941–953. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shirase T, Mori K, Okazaki Y, Itoh K,
Yamamoto M, Tabuchi M, Kishi F, Jiang L, Akatsuka S, Nakao K and
Toyokuni S: Suppression of SLC11A2 expression is essential to
maintain duodenal integrity during dietary iron overload. Am J
Pathol. 177:677–685. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Niitsu Y: Progress in the study of
molecular genetics of iron metabolism. Nihon Naika Gakkai Zasshi.
89:768–773. 2000.PubMed/NCBI(In Japanese).
|
|
25
|
Lin J, Liu C, Bai R, Zhang C, Pang J, Liu
Z, Ye X, Chen S, Liu X, Li H and Hu S: The effect of iron
absorption in ferrous gluconate form from enriched rice flour using
an in vitro digestion model and a Caco-2 cell model. Food Funct.
15:8788–8796. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mohammad-Zadeh LF, Moses L and
Gwaltney-Brant SM: Serotonin: A review. J Vet Pharmacol Ther.
31:187–199. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hoyer D, Clarke DE, Fozard JR, Hartig PR,
Martin GR, Mylecharane EJ, Saxena PR and Humphrey PP: International
union of pharmacology classification of receptors for
5-hydroxytryptamine (serotonin). Pharmacol Rev. 46:157–203.
1994.PubMed/NCBI
|
|
28
|
Manzella CR, Jayawardena D, Pagani W, Li
Y, Alrefai WA, Bauer J, Jung B, Weber CR and Gill RK: Serum
serotonin differentiates between disease activity states in Crohn's
patients. Inflamm Bowel Dis. 26:1607–1618. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jørandli JW, Thorsvik S, Skovdahl HK,
Kornfeld B, Sæterstad S, Gustafsson BI, Sandvik AK and van Beelen
Granlund A: The serotonin reuptake transporter is reduced in the
epithelium of active Crohn's disease and ulcerative colitis. Am J
Physiol Gastrointest Liver Physiol. 319:G761–G768. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ayme-Dietrich E, Lawson R, Côté F, de
Tapia C, Da Silva S, Ebel C, Hechler B, Gachet C, Guyonnet J,
Rouillard H, et al: The role of 5-HT2B receptors in
mitral valvulopathy: Bone marrow mobilization of endothelial
progenitors. Br J Pharmacol. 174:4123–4139. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dumotier BM and Urban L: Preclinical
mitigation of 5-HT2B agonism-related cardiac valvulopathy
revisited. J Pharmacol Toxicol Methods. 128(107542)2024.PubMed/NCBI View Article : Google Scholar
|