1. Introduction
Schizophrenia is an incapacitating mental disorder whose etiology is multifactorial. Within this context, both genetic and environmental factors contribute to its occurrence (1). Following adversities in life (2,3), gene-environment processes (3) and epigenetic changes (4,5) may affect the expression of genes implicated in synaptic function, neurodevelopment and stress response. Such factors could potentially promote the occurrence of schizophrenia through their effects on neurotransmitters and the immune system, resulting in oxidative stress (6,7). Genetic vulnerability in combination with prenatal and perinatal risk factors (including maternal stress, viral infection, malnutrition, zinc deficiency and hypoxia) may increase the susceptibility to environmental stressors (such as childhood abuse, urbanicity, migration and substance abuse) (8,9). Similarly, it has been proposed that early-life adversity may alter the hypothalamic-pituitary-adrenal axis function, leading to a modified stress response and increased stress sensitivity to potential stressors in adolescence and adulthood (9-11), thus advancing the occurrence of schizophrenia symptoms through dopaminergic hyperactivity (12). Furthermore, prolonged exposure to stress and to glucocorticoids may result in a reduction in hippocampal volume (13) and decreased levels of brain-derived neurotrophic factor (BDNF) in patients with schizophrenia (14-19).
Despite causing major disability worldwide, and the intense research performed over the last decades on the investigation of the molecular mechanisms underlying schizophrenia, its pathophysiology remains not fully understood. Numerous questions regarding the dysregulated signaling pathways and the molecular basis of differential personalised responses to various treatments remain unanswered. The importance of genetic risk factors and key cell signaling pathways is now confirmed; however, further research is required to fully delineate the underlying molecular mechanisms. Among the signaling pathways in schizophrenia that show dysregulation is the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, a pleiotropic pathway that plays a key role in multiple cellular functions (20-22). Since its discovery >30 years ago, JAK/STAT represents one of the most conserved pathways that transmits extracellular signals from over 50 cytokines, growth factors and hormones, to the nucleus, to tightly regulate target gene transcription (23,24). The specific JAK/STAT-dependent expression programs define important cellular responses linked to cell growth, proliferation, cell cycle progression, development, apoptosis, differentiation, migration, survival and malignancy (both solid and hematologic). Furthermore, JAK/STAT plays a pivotal role in immune, inflammatory and stress responses. The family of JAK kinases is composed of four members (JAK1, JAK2, JAK3, and TYK2), while the STAT family in mammals consists of seven members (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6). All STATs contain a conserved structure that includes the N-terminal, the coiled-coil, the Src-homology 2 (important for STAT dimerization), the linker, the DNA-binding and the transactivation domains (21).
The JAK/STAT pathway plays a key role in inflammation, cancer and neurodegenerative diseases (25). Its importance for brain disorders highlights the necessity to elucidate how it influences the functionality of neural cells. In the present review, insights into the dysregulation of the JAK/STAT signaling pathway in neurological dysfunction and schizophrenia are provided. Inflammation and signaling dysregulation, the crosstalk of non-coding RNAs (ncRNAs) with JAK/STAT, the involvement of the JAK/STAT pathway in patient stratification, management and treatment strategies, and models/approaches to study signaling and to improve therapeutic delivery of drugs in schizophrenia are discussed.
2. Methods
Searches across online databases, including PubMed, Google Scholar and Web of Science, were performed by employing specific keywords and their combinations [including JAK/STAT, signaling, schizophrenia, neurological dysfunction, inflammation/neuroinflammation, stress, ncRNAs (miRNAs, lncRNAs), biomarkers, therapies, organoids, nanotechnology] to identify publications relevant to this brief review. Due to space limitations, not all publications have been included. For efficient organisation, management and storage of the selected references, Zotero version 7.0.26, developed by the Corporation for Digital Scholarship, was used.
3. Inflammation and dysregulated signaling in schizophrenia
Various cell types mediate innate immunity, which participates in the defense of the immune system to various pathogens and is connected to the tightly controlled action of numerous signaling pathways, including the interferon (IFN), mitogen-activated protein kinase, JAK/STAT, interleukin (IL), chemokine receptor, and glycogen synthase kinase pathways. JAK/STAT is considered important in regulating inflammatory and immunological responses, thus promoting neuroinflammation in neurodegenerative diseases (25). Alterations to immune function have also been detected in psychosis, with increased proinflammatory cytokines in the serum of the patients, indicating activation of immune-related cells in the circulation (26).
Inflammation is considered a key factor for the onset and maintenance of schizophrenia, with a variety of cytokines being dysregulated depending on the disease stage (27-29). Cytokines, as key molecules of the immune system, control the brain/nervous system response to exogenous insults and mediate the communication of the nervous and immune systems (30). Cytokine imbalance has been detected in both blood and cerebrospinal fluid of patients with schizophrenia, particularly in T helper type 1 and type 2 cytokines, despite contradictory results in the literature (31). Infections, autoimmune diseases, and genetic predisposition are considered risk factors for schizophrenia. Furthermore, chronic stress is reported to contribute to a continued proinflammatory state that potentially promotes the disorder (31).
4. Dysregulated JAK/STAT signaling in neurological dysfunction and schizophrenia
JAK/STAT pathway in neurological dysfunction
Dysregulated cytokines bind to receptors that activate the JAK/STAT pathway, and thus are implicated in neuropsychiatric disorders through numerous processes, including neurogenesis, gliogenesis, synaptic plasticity and microglia activation (32,33). JAK/STAT signaling is reported to be linked with neurodegenerative and neuropsychiatric disorders. For example, JAK/STAT is reported to regulate many of the genes associated with epilepsy syndromes. Neurons respond to prolonged BDNF exposure, both in vivo and in vitro, by activating the JAK/STAT pathway. RNA-sequencing of neurons exposed to BDNF, in the presence or absence of JAK/STAT inhibitors, showed that the neuronal BDNF-induced JAK/STAT pathway involves more than just STAT3 phosphorylation, supporting an underlying non-canonical JAK/STAT mechanism (34). Furthermore, STAT3 plays an important role in neural function relevant to behaviour in both healthy and pathological conditions (35). However, numerous questions remain to be answered on STAT3 involvement in psychopathological neural mechanisms. Knockdown approaches suggested that STAT3 in the nuclei of the dorsal raphe (located in the midbrain and pons) controls behavioural reactivity, and serves as a functional connecting molecule between the upstream activators of STAT3, serotonergic neurotransmission and psychopathology (35). The JAK/STAT/Rho-associated coiled-coil containing protein kinase (JAK/STAT/ROCK) signaling pathway has been linked to lung-brain-related immune responses. JAK/STAT/ROCK is reported to be important for the crosstalk between uncontrolled inflammation following upper respiratory tract infections and the development of neurodegenerative diseases, providing novel avenues for the therapeutic management of the associated inflammation (36). Based on such findings, it cannot be excluded that similar mechanisms might exist in schizophrenia.
JAK/STAT pathway in schizophrenia
The JAK/STAT1 gene expression signature has been downregulated in early psychosis and in individuals with an acute exacerbation of psychosis who require hospitalisation. By contrast, this specific expression signature was normalised in individuals with longer psychosis duration and chronic psychopathology (26). Such findings validate the importance of the JAK/STAT pathway in neurophysiological circuits that are critical for the development of psychiatric diseases, including schizophrenia.
Stress triggers inflammation through the JAK/STAT pathway, leading to neuroinflammation that causes neuronal and synaptic dysfunction, and also cognitive impairments, which are hallmarks of schizophrenia and other stress-associated neuropsychiatric disorders. Cytokines released during the stress response regulate gliogenesis, neurogenesis, and synaptic plasticity. Chronic stress sustains JAK/STAT activation, leading to the chronic inflammation observed in schizophrenia (37).
Furthermore, a single-nucleotide polymorphism (SNP) in the ZNF804A gene has been associated with decreased transcript levels in the fetal brain and has been linked to schizophrenia and bipolar disorder. The formation of a protein complex of ZNF804A with IFN-activated STAT2 has indicated its function as a signal transducer that activates IFN-mediated gene expression programs, which are important for schizophrenia pathophysiology (38). Activation of STAT1 by signaling cascades initiated by receptors triggered by proinflammatory cytokines (such as IFNγ, IL-6, IL-2, IL-10), hypoxia, infections and peptide growth factors has also been reported to be dysregulated in schizophrenia (39). Such findings confirm the implication and importance of the dysregulated pleiotropic JAK/STAT pathway in schizophrenia, which influences several key disease-related cellular functions (Fig. 1).
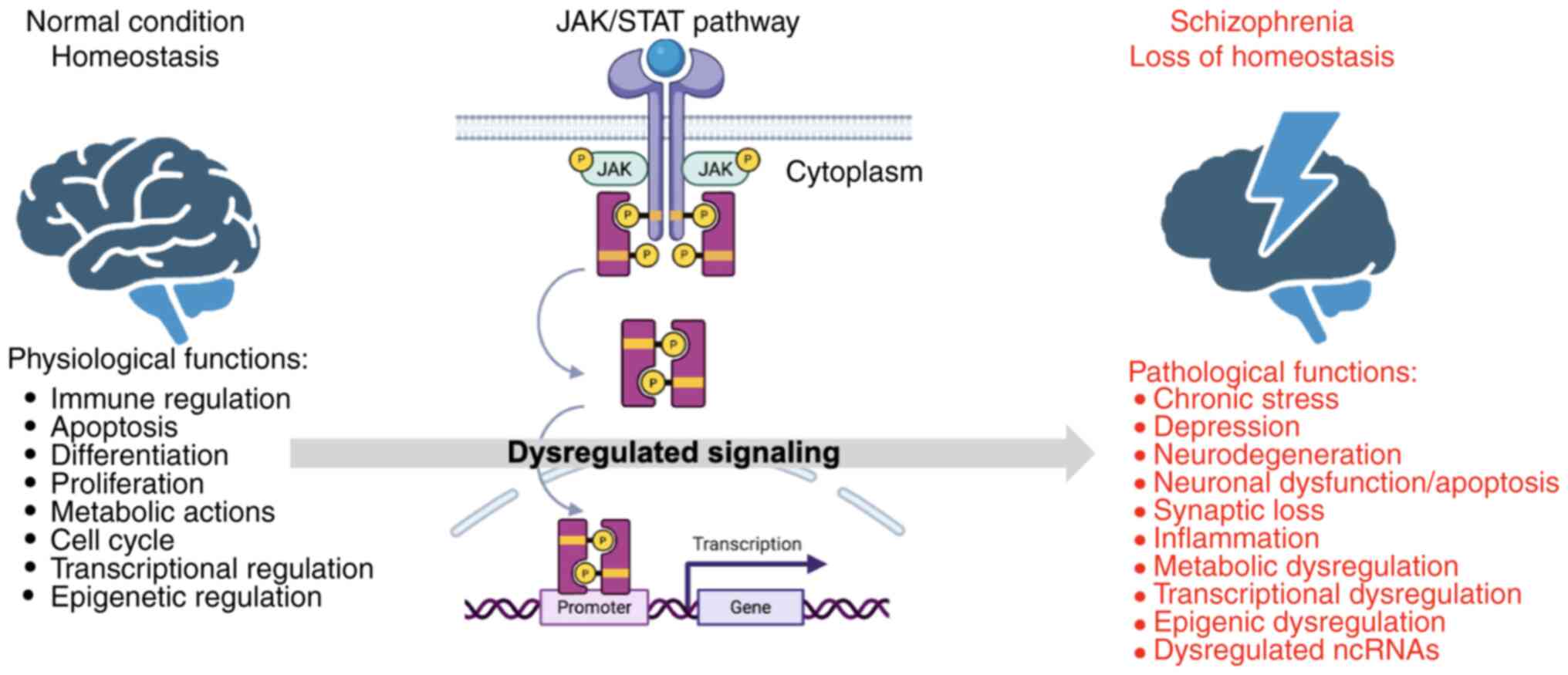 |
Figure 1
Dysregulation of JAK/STAT signaling pathway and other cellular functions in schizophrenia. The schematic diagram of the JAK/STAT signaling pathway is shown in the middle. The normal condition is depicted on the left, where no dysregulation is observed in the JAK/STAT pathway, and the cellular functions controlled by the JAK/STAT pathway (including inflammation, immune regulation and response, hematopoiesis, apoptosis, differentiation, proliferation, metabolic actions, cell cycle, and transcriptional and epigenetic regulation) are in homeostasis. In schizophrenia (on the right), upon dysregulation of the JAK/STAT and other pathways, metabolic, transcriptional and epigenetic dysregulation, chronic stress, depression, neurodegeneration, neuronal dysfunction and apoptosis, synaptic loss, inflammation and dysregulated ncRNAs are observed. JAK/STAT, Janus kinase/signal transducer and activator of transcription; ncRNA, non-coding RNAs. The figure was created using BioRender (https://BioRender.com).
|
5. Crosstalk of ncRNAs with the JAK/STAT pathway in schizophrenia
ncRNAs hold key roles in regulating gene expression and have been linked to neurological and neuropsychiatric disorders, including schizophrenia. ncRNAs regulate inflammation, and pro- and anti-inflammatory factors are regulated by microRNAs (miRNAs or miRs). Long ncRNAs (lncRNAs) also affect miRNAs (40). Dysregulation of ncRNAs in schizophrenia has been reported, with studies showing altered miRNA expression (including miR-132, miR-212, miR-34a/miR-34c, miR1307) and lncRNA changes in brain tissues from patients with schizophrenia (41,42). Among others, Gomafu, PINT, GAS5, IFNG-AS1, FAS-AS1, PVT1, and TUG1 have been reported as downregulated lncRNAs in schizophrenia. By contrast, MEG3, THRIL, HOXA-AS2, Linc-ROR, SPRY4-IT1, UCA1, and MALAT1 have been reported as upregulated. Furthermore, miR-30e, miR-130b, hsa-miR-130b, miR-193a-3p, hsa-miR-193a-3p, hsa-miR-181b, hsa-miR-34a, hsa-miR-346, and hsa-miR-7 have been shown to be dysregulated in blood or brain samples of patients with schizophrenia (43). Despite all these findings, the evidence for a crosstalk between ncRNAs and the JAK/STAT pathway in schizophrenia and other neuropsychiatric disorders is currently limited. ncRNAs can regulate JAK/STAT signaling by acting as downstream or upstream modulators (44). The lncRNAs AC006129.1 and RP5-998N21.4 are involved in immune responses and interact with pathways such as JAK/STAT, through the regulation of SOCS3 and STAT1, respectively (44-46).
Extensive ncRNA crosstalk with JAK/STAT pathway components is mainly documented in cancer (47). Evidence from neurological-related and other tumors shows that certain ncRNAs can regulate the JAK/STAT pathway by interacting with pathway regulators (such as SOCS1) or by directly affecting the expression and activation of JAK/STAT proteins, thereby influencing inflammation and cell signaling (48,49). Our previous studies demonstrated significant downregulation of specific miRNAs (let-7p-5p, miR-98-5p and miR-183-5p) in patients with cancer only, and cancer with schizophrenia, when compared to patients with schizophrenia only, suggesting an oncosuppressive role for these miRNAs (50-52). Based on our findings (50-52) and the extensively studied crosstalk of ncRNAs with JAK/STAT in various malignancies (53-57), it cannot be excluded that similar crosstalk participates in mechanisms that lead to schizophrenia, a hypothesis that needs further exploration and validation in future studies.
6. JAK/STAT pathway in the therapeutic management of schizophrenia
Given the importance of the JAK/STAT pathway in neurodegenerative and neuropsychiatric diseases, the use of JAK/STAT pathway components as biomarkers for monitoring disease progression and response to therapies, or for therapeutic targeting of schizophrenia and other relevant diseases, constitutes a clinical necessity.
JAK/STAT components as biomarkers
Components of the JAK/STAT signaling pathway have already been utilised as biomarkers of disease state. Patients with schizophrenia with poorer cognitive performance have expressed significantly higher levels of activated pSTAT1 when compared with controls, providing a biomarker of biological significance (39). Phospho-specific cell signaling epitope expression in peripheral blood mononuclear cell (PBMC) subtypes, in drug-naïve schizophrenia patients compared with controls, has identified specific disease alterations. In combination with serum immune response proteins, polygenic risk scores, drug response and side effects, such alterations have been analysed throughout the antipsychotic therapy. Among others, Stat3 (pS727), Stat3 (pY705) and Stat5 (pY694) across PBMC subtypes have been linked with schizophrenia at disease onset, and have been correlated with the type I IFN-related serum molecules CD40 and CXCL11. Alterations in Stat3 (pS727), among other molecules, predicted the development of metabolic and cardiovascular side effects following antipsychotic treatment, together with side effects and early improvements in general psychopathology scores (58). Such findings validate the importance of using PBMCs as a valuable model for detecting intracellular signaling alterations in schizophrenia for stratification of patients with differential clinical outcomes or risk for side effects of antipsychotic treatment (58). Furthermore, highly sensitive C-reactive protein, a biomarker for detecting inflammation in schizophrenia (33), has been used, and elevated levels of pro-inflammatory and anti-inflammatory cytokines have been detected in the peripheral blood of patients with first-episode schizophrenia and relapsed, when compared with healthy controls. Specifically, IL-6, IL-1, TNF, and IFN have been demonstrated to be dysregulated in schizophrenia (33). It cannot be excluded that such findings may be linked to dysregulation of the JAK/STAT pathway or can be used in combination with detected dysregulation of JAK/STAT components or relevant ncRNAs for more accurate stratification, therapeutic management, and prediction of therapeutic response in schizophrenia.
JAK/STAT targeting for therapeutic purposes
Anti-inflammatory agents inhibiting relevant signaling pathways might serve as useful treatments for inflammatory schizophrenia (33). Such agents have been effective in reducing disease symptoms and increasing the functionality of patients. Despite encouraging results, further research is needed towards the personalised approach of such treatments (33).
Small-molecule inhibitors of JAKs (jakinibs) have been safe and efficient for the treatment of diseases with underlying inflammation (rheumatoid arthritis, psoriasis, and inflammatory bowel disease) (59), and may constitute promising treatments for schizophrenia in the future.
Symptoms of depression, frequent in schizophrenia, and the antidepressant actions of current treatments have been mediated by JAK/STAT-dependent mechanisms (60). Furthermore, inflammation and oxidative stress have been targeted for the development of novel therapies for depression. The finding that the JAK/STAT pathway might mediate antidepressant-like effects of N-acetylcysteine, suggests the importance of targeting this pathway for novel and effective therapies for depression (60).
The pathogenesis of schizophrenia involves abnormal activation of microglia. Minocycline and antipsychotics have been effective in blocking the activation of microglia and thus reducing the negative symptoms of schizophrenia. When microglia cells were activated by lipopolysaccharide and treated with minocycline, haloperidol, and risperidone, various effects were detected in cytokine production and signaling pathway dysregulation. Minocycline was shown to suppress JAK2 and STAT3 activation. Risperidone and haloperidol only suppressed STAT3 activation. These findings have shed light on the underlying mechanisms of minocycline and atypical antipsychotics for the treatment of schizophrenia (61) and indicated the importance of targeting the JAK/STAT pathway.
Therapeutic agents that target the JAK/STAT pathway involve cytokine or receptor antibodies, JAK inhibitors, and STAT inhibitors, which have been applied to various cancers and autoimmune diseases. Pimozide, an antipsychotic drug belonging to the diphenylbutylpiperidine class, has been shown to inhibit the action of STAT proteins, mainly STAT3 and STAT5(62). When pimozide was used to treat schizophrenia, the patients demonstrated a lower incidence of solid malignancies. Further studies have demonstrated that pimozide induces apoptosis and decreases metastasis through mechanisms involving STAT3, STAT5 and other molecules (63,64). As inflammation plays a major role in the onset and maintenance of schizophrenia, and the dysregulated cytokines depend on the phase of the illness (33), a wide variety of anti-inflammatory agents may inhibit subsequent pathways and have been effective for treatment. Hormonal therapies, antioxidants, omega-3 fatty acids, and minocycline have improved symptoms and everyday functioning of the patients (33); however, many questions remain to be answered on the crosstalk of such anti-inflammatory agents with the JAK/STAT pathway. Furthermore, their action in other cellular processes beyond inflammation requires further investigation and questions their efficacy for the improvement of schizophrenia. Current therapies to mitigate inflammation in schizophrenia also include antibiotics and emerging biological agents that target specific inflammatory pathways (27,61). Additional strategies involve probiotics and advanced techniques, such as nanotechnology, to effectively deliver therapeutic agents (65). These are often used as adjunctive medication, added to traditional treatments to improve symptoms (65). The therapeutic targeting of ncRNAs to reduce inflammation is also of importance, as ncRNAs can serve as therapeutic targets in inflammation-related diseases (40), including schizophrenia.
It is expected that targeting the dysregulated JAK/STAT pathway alone, or in combination with the already reported anti-inflammatory therapies, or the ncRNAs regulating the JAK/STAT pathway, will contribute to the development of efficient and personalised therapeutic management approaches for schizophrenia.
7. Models and approaches to study signaling pathways in schizophrenia
Organoids
Organoid models of schizophrenia have been successfully used to study dysregulated signaling pathways, providing pioneering tools in understanding the underlying disease mechanisms. Patient-derived induced pluripotent stem cell (iPSC) organoids have been used. Such models in schizophrenia confirmed dysregulation of signaling (FGFR1) that affects cortical development (66), and dysregulation of genes functionally relevant to synapses, neurodevelopment, axonal guidance, synaptogenesis and other pathways (67-69). Such studies demonstrate the reliability of organoid models to recapitulate signaling and other pathway disruptions in schizophrenia. However, the study of the JAK/STAT signaling field, using organoid models, remains unexplored. Research using organoids is expected to address the role of JAK/STAT in schizophrenia in the near future.
Nanotechnology
Nanotechnology describes various devices, carriers, and materials that facilitate the transportation of drugs for therapeutic purposes, and includes nanomaterials and nanodevices. Nanotechnology, encompassing engineered small-scale objects that match the sizes of biomolecules, their assemblies, and cellular parts (70), provides unique opportunities to understand brain functions. Nanomaterials, acting as nano-sized lipophilic carriers, facilitate the transportation of drugs across the blood-brain barrier (71). As an example, when miR-137, which controls key cognitive and sensory processing genes that are dysregulated in schizophrenia, was encapsulated in nanoparticles, nontoxic delivery and easy incorporation into cells was achieved (72). Nanopsychiatry aims to improve agents for the treatment of psychiatric diseases through the use of nanoscaled carriers. Devices capable of detecting levels of dopamine and serotonin in patients, which could improve schizophrenia diagnostics, have already been developed. Novel nano-sized drug delivery systems, by increasing the efficacy and pharmacokinetics of traditional schizophrenia drug treatments, could improve the therapeutic landscape of this complex disease (73). The use of nanotechnology systems to study dysregulated signaling pathways, including JAK/STAT in schizophrenia, is currently limited; however, it is expected to expand in the near future and to improve the delivery of drugs targeting JAK/STAT or other pathways for the therapy of schizophrenia.
8. Conclusion
Schizophrenia, a traditionally defined brain disorder, is now known to be controlled by the periphery and the immune system. The JAK/STAT pathway plays a key role in inflammation and neurodegenerative diseases. Its importance for most brain disorders highlights the necessity to elucidate how it functionally modulates molecular pathways in neural cells. In this short review, insights into the dysregulation of the JAK/STAT signaling pathway in schizophrenia are provided. Aspects of the dysregulated signaling in inflammation, the cross-talk of ncRNAs with JAK/STAT, the involvement of JAK/STAT in therapeutic management strategies, and the models/approaches to study signaling and to efficiently deliver treatments in schizophrenia are discussed.
Future research on the dysregulated JAK/STAT signaling pathway using organoid models will generate scientific knowledge to further elucidate the pathophysiology of this complex disorder. Additionally, it is expected to provide useful biomarkers or therapeutic targets to develop novel and more efficient stratification and therapeutic management strategies for schizophrenia. Nanopsychiatry and nanotechnology are also expected to improve the delivery of drugs targeting JAK/STAT or other pathways towards efficient and personalised therapies for schizophrenia.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ER and EK wrote and edited the manuscript. CT, JNT and DAS edited the manuscript. All authors contributed to the article, and read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
|
1
|
Sawa A and Snyder SH: Schizophrenia: Diverse approaches to a complex disease. Science. 296:692–695. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Giannopoulou I, Georgiades S, Stefanou MI, Spandidos DA and Rizos E: Links between trauma and psychosis (Review). Exp Ther Med. 26(386)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Morgan C, Charalambides M, Hutchinson G and Murray RM: Migration, ethnicity, and psychosis: Toward a sociodevelopmental model. Schizophr Bull. 36:655–664. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Davis EG, Humphreys KL, McEwen LM, Sacchet MD, Camacho MC, MacIsaac JL, Lin DTS, Kobor MS and Gotlib IH: Accelerated DNA methylation age in adolescent girls: Associations with elevated diurnal cortisol and reduced hippocampal volume. Transl Psychiatry. 7(e1223)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen Q, Li D, Jin W, Shi Y, Li Z, Ma P, Sun J, Chen S, Li P and Lin P: Research progress on the correlation between epigenetics and Schizophrenia. Front Neurosci. 15(688727)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Alameda L, Rodriguez V, Carr E, Aas M, Trotta G, Marino P, Vorontsova N, Herane-Vives A, Gadelrab R, Spinazzola E, et al: A systematic review on mediators between adversity and psychosis: Potential targets for treatment. Psychol Med. 50:1966–1976. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Theleritis C, Stefanou MI, Demetriou M, Alevyzakis E, Triantafyllou K, Smyrnis N, Spandidos DA and Rizos E: Association of gut dysbiosis with first-episode psychosis (Review). Mol Med Rep. 30(130)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bayer TA, Falkai P and Maier W: Genetic and non-genetic vulnerability factors in schizophrenia: The basis of the ‘two hit hypothesis.’. J Psychiatr Res. 33:543–548. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Theleritis C, Demetriou M, Stefanou MI, Alevyzakis E, Makris M, Zoumpourlis V, Peppa M, Smyrnis N, Spandidos DA and Rizos E: Zinc in psychosis (Review). Mol Med Rep. 32(201)2025.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lardinois M, Lataster T, Mengelers R, Van Os J and Myin-Germeys I: Childhood trauma and increased stress sensitivity in psychosis. Acta Psychiatr Scand. 123:28–35. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, Heinssen R, Mathalon DH, Perkins DO, Seidman LJ, et al: Cortisol levels and risk for psychosis: Initial findings from the North American prodrome longitudinal study. Biol Psychiatry. 74:410–417. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sapolsky RM: Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 57:925–935. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vita A, De Peri L, Silenzi C and Dieci M: Brain morphology in first-episode schizophrenia: A meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 82:75–88. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Thompson Ray M, Weickert CS, Wyatt E and Webster MJ: Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci JPN. 36:195–203. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Daskalakis NP, De Kloet ER, Yehuda R, Malaspina D and Kranz TM: Early life stress effects on Glucocorticoid-BDNF interplay in the hippocampus. Front Mol Neurosci. 8(68)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rizos EN, Rontos I, Laskos E, Arsenis G, Michalopoulou PG, Vasilopoulos D, Gournellis R and Lykouras L: Investigation of serum BDNF levels in drug-naive patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 32:1308–1311. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rizos EN, Papathanasiou M, Michalopoulou PG, Mazioti A, Douzenis A, Kastania A, Nikolaidou P, Laskos E, Vasilopoulou K and Lykouras L: Association of serum BDNF levels with hippocampal volumes in first psychotic episode drug-naive schizophrenic patients. Schizophr Res. 129:201–204. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rizos EN, Michalopoulou PG, Siafakas N, Stefanis N, Douzenis A, Rontos I, Laskos E, Kastania A, Zoumpourlis V and Lykouras L: Association of serum brain-derived neurotrophic factor and duration of untreated psychosis in first-episode patients with schizophrenia. Neuropsychobiology. 62:87–90. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Theleritis C, Fisher HL, Shäfer I, Winters L, Stahl D, Morgan C, Dazzan P, Breedvelt J, Sambath I, Vitoratou S, et al: Brain derived Neurotropic Factor (BDNF) is associated with childhood abuse but not cognitive domains in first episode psychosis. Schizophr Res. 159:56–61. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fasouli ES and Katsantoni E: Age-associated myeloid malignancies-the role of STAT3 and STAT5 in myelodysplastic syndrome and acute myeloid leukemia. FEBS Lett. 598:2809–2828. 2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fasouli ES and Katsantoni E: JAK-STAT in Early Hematopoiesis and Leukemia. Front Cell Dev Biol. 9(669363)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Katsantoni E: Protein complexes and target genes identification by in vivo biotinylation: The STAT5 paradigm. Sci Signal. 5(pt13)2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Theodorou M, Speletas M, Mamara A, Papachristopoulou G, Lazou V, Scorilas A and Katsantoni E: Identification of a STAT5 target gene, Dpf3, provides novel insights in chronic lymphocytic leukemia. PLoS One. 8(e76155)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nanou A, Toumpeki C, Lavigne MD, Lazou V, Demmers J, Paparountas T, Thanos D and Katsantoni E: The dual role of LSD1 and HDAC3 in STAT5-dependent transcription is determined by protein interactions, binding affinities, motifs and genomic positions. Nucleic Acids Res. 45:142–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jain M, Singh MK, Shyam H, Mishra A, Kumar S, Kumar A and Kushwaha J: Role of JAK/STAT in the Neuroinflammation and its association with neurological disorders. Ann Neurosci. 28:191–200. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Melbourne JK, Rosen C, Feiner B, Pang Y and Sharma RP: The JAK-STAT1 transcriptional signature in peripheral immune cells reveals alterations related to illness duration and acuity in psychosis. Brain Behav Immun. 77:37–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Müller N, Weidinger E, Leitner B and Schwarz MJ: The role of inflammation in schizophrenia. Front Neurosci. 9(372)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chaves C, Dursun SM, Tusconi M and Hallak JEC: Neuroinflammation and schizophrenia-is there a link? Front Psychiatry. 15(1356975)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Miller BJ and Goldsmith DR: Evaluating the hypothesis that schizophrenia is an inflammatory disorder. Focus. 18:391–401. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Konsman JP: Cytokines in the brain and neuroinflammation: We Didn't Starve the Fire! Pharm Basel Switz. 15(140)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Reale M, Costantini E and Greig NH: Cytokine imbalance in schizophrenia. From research to clinic: Potential implications for treatment. Front Psychiatry. 12(536257)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shariq AS, Brietzke E, Rosenblat JD, Pan Z, Rong C, Ragguett RM, Park C and McIntyre RS: Therapeutic potential of JAK/STAT pathway modulation in mood disorders. Rev Neurosci. 30:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fond G, Lançon C, Korchia T, Auquier P and Boyer L: The role of inflammation in the treatment of Schizophrenia. Front Psychiatry. 11(160)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hixson KM, Cogswell M, Brooks-Kayal AR and Russek SJ: Evidence for a non-canonical JAK/STAT signaling pathway in the synthesis of the brain's major ion channels and neurotransmitter receptors. BMC Genomics. 20(677)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Reisinger SN, Sideromenos S, Horvath O, Derdak S, Cicvaric A, Monje FJ, Bilban M, Häring M, Glat M and Pollak DD: STAT3 in the dorsal raphe gates behavioural reactivity and regulates gene networks associated with psychopathology. Mol Psychiatry. 26:2886–2899. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li J, Mao N, Wang Y, Deng S and Chen K: Novel insights into the ROCK-JAK-STAT signaling pathway in upper respiratory tract infections and neurodegenerative diseases. Mol Ther J Am Soc Gene Ther. 33:32–50. 2025.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sarapultsev A, Gusev E, Komelkova M, Utepova I, Luo S and Hu D: JAK-STAT signaling in inflammation and stress-related diseases: Implications for therapeutic interventions. Mol Biomed. 4(40)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Klockmeier K, Silva Ramos E, Raskó T, Martí Pastor A and Wanker EE: Schizophrenia risk candidate protein ZNF804A interacts with STAT2 and influences interferon-mediated gene transcription in mammalian cells. J Mol Biol. 433(167184)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sharma RP, Rosen C, Melbourne JK, Feiner B and Chase KA: Activated phosphorylated STAT1 levels as a biologically relevant immune signal in Schizophrenia. Neuroimmunomodulation. 23:224–229. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ashrafizadeh M, Zarrabi A, Mostafavi E, Aref AR, Sethi G, Wang L and Tergaonkar V: Non-coding RNA-based regulation of inflammation. Semin Immunol. 59(101606)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gibbons A, Udawela M and Dean B: Non-coding RNA as novel players in the pathophysiology of schizophrenia. Noncoding RNA. 4(11)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu Y, Chang X, Hahn CG, Gur RE, Sleiman PAM and Hakonarson H: Non-coding RNA dysregulation in the amygdala region of schizophrenia patients contributes to the pathogenesis of the disease. Transl Psychiatry. 8(44)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ghafouri-Fard S, Eghtedarian R, Taheri M, Beatrix Brühl A, Sadeghi-Bahmani D and Brand S: A review on the expression pattern of Non-coding RNAs in patients with schizophrenia: With a special focus on peripheral blood as a source of expression analysis. Front Psychiatry. 12(640463)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu G, Du X, Li Z, Du Y, Lv J, Li X, Xu Y and Liu S: The emerging role of long non-coding RNAs in schizophrenia. Front Psychiatry. 13(995956)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Guo B, Jiang T, Wu F, Ni H, Ye J, Wu X, Ni C, Jiang M, Ye L, Li Z, et al: LncRNA RP5-998N21.4 promotes immune defense through upregulation of IFIT2 and IFIT3 in schizophrenia. Schizophrenia (Heidelb). 8(11)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ni C, Jiang W, Wang Z, Wang Z, Zhang J, Zheng X, Liu Z, Ou H, Jiang T, Liang W, et al: LncRNA-AC006129.1 reactivates a SOCS3-mediated anti-inflammatory response through DNA methylation-mediated CIC downregulation in schizophrenia. Mol Psychiatry. 26:4511–4528. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hjazi A, Obaid RF, Ali SS, Abdullaev B, Alsaab HO, Huldani H, Romero-Parra RM, Mustafa YF, Hussien BM and Saadoon SJ: The cross-talk between LncRNAs and JAK-STAT signaling pathway in cancer. Pathol Res Pract. 248(154657)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bian Z, Ji W, Xu B, Huo Z, Huang H, Huang J, Jiao J, Shao J and Zhang X: Noncoding RNAs involved in the STAT3 pathway in glioma. Cancer Cell Int. 21(445)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Qing X, Tan GL, Liu HW, Li W, Ai JG, Xiong SS, Yang MQ and Wang TS: LINC00669 insulates the JAK/STAT suppressor SOCS1 to promote nasopharyngeal cancer cell proliferation and invasion. J Exp Clin Cancer Res. 39(166)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rizos E, Siafakas N, Skourti E, Papageorgiou C, Tsoporis J, Parker TH, Christodoulou DI, Spandidos DA, Katsantoni E and Zoumpourlis V: miRNAs and their role in the correlation between schizophrenia and cancer. Mol Med Rep. 14:4942–4946. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rizos E, Siafakas N, Katsantoni E, Skourti E, Salpeas V, Rizos I, Tsoporis JN, Kastania A, Filippopoulou A, Xiros N, et al: Let-7, Mir-98 and Mir-181 as biomarkers for cancer and schizophrenia. PLoS One. 10(e0123522)2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rizos E, Siafakas N, Koumarianou A, Katsantoni E, Filippopoulou A, Ntounas P, Touloumis Ch, Kastania A and Zoumpourlis V: miR-183 as a molecular and protective biomarker for cancer in schizophrenic subjects. Oncol Rep. 28:2200–2204. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Dhiflaoui A and Almawi WY: Mechanisms and clinical implications of microRNA associations and signaling pathways in B-cell acute lymphoblastic leukemia. Gene. 967(149730)2025.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Shenoy A, Danial M and Blelloch RH: Let-7 and miR-125 cooperate to prime progenitors for astrogliogenesis. EMBO J. 34:1180–1194. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Patel K, Kollory A, Takashima A, Sarkar S, Faller DV and Ghosh SK: MicroRNA let-7 downregulates STAT3 phosphorylation in pancreatic cancer cells by increasing SOCS3 expression. Cancer Lett. 347:54–64. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang Y, Lu Y, Toh ST, Sung WK, Tan P, Chow P, Chung AY, Jooi LL and Lee CG: Lethal-7 is down-regulated by the hepatitis B virus x protein and targets signal transducer and activator of transcription 3. J Hepatol. 53:57–66. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Cao Q, Li YY, He WF, Zhang ZZ, Zhou Q, Liu X, Shen Y and Huang TT: Interplay between microRNAs and the STAT3 signaling pathway in human cancers. Physiol Genomics. 45:1206–1214. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lago SG, Tomasik J, van Rees GF, Rustogi N, Vázquez-Bourgon J, Papiol S, Suarez-Pinilla P, Crespo-Facorro B and Bahn S: Peripheral lymphocyte signaling pathway deficiencies predict treatment response in first-onset drug-naïve schizophrenia. Brain Behav Immun. 103:37–49. 2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M and O'Shea JJ: JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 16:843–862. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Al-Samhari MM, Al-Rasheed NM, Al-Rejaie S, Al-Rasheed NM, Hasan IH, Mahmoud AM and Dzimiri N: Possible involvement of the JAK/STAT signaling pathway in N-acetylcysteine-mediated antidepressant-like effects. Exp Biol Med. 241:509–518. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Long Y, Wang Y, Shen Y, Huang J, Li Y, Wu R and Zhao J: Minocycline and antipsychotics inhibit inflammatory responses in BV-2 microglia activated by LPS via regulating the MAPKs/JAK-STAT signaling pathway. BMC Psychiatry. 23(514)2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Elmaci I and Altinoz MA: Targeting the cellular schizophrenia. Likely employment of the antipsychotic agent pimozide in treatment of refractory cancers and glioblastoma. Crit Rev Oncol Hematol. 128:96–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Shaw V, Srivastava S and Srivastava SK: Repurposing antipsychotics of the diphenylbutylpiperidine class for cancer therapy. Semin Cancer Biol. 68:75–83. 2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hu X, Li J, Fu M, Zhao X and Wang W: The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct Target Ther. 6(402)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Mosquera FEC, Guevara-Montoya MC, Serna-Ramirez V and Liscano Y: Neuroinflammation and schizophrenia: New therapeutic strategies through psychobiotics, nanotechnology, and artificial intelligence (AI). J Personalized Med. 14(391)2024.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Stachowiak EK, Benson CA, Narla ST, Dimitri A, Chuye LEB, Dhiman S, Harikrishnan K, Elahi S, Freedman D, Brennand KJ, et al: Cerebral organoids reveal early cortical maldevelopment in schizophrenia-computational anatomy and genomics, role of FGFR1. Transl Psychiatry. 7(6)2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Nascimento JM, Saia-Cereda VM, Zuccoli GS, Reis-de-Oliveira G, Carregari VC, Smith BJ, Rehen SK and Martins-de-Souza D: Proteomic signatures of schizophrenia-sourced iPSC-derived neural cells and brain organoids are similar to patients' postmortem brains. Cell Biosci. 12(189)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kathuria A, Lopez-Lengowski K, Jagtap SS, McPhie D, Perlis RH, Cohen BM and Karmacharya R: Transcriptomic landscape and functional characterization of induced pluripotent stem Cell-derived cerebral organoids in schizophrenia. JAMA Psychiatry. 77:745–754. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Sebastian R, Jin K, Pavon N, Bansal R, Potter A, Song Y, Babu J, Gabriel R, Sun Y, Aronow B and Pak C: Schizophrenia-associated NRXN1 deletions induce developmental-timing- and cell-type-specific vulnerabilities in human brain organoids. Nat Commun. 14(3770)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ahmed AAA, Alegret N, Almeida B, Alvarez-Puebla R, Andrews AM, Ballerini L, Barrios-Capuchino JJ, Becker C, Blick RH, Bonakdar S, et al: Interfacing with the Brain: How nanotechnology can contribute. ACS Nano. 19:10630–10717. 2025.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rajendran R, Menon KN and Nair SC: Nanotechnology approaches for enhanced CNS drug delivery in the management of schizophrenia. Adv Pharm Bull. 12:490–508. 2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Palumbo M and Janowsky A: Delivering MicroRNA-137 to the brain via nanoparticles. FASEB J. 36(fasebj.2022.36.S1.R5716)2022.
|
|
73
|
Radaic A and Martins-de-Souza D: The state of the art of nanopsychiatry for schizophrenia diagnostics and treatment. Nanomedicine Nanotechnol Biol Med. 28(102222)2020.PubMed/NCBI View Article : Google Scholar
|















