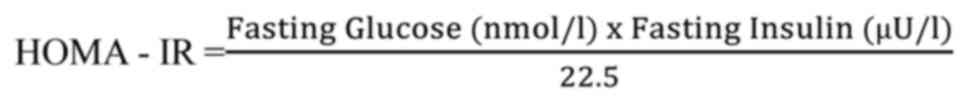Introduction
South Africa is facing a fourfold burden of health
issues including maternal, newborn and child health conditions,
human immunodeficiency virus (HIV)/acquired immunodeficiency
syndrome (AIDS) and tuberculosis (TB), non-communicable diseases
(NCDs), alongside violence and injuries (1). Despite measures taken to curb these
infectious diseases, HIV/AIDS remain prominent globally, with South
Africa accounting for 20% of all new infections worldwide in 2021.
Moreover, the prevalence of HIV was increased from an estimated 13%
(~7.8 million) in 2020 to 13.9% in 2022 (8.45 million) in South
Africa (2). Having the largest
number of people enrolled on antiretroviral (ARV) therapy (ART)
worldwide, South Africa has been able to reduce premature deaths
from AIDS, increasing the life expectancy of people living with HIV
(PLWH). However, the ageing population of HIV-infected individuals
are more prone to cardiometabolic diseases (CMDs) and other
age-associated diseases including Alzheimer's disease, Parkinson's
disease and cancer (3). CMDs are
the leading causes of mortality in the NCD category and include
diabetes mellitus (DM), hypertension, cerebrovascular diseases, and
other forms of heart diseases such as cardiomyopathies, coronary
artery disease and heart failure (4).
The pathway linking HIV/AIDS and CMDs has previously
been investigated (5). The virus
activates inflammatory responses, cellular apoptosis and
mitochondrial dysfunction, which enhances the production of
proinflammatory cytokines [tumor necrosis factor (TNF)-α,
interleukins (ILs) and C-reactive protein (CRP)], while suppressing
the release of the anti-inflammatory cytokine adiponectin, leading
to the development of insulin resistance, dyslipidemia, obesity and
DM (6). Conversely, ARVs, more
specifically non-nucleoside reverse transcriptase inhibitors
(NNRTIs) and protease inhibitors (PIs) after long term use alter
glucose homeostasis, causing dyslipidemia, lipodystrophy and
mitochondrial dysfunction (6).
These dysregulations lead to the development of CMDs (7). The underlying pathways are regulated
by various genes, including the phospholipase A2 (PLA2)
gene.
The PLA2 gene belongs to the family of
phospholipases which encode for enzymes involved in the hydrolysis
of phospholipid substrates at specific sn-2 bonds to produce free
fatty acids and lysophospholipids. Among the free fatty acids,
arachidonic acid, which is a precursor of eicosanoids, including
the inflammatory markers prostaglandins and leukotrienes, is the
most widely produced. Several isoforms of the PLA2 enzyme have been
discovered with the secreted PLA2 (sPLA2) and cytosolic PLA2
(cPLA2) being the most studied (8).
These two differ in that, cPLA2 is specific to arachidonic acid
while sPLA2 produces various fatty acids (9). A total of about 14 isoforms of sPLA2
have been identified, with 10 characterized in the human genome
including group IB, IIA, IID-F, III, V, X, XIIA and XIIB (10).
Studies on the expression of sPLA2 have
mostly been carried out in knockout mice and other animal models
with the expression of sPLA2 group IIA observed to be
upregulated by IL-1, IL-6, TNF-α, lipopolysaccharides (LPS)
(11,12) and a high fat diet (HFD) in male
Wistar rats (13). When the
phospholipase A2 group IIA (PLA2G2A) inhibitor KH064 was
administered orally to these rats, there was a reduction in weight
gain, fat mass and improvement in glucose tolerance and insulin
sensitivity, indicating that the increase in expression was
associated with metabolic abnormalities (13). However, overexpression of human
PLA2G2A in male C57BL/6 mice protected them from weight gain
on an HFD, enhanced energy expenditure and oxygen consumption, and
improved glucose clearance and insulin sensitivity, as determined
using glucose tolerance tests and insulin tolerance tests, thereby
alleviating obesogenic symptoms in response to an HFD (14,15).
While sPLA2 contributes to dyslipidemia, insulin resistance
and obesity through the breakdown of oxidized lipid contained in
low-density lipoprotein (LDL) and high-density lipoprotein (HDL)
(16), cPLA2 contributes to
metabolic diseases through the expansion of lipid droplets and
adipogenesis (17,18). Therefore, alteration in the
expression of PLA2 genes will affect their corresponding
proteins and downstream targets, leading to the development of CMDs
(19).
Single nucleotide polymorphism (SNP) is the most
common type of genetic variation among humans. It occurs when a
base in a given portion of a gene is substituted by another. These
changes have been shown to affect mRNA expression, modify the
activity of a protein, leading to the development of a disease.
Regarding PLA2, a previous study showed that SNPs of this gene are
associated with a lower risk of hypertension and CVD, weight loss
in patients with chronic obstructive pulmonary disease, stroke,
dyslipidemia, and atherosclerosis (19). Specifically, the rs4744 SNP has been
associated with increased serum PLA2G2A levels and with increased
CVD events (20). However, there is
sparse literature from Africa despite the high prevalence of HIV
and cardiometabolic diseases. The present study therefore aimed to
examine the association of nine SNPs in the PLA2 gene
(PLA2G2A, PLA2G2C and PLA2G4E) with CMDs in
South African adults living with HIV infection.
Patients and methods
Study type and population
The present study was a cross-sectional study
consisting of 716 HIV-infected individuals aged ≥18 years and
receiving ART. The participants were recruited from primary health
care facilities in the Western Cape province of South Africa
between March 2014 and February 2015. A total of 42 facilities in
Cape Town and 20 in the surrounding rural municipalities met the
criteria of provision of ART to a minimum of 325 patients per month
and were included in the present study. Among these 62 facilities,
13 urban and 4 rural were randomly selected, with 15-60
participants recruited from each facility. Approval was obtained
from the South African Medical Research Council Ethics Committee
(approval no. EC021-11/2013) in Cape Town, South Africa and the
study was conducted in accordance with the principles of the
Declaration of Helsinki. The Health Research Office of the Western
Cape Department of Health and the selected healthcare facilities in
Cape Town, South Africa granted permission for the recruitment of
participants. Written informed consent was obtained from all
participants before inclusion in the study.
Inclusion and exclusion criteria
HIV-positive adults (18 years and older) attending
facilities with directed HIV clinics, and who were willing to
participate and provided informed consent were included in the
present study. Participants were excluded from the study if they
were: Bedridden, patients with active malignancy or currently
undergoing treatment for malignancy, patients on chronic
corticosteroid treatment, pregnant or breastfeeding women, and
patients unwilling or unable to provide informed consent.
Data collection and sampling
A structured interviewer-administered questionnaire
adapted from the WHO STEP-wise approach surveillance tool (21) was used for data collection. On
recruitment day, sociodemographic information, anthropometric and
blood pressure measurements, medical history of HIV infection
including duration of diagnosis of HIV infection, CD4 counts and
ARV regimen were recorded in the questionnaire by trained
fieldworkers. Socio-demographic information, medical history of HIV
infection, anthropometric and blood pressure measurements were
obtained as previously described (22). All participants who consented for
the study were invited for blood sample collection the following
day. Blood samples were collected in EDTA tubes and tubes without
anti-coagulant after participants had fasted for at least 8 h, and
a portion was processed for biochemical analysis. Plasma glucose
(hexokinase method), serum creatinine (Cayman Chemical) and gamma
glutamyl transferase (Abcam) were measured using colorimetric
methods according to the manufacturers' protocols. Estimated
glomerular filtration rate (eGFR) was calculated using the
IDMS-traceable Modification of Diet in Renal Disease (MDRD) Study
equation (23). Total cholesterol
(TC), HDL-cholesterol (HDL-C) and triglycerides were measured in
serum samples by colorimetric methods using enzymatic techniques
(24-26),
LDL-cholesterol (LDL-C) was calculated using the formula described
by Friedewald et al (27),
and non HDL-C was calculated using the formula: TC-HDL-C. Liver
function enzymes (alanine transaminase and aspartate transaminase)
were quantified using standardized methods according to the
manufacturer's protocols (Thermo-Fisher Scientific, Inc.). All
colorimetric assays were performed using a Beckman Coulter AU 500
spectrophotometer (Beckman Coulter, Inc.). Plasma insulin was
quantified by chemiluminescence immunoassay (Human Insulin CLIA
kit; Abnova Corporation) and glycated haemoglobin (HbA1c) was
determined using high-performance liquid chromatography in
accordance with the National Glycohaemoglobin Standardization
Programme. Highly sensitive CRP (hs-CRP), TNF-α, IL-2 and IL-10
were measured by ELISA (Biomatik kit). All biochemical analyses
were performed at an ISO 15189 accredited pathology laboratory
(PathCare, Reference Laboratory, Cape Town, South Africa) which had
no access to the clinical information of the participants.
The remaining portion of blood samples were frozen
at -80˚C for DNA extraction and SNP genotyping. DNA was extracted
from whole blood samples by the salt extraction method (28).
Genotyping of SNPs was carried out using the
TaqMan® Genotyping Master Mix Protocol from ThermoFisher
Scientific, Inc. A total of nine SNPs of the PLA2 gene were
genotyped (rs11573156, rs4744, rs10732279, rs6426616, rs2301475,
rs12139100, rs116431025, rs193222555 and rs149056482). The
PLA2 gene was selected as it encodes for enzymes which are
involved in the regulation of inflammation and have the potential
to be used as markers of respiratory, neurodegenerative and
cardiometabolic diseases (20). The
SNPs were selected from the dbSNP-polymorphism repository
(https://www.ncbi.nlm.nih.gov/snp). In
total, six secreted PLA2 SNPs (three PLA2G2A and
three PLA2G2C SNPs) and three cPLA2 SNPs (three
PLA2G4E SNPs), which are widely studied, and the minor
allele have been reported to be associated with increased or
reduced serum/activity levels (20,29,30).
A sufficient amount of PCR Master Mix (Applied
Biosystems®; Thermo Fisher Scientific, Inc.) for the
requisite number of reactions was produced in a 1.5-ml tube
containing 5 µl of 2X Genotyping Mix (cat. no. 4381656; Applied
Biosystems®; Thermo Fisher Scientific, Inc.), 0.125 µl
of 40X SNP assay mix (cat. no. 4331349; Applied
Biosystems®; Thermo Fisher Scientific, Inc.; containing
the forward and reverse primers specific for each SNP; Table I), and 2.875 µl ddH20 for
each reaction. The mixture was pulse vortexed and then briefly
centrifuged (1,000 x g for 30 sec at room temperature).
Subsequently, 8 µl of the Master Mix were pipetted into each of the
wells of the 96-well plate. Then, 2 µl of the template DNA sample
(5 ng/µl concentration) were pipetted into the appropriate well.
For quality control purposes, a non-template control was also
included in each PCR run, with 2 µl of ddH20 used in
place of template DNA. When all components of the PCR had been
pipetted into the appropriate wells, the 96-well plate was covered
with MicroAmp optical caps (Applied Biosystems®; Thermo
Fisher Scientific, Inc.), and PCR was performed using Applied
Biosystems® Quant Studio™ 7 Flex Real-time PCR system
(ThermoFisher Scientific, Inc.) as follows: Initial denaturation at
95˚C for 10 min followed by 40 cycles of denaturation at 95˚C for
15 sec, annealing at 60˚C for 90 sec and extension at 60˚C for 90
sec.
 | Table IPrimer sequences for SNP
genotyping. |
Table I
Primer sequences for SNP
genotyping.
| SNP | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| rs11573156 |
TTGCATATCCCCACACTGGA |
TTGGTAGTCCCTTTGGGTGTG |
| rs4744 |
CTGAGACCTCTGCGCCCATC |
CCCATCACCAGACAACTCCCA |
| rs10732279 |
ATAACTGAGTGCGGCTTCCTT |
GGTAAGAGCTGACCCTGACCT |
| rs6426616 |
GGCCTGTTGGGGATGATCTG |
TCCTTAACCCTTGGCCCCTT |
| rs2301475 |
TGATAGACCCAGGACACAAAC |
CCTCCCCTGTCAAAGTCCAAA |
| rs12139100 |
CCCATCCTCCAAATCCCGTT |
CTAGCTGTGTTACAGGGGCA |
| rs193222555 |
CTTCTTCTCCACTGCGAGCC |
TACTGTGCTTGGTGCTAGGG |
| rs149056482 |
GCTCACCTGGTGCCTTGTATTT |
GCTGAGTTGTTAGCTGGGGAA |
| rs116431025 |
TGGGAAGAAAGTCACGATGGG |
GCTGAGTTGTTAGCTGGGGA |
Genotypes were confirmed by randomly selecting 20
samples which were sequenced by Inqaba Biotec using the Sanger
sequencing method. The chromatograms showing the GG, GA and AA
genotypes of the rs4744 SNP are provided as Fig. S1, Fig.
S2 and Fig. S3,
respectively.
Calculations and definitions
Body mass index (BMI) was calculated as weight
(kg)/height (m2). Participants were categorized
according to BMI as normal weight (BMI <25 kg/m2),
overweight (BMI ≥25 kg/m2 and BMI <30
kg/m2) and obese (BMI ≥30 kg/m2) (31). Central obesity was determined using
the following criteria: Waist circumference (WC) >94 cm in men
and >80 cm in women (32).
Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg
or diastolic blood pressure (DBP) ≥90 mmHg or known hypertension on
treatment (33). Dyslipidemia was
defined as TC >5 mmol/l, triglycerides >1.5 mmol/l, HDL-C
<1.2 mmol/l, LDL-C >3.0 mmol/l and non-HDL-C >3.37 mmol/l
or taking anti-lipid agents (34).
Diabetes was defined as fasting plasma glucose ≥7.0 mmol/l and/or
2-h post glucose load ≥11.1 mmol/l, previously diagnosed or taking
antidiabetic medications (35).
Insulin resistance (IR) was based on the homeostasis
model assessment (HOMA) using the formula:
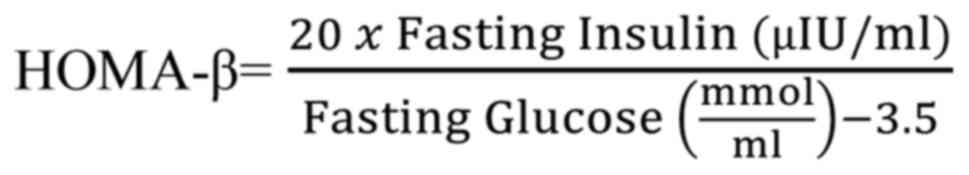
Beta cell function was determined by HOMA-β using
the formula (36):
Metabolic syndrome (MetS) was defined using the
Joint Interim Statement (JIS) criteria (37) when three of the following conditions
were met: i) A waist circumference ≥80 cm for women or ≥94 cm for
men; ii) triglyceride level ≥1.7 mmol/l; HDL-C level <1.04
mmol/l in men or <1.3 mmol/l in women; iii) high blood pressure
defined by SBP ≥130 mmHg and/or DBP ≥85 mmHg or receiving
hypertensive medication; and iv) fasting plasma glucose ≥5.6 mmol/l
or receiving diabetic medications.
The three genotypes were determined from the PCR
amplification output as follows: A sample was homozygous for the
dominant allele when amplification curves were observed only in the
VIC channel, amplification signal in the ROX channel was an
indication for homozygous recessive and amplification in both
channels was an indication of heterozygous genotype.
Statistical analysis
Data were entered onto an Excel spread sheet and
exported into the IBM Statistical Package for Social Sciences
(SPSS) version 25 software (IBM Corp.) for analysis. Continuous
variables which were skewed, are reported as median (25-75th
percentile) and compared using the median test, while categorical
variables are reported as ratio and percentages and compared using
the chi squared test. Hardy Weinberg equilibrium (HWE) was assessed
for all nine SNPs, and the rs4744 which was in HWE was used for
further analysis. The interactions between genotypes of the rs4744
SNP and cardiometabolic risk profile were determined using linear
and logistic regression analysis, by incorporating the dominant,
recessive and additive models on the predictive variable, as well
as their interaction with age and sex. All analysis were carried
out at 95% confidence interval and a 2-tailed P<0.05 was
considered to indicate a statistically significant difference.
Results
General characteristics of study
participants
A total of 716 participants were involved in the
present study, with 72.9% being women and the median duration of
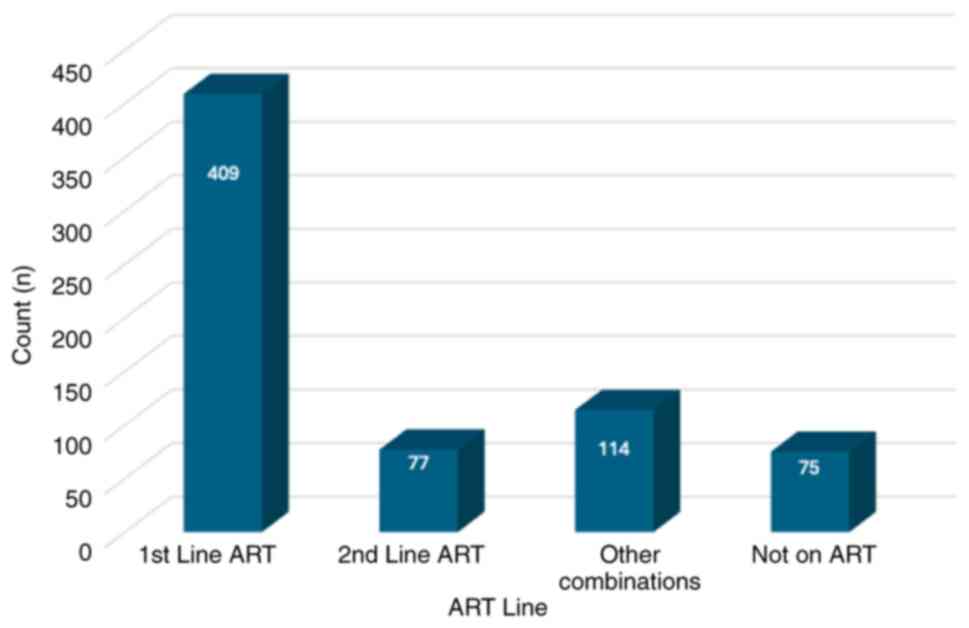
HIV was 60 months (25-75th percentile: 24-108) (Table II). The majority of participants
(60.6%, n=409) were on first-line ART, 11.4% (77 participants) were
on second-line ART, 16.9% (114 participants) were on other ART
combinations and 11.1% of participants (n=75) were not on HIV
medications (Fig. 1). The
prevalence of type 2 diabetes, obesity (BMI ≥30 kg/m2),
hypertension and metabolic syndrome were 8.5, 34.4, 24.2 and 27.5%,
respectively (Table II). Age,
weight, height, BMI, waist circumference, hip circumference,
waist-to-hip ratio, waist-to-height ratio, SBP, DBP, insulin,
homeostasis model assessment of insulin resistance (HOMA-IR),
glycated haemoglobin (HbA1c), and gamma glutamyl transferase (GGT)
were significantly higher in participants with MetS when compared
with those without MetS (Table
II). Moreover, the prevalence of MetS was significantly higher
amongst the female participants (31.0%) compared with the male
participants (16.9%); P<0.001 (Table II).
 | Table IIClinical characteristics of study
participants across MetS status. |
Table II
Clinical characteristics of study
participants across MetS status.
|
Variablea | N | Total: 716
(100%) | With MetS: 197
(27.5%) | Without MetS: 519
(72.5%) | P-value |
|---|
| Age (years) | 716 | 38.0
(32.0-45.0) | 39.0
(34.0-45.5) | 37.0
(30.5-42.0) | <0.001 |
| Weight (kg) | 715 | 68.4
(58.1-81.8) | 80.0
(70.3-93.5) | 65.9
(56.0-80.3) | <0.001 |
| Height (cm) | 715 | 160.6
(156.0-166.3) | 161.3
(156.4-165.6) | 160.1
(155.5-165.1) | 0.012 |
| BMI
(kg/m2) | 715 | 26.3
(22.1-32.1) | 30.5
(27.5-36.3) | 24.9
(21.5-31.7) | <0.001 |
| Waist circumference
(cm) | 715 | 88.0
(77.5-98.0) | 98.5
(92.6-106.8) | 84.7
(76.5-96.3) | <0.001 |
| Hip circumference
(cm) | 715 | 102.1
(92.6-112.1) | 109.2
(103.0-120.2) | 100.5
(92.0-111.3) | <0.001 |
| Waist to hip
ratio | 715 | 0.9 (0.8-0.9) | 0.9 (0.8-1.0) | 0.8 (0.8-0.9) | <0.001 |
| Waist to height
ratio | 715 | 0.6 (0.5-0.6) | 0.6 (0.6-0.7) | 0.5 (0.5-0.6) | <0.001 |
| SBP (mmHg) | 715 | 117.0
(107.0-129.5) | 124.3
(116.0-135.8) | 114.0
(105.0-124.3) | <0.001 |
| DBP (mmHg) | 725 | 82.0
(75.0-90.5) | 89.8
(82.3-97.3) | 80.3
(73.5-86.5) | <0.001 |
| Heart rate
(beats/min) | 725 | 74.5
(66.5-82.5) | 76.0
(70.0-82.8) | 75.0
(67.0-82.5) | 0.016 |
| CD4 count
(cells/mm3) | 371 | 395.0
(241.0-604.0) | 429.5
(275.0-670.5) | 358.0
(225.0-558.0) | 0.187 |
| HIV diagnosis
(duration in months) | NA | 60.0
(24.0-108.0) | 74.5
(48.0-120.0) | 53.0
(24.0-96.0) | <0.001 |
| Alanine
transaminase (IU/l) | 712 | 23.0
(17.0-34.0) | 24.0
(17.5-36.5) | 22.0
(16.0-32.0) | 0.132 |
| Aspartate
transaminase (IU/l) | 712 | 29.0
(24.0-38.0) | 29.5
(24.0-37.5) | 29.0
(24.5-38.0) | 0.916 |
| Total cholesterol
(mmol/l) | 711 | 4.3 (3.7-5.1) | 4.3 (3.7-5.0) | 4.4 (3.8-5.2) | 0.024 |
| HDL-C (mmol/l) | 711 | 1.3 (1.0-1.5) | 1.1 (1.0-1.2) | 1.4 (1.1-1.7) | <0.001 |
| Triglycerides
(mmol/l) | 710 | 1.0 (0.8-1.4) | 1.2 (0.9-1.8) | 0.9 (0.7-1.2) | <0.001 |
| LDL-C (mmol/l) | 711 | 2.5 (2.0-3.1) | 2.5 (2.1-3.2) | 2.5 (2.0-3.0) | <0.001 |
| Non-HDL-C
(mmol/l) | 665 | 3.0 (2.5-3.7) | 3.1 (2.6-3.8) | 3.0 (2.4-3.5) | <0.001 |
| Glucose
(mmol/l) | 711 | 5.0 (4.6-5.4) | 5.3 (4.7-6.0) | 4.9 (4.6-5.2) | <0.001 |
| Insulin (µU/l) | 679 | 6.1 (4.0-9.6) | 8.3 (6.0-12.3) | 5.4 (3.6-8.8) | <0.001 |
| HOMA-β
(µU/mmol) | 677 | 81.3
(48.6-130.1) | 99.2
(55.6-155.7) | 81.0
(49.7-120.8) | 0.220 |
| HOMA-IR (µU x
mmol/l2) | 678 | 1.4 (0.9-2.3) | 2.0 (1.4-3.4) | 1.2 (0.8-1.9) | <0.001 |
| HbA1c (%) | 712 | 5.4 (5.2-5.7) | 5.6 (5.3-6.0) | 5.4 (5.2-5.7) | <0.001 |
| Creatinine
(µmol/l) | 710 | 58.0
(51.0-67.0) | 59.5
(51.0-65.5) | 58.0
(51.0-66.0) | 0.456 |
| GGT (U/l) | 711 | 39.0
(26.0-66.0) | 47 (26.5-73.5) | 37 (24.0-61.0) | 0.019 |
| hs-CRP (mg/l) | 711 | 5.4 (2.4-14.0) | 6.4 (2.8-15.4) | 5.0 (1.7-12.3) | 0.075 |
| INF-γ (pg/ml) | 660 | 14 (12-16) | 13.5
(12.0-16.0) | 14 (12.0-16.0) | 0.104 |
| IL-10 (pg/ml) | 660 | 18 (14.5-24) | 17 (14.0-22.0) | 18.5 (14.8-25) | 0.062 |
| 1L-2 (pg/ml) | 660 | 9.5 (9-10) | 9 (9-10) | 9.5 (9-10) | 0.402 |
| TNF-α (pg/ml) | 660 | 19 (15-23.5) | 19 (15.5-22.5) | 19 (15-24) | 0.954 |
| eGFR (ml/min) | 701 | 91.0
(91.0-91.0) | 91.0
(91.0-91.0) | 91.0
(91.0-91.0) | NA |
| Sex | | | | | <0.001 |
|
Female | 710 | 562 (79.2) | 174 (31.0) | 388 (69.0) | |
|
Male | | 148 (20.8) | 25 (16.9) | 123 (83.1) | |
| Obese (BMI ≥30
kg/m2) | 715 | 246.0 (34.4) | 107.0 (54.3) | 139.0 (26.8) | <0.001 |
| WC (cm): men
>94; women >80 | 715 | 440.0 (61.5) | 186.0 (94.4) | 254.0 (49.0) | <0.001 |
| Hypertension | 715 | 173.0 (24.2) | 75.0 (38.1) | 98.0 (18.9) | <0.001 |
| Type 2
diabetes | 709 | 60 (8.5) | 46 (23.1) | 14 (2.7) | <0.001 |
| Hyperglycemia | 711 | 148.0 (20.8) | 100.0 (50.8) | 48.0 (9.3) | <0.001 |
| Total cholesterol
>5.0 mmol/l | 711 | 181.0 (25.5) | 55.0 (28.1) | 126.0 (24.5) | 0.325 |
| HDL-C <1.2
mmol/l | 711 | 265.0 (37.3) | 115.0 (58.7) | 150.0 (29.1) | <0.001 |
| Triglycerides
>1.5 mmol/l | 710 | 129.0 (18.2) | 85.0 (43.4) | 44.0 (8.6) | <0.001 |
| LDL-C >3.0
mmol/l | 711 | 199.0 (28.0) | 81.0 (41.3) | 118.0 (22.9) | <0.001 |
| Non-HDL-C >3.37
mmol/l | 665 | 243.0 (36.5) | 94.0 (50.5) | 149.0 (31.1) | <0.001 |
Genotypic distribution and minor
allele frequency of single nucleotide polymorphisms
The genotypic distribution and minor allele
frequency of the nine SNPs are presented in Table III. The percentage of successful
genotyping of the nine SNPs ranged between 82.3 and 98.6%. All
three genotypes were present for rs11573156 (C/G), rs4744 (G/A),
rs10732279 (A/G), rs6426616 (G/A), rs2301475 (A/G), rs12139100
(C/T), rs193222555 (T/C) and rs149056482 (T/C), while rs116431025
(C/T) contained the CC and CT genotype. Amongst the nine SNPs
investigated, only rs4744 was in HWE (Table III). All the other eight SNPs
significantly deviated from HWE (all P<0.001). Although positive
controls were used during genotyping and the genotypes were
confirmed by sequencing 20 samples, the absence of genotyping error
or copy number variation for SNPs deviating from HWE cannot be
excluded. As such, SNPs not in HWE were excluded from further
analysis, and only rs4744 was investigated on its association with
cardiometabolic diseases.
 | Table IIIFrequency of the PLA2 gene SNP
genotypes and minor allele frequency. |
Table III
Frequency of the PLA2 gene SNP
genotypes and minor allele frequency.
| SNP | Homozygous for
major allele, n (%) | Heterozygous, n
(%) | Homozygous for
minor allele, n (%) | Minor allele
frequency (%) | Deviation from HWE
(P-value) |
|---|
| rs11573156
(n=589) | 304.0 (51.6) | 283.0 (48.1) | 2.0 (0.3) | 24.4 | <0.0001 |
| rs4744 (n=661) | 549.0 (83.1) | 106.0 (16.0) | 6.0 (0.9) | 8.9 | 0.940 |
| rs10732279
(n=702) | 312.0 (44.4) | 379.0 (54.0) | 11.0 (1.6) | 28.0 | <0.0001 |
| rs6426616
(n=684) | 50.0 (7.3) | 580.0 (84.8) | 54.0 (7.9) | 49.7 | <0.0001 |
| rs2301475
(n=688) | 313.0 (45.5) | 333.0 (48.4) | 42.0 (6.1) | 30.3 | 0.00067 |
| rs12139100
(n=703) | 346.0 (49.2) | 344.0 (48.9) | 13.0 (1.9) | 26.1 | <0.0001 |
| rs116431025
(n=680) | 273.0 (40.1) | 407.0 (59.9) | 0.0 (0.0) | 29.9 | <0.0001 |
| rs193222555
(n=694) | 4.0 (0.6) | 349.0 (50.3) | 341.0 (49.1) | 25.7 | <0.0001 |
| rs149056482
(n=706) | 5.0 (0.7) | 445.0 (63.0) | 256.0 (36.3) | 32.3 | <0.0001 |
Participants characteristics across
rs4744 genotypes
In the present study, 647 participants with
anthropometric and biochemical measurements were successfully
genotyped for rs4744. The GG genotype was the most prevalent
representing 83.1% followed by the heterozygous GA representing
16.0%, while the AA genotype was found in 0.9% of the participants
(Table III). When clinical
measurements were compared across genotypes of the rs4744 SNP of
the PLA2G2A gene, the recessive AA genotype was associated
with low median HDL-C (P=0.044), high median TNF-α (P=0.041), HDL-C
<1.2 mmol/l (P=0.023) and the dominant GG genotype was
associated with BMI ≥30 kg/m2 (P=0.037), while no
significant differences were observed with all other measurements
(Table IV). No significant
differences were observed with type 2 diabetes and traits of
dysglycemia, high blood pressure and other markers of dyslipidemia
between the genotypes (Table
IV).
 | Table IVClinical characteristics compared
across PLA2G2A rs4744 genotypes. |
Table IV
Clinical characteristics compared
across PLA2G2A rs4744 genotypes.
|
Variablea | N | GG (n=536) | AA (n=6) | GA (n=105) | P-value |
|---|
| Age (years) | 716 | 37.5
(31.0-43.0) | 46.0
(37.0-55.0) | 36.0
(31.0-43.0) | 0.350 |
| Weight (kg) | 715 | 71.3
(59.2-88.4) | 73.3
(73.2-73.5) | 67.5
(56.2-84.6) | 0.165 |
| Height (cm) | 715 | 160.8
(155.7-165.5) | 165.9
(160.6-171.3) | 159.9
(156.8-163.5) | 0.898 |
| BMI
(kg/m2) | 715 | 27.7
(22.9-33.7) | 26.7
(25.0-28.4) | 27.6
(21.8-31.8) | 0.202 |
| Waist circumference
(cm) | 715 | 89.5
(79.5-101.6) | 95.5
(91.5-99.5) | 88.0
(78.2-97.8) | 0.325 |
| Hip circumference
(cm) | 715 | 104.5
(94.4-115.7) | 107.23
(100.3-114.3) | 103.0
(90.5-111.3) | 0.081 |
| Waist to hip
ratio | 715 | 0.9 (0.8-0.9) | 0.9 (0.8-0.9) | 0.8 (0.8-0.9) | 8.813 |
| Waist to height
ratio | 715 | 0.6 (0.5-0.6) | 0.6 (0.6-0.6) | 0.6 (0.5-0.6) | 0.438 |
| SBP (mmHg) | 715 | 117.0
(107.5-128.5) | 120.8
(114.5-127.0) | 112.8
(104.0-124.0) | 0.263 |
| DBP (mmHg) | 725 | 82.5
(75.5-89.5) | 83.8
(80.0-87.5) | 82.0
(71.5-88.5) | 0.185 |
| Heart rate
(beats/min) | 725 | 75.3
(67.5-81.5) | 71.5
(67.0-76.0) | 78.0
(66.5-85.5) | 0.176 |
| CD4 count
(cells/mm3) | 371 | 375.0
(229.0-594.0) | 660.0
(476.0-884.0) | 408.5
(254.0-545.0) | 0.358 |
| Duration of HIV in
months | 659 | 60 (24-108) | 66.0 (12-84) | 60 (36-96) | 0.846 |
| Alanine
transaminase (IU/l) | 712 | 23.0
(17.0-34.0) | 19.0
(16.0-22.0) | 23.0
(16.0-34.0) | 0.790 |
| Aspartate
transaminase (IU/l) | 712 | 30.0
(25.0-38.0) | 26.5
(20.0-33.0) | 30.0
(24.0-37.0) | 0.619 |
| Total cholesterol
(mmol/l) | 711 | 4.4 (3.8-5.1) | 3.9 (3.4-4.4) | 4.3 (3.5-5.0) | 0.792 |
| HDL-C (mmol/l) | 711 | 1.3 (1.1-1.6) | 1.2 (1.1-1.3) | 1.3 (1.0-1.5) | 0.044b |
| Triglycerides
(mmol/l) | 710 | 1.0 (0.8-1.3) | 0.8 (0.7-0.9) | 0.9 (0.6-1.3) | 0.259 |
| LDL-C (mmol/l) | 711 | 2.5 (2.0-3.1) | 2.2 (1.7-2.7) | 2.3 (1.9-2.8) | 0.531 |
| Non-HDL cholesterol
(mmol/l) | 665 | 3.0
(2.52-3.67) | 2.7 (2.2-3.1) | 2.9 (2.5-3.2) | 0.388 |
| Glucose
(mmol/l) | 711 | 5.0 (4.6-5.4) | 5.1 (4.5-5.6) | 4.9 (4.6-5.2) | 0.776 |
| Insulin (µU/l) | 679 | 6.2 (4.0-9.80) | 4.9 (3.6-6.1) | 5.4 (4.0-8.7) | 0.073 |
| HOMA-β
(µU/mmol) | 677 | 86.7
(50.0-130.8) | 78.1
(34.3-122.0) | 86.0
(60.0-145.0) | 0.247 |
| HOMA-IR (µIU x
mmol/l2) | 678 | 1.4 (0.9-2.3) | 1.1 (0.9-1.2) | 1.2 (0.9-1.8) | 0.207 |
| HbA1c (%) | 712 | 5.4 (5.2-5.7) | 5.4 (5.3-5.5) | 5.5 (5.2-5.6) | 0.693 |
| Creatinine
(µmol/l) | 710 | 58.0
(51.0-65.0) | 48.0
(44.0-52.0) | 56.0
(49.0-67.0) | 0.240 |
| GGT (U/l) | 711 | 39.0
(25.0-66.0) | 73.0
(19.0-127.0) | 27.0
(19.0-40.0) | 0.281 |
| hs-CRP (mg/l) | 711 | 5.1 (1.9-12.9) | 3.1 (2.0-4.2) | 4.8 (1.8-10.1) | 0.992 |
| INF-γ (pg/ml) | 613 | 14 (12-16.3) | 16 (11-17) | 13.5 (12-15) | 0.323 |
| IL-10 (pg/ml) | 613 | 18.5 (14.5-24) | 16 (16-43) | 18 (14-23) | 0.414 |
| 1L-2 (pg/ml) | 613 | 9.5 (9.5-10) | 9 (8.5-10) | 9 (9-10) | 0.882 |
| TNF-α (pg/ml) | 613 | 19 (15-23) | 22.5 (21.5-28) | 19 (16-23) | 0.041b |
| eGFR (ml/min) | 701 | 91.0
(91.0-91.0) | 91.0
(91.0-91.0) | 91.0
(87.0-91.0) | NA |
| Sex (Female) | 647 | 429 (80.0) | 6(100) | 79 (75.2) | 0.246 |
| BMI ≥30
kg/m2 | 646 | 195 (36.4) | 0 (0.0) | 27 (26.0) | 0.037b |
| WC (cm): men
>94; women >80 | 646 | 335 (62.5) | 3 (50.0) | 59 (56.7) | 0.459 |
| Hypertension | 646 | 144 (26.9) | 0 (0.0) | 30 (28.8) | 0.30 |
| Type 2
diabetes | 643 | 46 (8.6) | 0 (0.0) | 7 (6.7) | 0.367 |
| Total cholesterol
>5.0 mmol/l | 643 | 138 (25.9) | 0 (0.0) | 25 (23.8) | 0.322 |
| HDL-C <1.2
mmol/l | 643 | 234 (44.0) | 6(100) | 47 (44.8) | 0.023b |
| Triglyceride
>1.5 mmol/l | 642 | 73 (13.7) | 2 (33.3) | 12 (11.4) | 0.297 |
| LDL-C >3.0
mmol/l | 643 | 150 (28.2) | 1 (16.7) | 28 (15.6) | 0.787 |
| Non HDL-C >3.37
mmol/l | 598 | 184 (37.2) | 2 (33.3) | 30 (30.9) | 0.49 |
Linear and logistic regression
analysis for the association of rs4744 SNP genotypes with
cardiometabolic traits
Linear regression adjusting for age, sex and BMI was
used to explore the relationship between the rs4744 recessive
genotype of the PLA2G2A gene and cardiometabolic traits. The
results showed that the recessive genotype was associated with
HDL-C (β=0.366; P=0.024) when compared with the homozygous dominant
genotype and (β=0.371; P=0.025) when compared with the heterozygous
genotype. There was no association between the genotypes and all
other cardiometabolic traits (Table
V).
 | Table VSimple linear regression analysis of
rs4744 SNP genotypes and cardiometabolic traits. |
Table V
Simple linear regression analysis of
rs4744 SNP genotypes and cardiometabolic traits.
| Variable | Age | Sex
(Female=Reference) | BMI | rs4744 major | rs4744 hetero | R2 Model 1 | R2 Model 2 |
|---|
| SBP (mmHg) | 0.735
(0.079)a | 7.841
(1.941)a | 0.173 (0.113) | 0.586 (1.933) | 9.431 (7.439) | 0.002 | 0.155 |
| DBP (mmHg) | 0.275
(0.053)a | 2.466 (1.292) | 0.227
(0.075)b | 7.142 (4.951) | 0.972 (1.287) | 0.004 | 0.059 |
| TC (mmol/l) | 0.026
(0.004)a | -0.211
(0.109)c | 0.011 (0.006) | 0.615 (0.418) | -0.072 (0.109) | 0.002 | 0.06 |
| HDL-C (mmol/l) | 0.004
(0.02)c | -0.176
(0.042)a | -0.013
(0.002)a | 0.366
(0.162)c | 0.371
(0.165)c | 0.001 | 0.053 |
| TG (mmol/l) | 0.012
(0.003)a | 0.275
(0.063)a | 0.016
(0.004)a | -0.253 (0.241) | -0.068 (0.063) | -0.001 | 0.077 |
| LDL-C (mmol/l) | 0.020
(0.004)a | -0.110 (0.094) | 0.021
(0.005)a | 0.390 (0.395) | -0.077 (0.093) | 0.002 | 0.075 |
| Non HDL-C
(mmol/l) | 0.022
(0.004)a | -0.028 (0.101) | 0.025
(0.006)a | 0.233 (0.377) | -0.077 (0.102) | 0.000 | 0.072 |
| Glucose
(mmol/l) | 0.029
(0.007)a | 0.233 (0.181) | 0.038
(0.010)a | 0.310 (0.691) | -0.016 (0.180) | -0.002 | 0.040 |
| Insulin | -0.062 (0.032) | -0.961 (0.790) | 0.350
(0.045)a | 2.144 (3.198) | -1.480 (0.786) | 0.008 | 0.128 |
| HOMA-β | -0.627 (0.927) | -20.258
(22.993) | 4.495
(1.324)a | 19.828
(103.649) | -4.870
(22.878) | -0.003 | 0.011 |
| HOMA-IR | -0.004 (0.010) | -0.139 (0.239) | 0.104
(0.014)a | 0.540 (0.968) | -0.379 (0.238) | 0.005 | 0.166 |
| HbA1c | 0.015
(0.003)a | 0.086 (0.083) | 0.020
(0.005)a | -0.072 (0.318) | 0.046 (0.083) | -0.003 | 0.052 |
Binary logistic regression was used to examine
whether the recessive genotype of the rs4744 SNP of the
PLA2G2A gene could be used to predict CMDs and traits
including obesity, diabetes, hypertension, MetS or abnormal lipid
levels in the study population. The odds of prevalent MetS for
individuals with the recessive genotype were 7 times higher
(P=0.017) and 10 times higher (P=0.036) when compared with
individuals of the heterozygous genotype and dominant genotype,
respectively (Table VI). The odds
of dyslipidemia characterized by low HDL-C were 5 times higher for
individuals with the recessive genotype compared with those with
the heterozygous and dominant genotypes (P=0.049).
 | Table VILogistic regression analysis of
rs4744 SNP genotypes and cardiometabolic diseases. |
Table VI
Logistic regression analysis of
rs4744 SNP genotypes and cardiometabolic diseases.
| Variable | Age | Sex
(Female=Reference) | BMI | rs4744 major | rs4744 hetero |
|---|
| Diabetes | 1.07
(1.03-1.10)a | 1.49
(0.68-3.26) | 1.07
(1.02-1.11)b | Undetermined | Undetermined |
| Obesity (WC) | 0.96
(0.92-0.99)b | 13.0
(5.37-31.5)a | 0.51
(0.45-0.58)a | 2.70
(0.09-85.6) | 3.53
(0.10-119.5) |
| Hypertension | 1.02
(1.04-1.08)a | 1.17
(0.85-2.21) | 1.02
(1.00-1.05) | Undetermined | Undetermined |
| MetS | 1.07
(1.04-1.09)a | 0.70
(0.39-1.27) | 1.13
(1.09-1.16)a | 0.14
(0.02-0.88)c | 0.10
(0.02-0.67)c |
| High TC
(mmol/l) | 1.05
(1.03-1.07)a | 0.89
(0.54-1.46) | 1.02
(0.99-1.05) | Undetermined | Undetermined |
| Low HDL-C
(mmol/l) | 0.98
(0.96-1.00)c | 2.54
(1.62-3.97)a | 1.05
(1.02-1.08)a | 0.19
(0.03-1.07)c | 0.19
(0.03-0.99)c |
| High TG
(mmol/l) | 1.05
(1.03-1.08)a | 3.08
(1.79-5.31)a | 1.06
(1.02-1.09)c | 0.19
(0.03-1.18) | 0.18
(0.03-1.20) |
| High LDL-C
(mmol/l) | 1.05
(1.03-1.07)a | 0.84
(0.51-1.38) | 1.04
(1.01-1.07)b | 1.52
(0.17-13.96) | 1.48
(0.16-14.05) |
| High non-HDL-C
(mmol/l) | 1.05
(1.03-1.07)a | 1.00
(0.63-1.61) | 1.04
(1.01-1.07)b | 0.85
(0.14-5.06) | 0.67
(0.11-4.19) |
Discussion
The present study sought to examine the association
between nine SNPs of the PLA2 gene and cardiometabolic
diseases including diabetes, obesity, hypertension and MetS in a
black South African population with HIV. This population consisted
of more female participants (79.3%) than male participants (20.7%),
a disparity which cannot be explained by the sex distribution in
Cape Town, where females comprised 51% and males 49% in
2015(38). The high representation
of females could result from the high prevalence of HIV as well as
the willingness of women to participate in research, and the
reluctance of providing blood samples by potential male
participants. The prevalence of these cardiometabolic diseases were
8.4, 34.4, 24.2 and 27.5% for diabetes, obesity, hypertension and
MetS, respectively. Amongst the nine SNPs examined, only the rs4744
SNP was in HWE equilibrium. Given that non-random mating and
genotyping errors may result to deviation from HWE and lead to
spurious associations, all SNPs not in HWE were not analyzed for
their association with cardiometabolic traits. As such, only rs4744
SNP of the PLA2G2A gene was investigated for its association
with cardiometabolic diseases. The recessive (AA) genotype of the
rs4744 SNP was significantly associated with low HDL-C and high
TNF-α levels. Moreover, linear and logistic regression adjusting
for age, sex and BMI revealed that carrying the AA genotype of the
SNP was associated with low HDL-C levels and an increased risk of
MetS.
Non-synonymous SNPs can influence the expression of
a gene and mRNA levels leading to an increase or a decrease in the
level of the translated protein/enzyme (30,39).
The association between SNPs in the PLA2 gene and its
corresponding enzyme activity has been extensively studied
(40-42).
In Caucasians with and without coronary artery disease (CAD) and
diabetes, two SNPs of the PLA2G2A gene (rs11573156, and
rs1774131 rare alleles) were associated with high enzyme activity
and three SNPs (rs3767221, rs3753827 and rs2236771 rare alleles)
were associated with low enzyme activities (40). Similarly, sPLA2-IIa levels were
almost 200% higher in carriers of the rs4744 recessive genotype
when compared with carriers of the wild type homozygous genotype in
patients with stable CAD (20).
Similarly, the rare allele of the rs11573156 SNP was associated
with high enzyme activity in French patients with myocardial
infarction (41). Moreover, altered
activities and levels of PLA2G2A proteins have been observed to be
associated with cardiometabolic traits and diseases including
dyslipidemia, insulin resistance (42), obesity, CVD, stroke and type 2
diabetes (43). Lower levels of
sPLA2 enzyme activity and sPLA2-IIA mass were associated with a
reduced risk of cardiovascular events in the general European
population (29). Similarly the A/A
genotype of rs4744 SNP was associated with a higher risk of acute
cardiovascular events (acute coronary syndrome, myocardial
infarction, coronary revascularization) (20). As such, changes in the expression of
genes resulting from SNPs could possibly alter downstream pathways
which are associated with the development of cardiometabolic
diseases. The present findings could therefore be indicative that
the recessive genotype of the rs4744 SNP of the PLA2G2A gene
alters downstream targets leading to the development of
dyslipidemia characterized by low HDL-C and MetS.
The various forms of the PLA2 gene have been
reported to be associated differently with insulin sensitivity and
adiposity. The expression of PLA2G1B and PLA2G2E was
shown to be positively associated with the risk of obesity and
insulin resistance (17,44). Similarly, an inhibitor of the
PLA2G2A gene reduced the overexpression of the gene and
attenuated visceral adiposity, and reversed most characteristics of
MetS, including insulin sensitivity, glucose intolerance and
cardiovascular abnormalities in male Wistar rats (13). Conversely, overexpression of
PLA2G2A improved insulin sensitivity, glucose tolerance, and
adiposity in IIA+ mice (mice expressing the human
PLA2G2A gene), indicating that the metabolic effect is
dependent on the SNP studied (44).
Given that rs4744 SNP has been associated with increased serum
PLA2G2A levels leading to the development of CVD (20), a similar mechanism might be
postulated in this present study whereby the recessive genotype
contributed to the dysregulation of the enzyme or activity level
leading to the development of MetS.
Another pathway through which the recessive genotype
of the rs4744 SNP of the PLA2G2A gene could increase the
risk of MetS is the inflammatory pathway and dyslipidemia. Notably,
100% of participants with the minor AA genotype had low HDL-C
levels, characteristic of dyslipidemia compared with 44% of the
major (GG) genotype carriers. The dyslipidemia could also result
from the ARVs, given that >80% of the study participants were on
either protease inhibitors, NRTIs, NNRTIs or a combination of these
ARVs, which contribute to abnormal lipid metabolism. Moreover, the
virus could contribute to increased inflammation in carriers of the
minor genotype. This is because the inflammatory marker, TNF-α was
higher in carriers of the recessive genotype when compared with
carriers of the dominant genotype. The absence of association with
IL-10 might indicate that the SNP investigated induced inflammation
by targeting the pro-inflammatory pathway without affecting the
anti-inflammatory pathway.
The limitations of the present study include the
small sample size and low representation of male participants. Due
to the limited sample size, only 6 participants with the recessive
genotype were genotyped, and none were found in the diabetes,
obesity, and hypertension group. As such, logistic regression to
predict diabetes, obesity, and hypertension could not be computed.
Furthermore, the present study did not assess the expression and
activity levels of serum PLA2G2A, making it impossible to establish
a correlation between the genotypes and the expression levels of
the enzyme. Moreover, the levels of prostaglandins and
leukotrienes, which are the main inflammatory markers produced from
hydrolysis of phospholipids by PLA2G2A enzymes, were not
determined. Additionally, the study only involved HIV participants,
limiting the possibility to explore and compare the effects between
HIV and non-HIV populations. Therefore, further studies to mitigate
these limitations are warranted.
In conclusion, in a black South African population
with HIV, the minor AA genotype of the rs4744 SNP of the
PLA2G2A gene could potentially contribute to cardiometabolic
risk evaluation. This minor genotype was revealed to be associated
with a high PLA2 enzyme activity level. In addition, this enzyme
mediates lipid signaling (20), and
in the South African population may contribute to CMDs by inducing
inflammation and dyslipidemia. Given that, to the best of our
knowledge, this is the first study to report such findings and no
independent validation was carried out, further studies in
independent cohorts are warranted to confirm these results, while
investigating other SNPs and protein expression levels.
Furthermore, functional studies in animal models will contribute to
identify the molecular pathways which may be essential in the
establishment of therapeutic targets.
Supplementary Material
Homozygous dominant GG genotype of the
rs4744 single nucleotide polymorphism.
Heterozygous GA genotype of the rs4744
single nucleotide polymorphism.
Homozygous recessive AA genotype of
the rs4744 single nucleotide polymorphism.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Grand Challenges
Canada, through the Global Alliance on Chronic Diseases Initiative
(Hypertension grant no. 0169-04); and the South African Medical
Research Council (SAMRC) through baseline allocation to the
Non-communicable Diseases Research Unit (NCDRU).
Availability of data and materials
The data SNP dataset is available at: https://esango.cput.ac.za/articles/dataset/_b_Investigating_PLA2G2A_SNPs_and_cardiometabolic_diseases_in_South_Africa_b_/29480720?file=55989242).
Authors' contributions
TEM and APK conceived the study and acquired the
funding. NEN performed the single nucleotide polymorphism
genotyping. NEN and UN confirm the authenticity of all the raw
data, performed the data analysis and interpretation, and wrote the
original draft of the manuscript. All authors participated in the
revision of the manuscript, and read and approved the final
version.
Ethics approval and consent to
participate
Approval was obtained from the South African Medical
Research Council Ethics Committee (approval no. EC021-11/2013) in
Cape Town, South Africa and the study was conducted in accordance
with the principles of the Declaration of Helsinki. The Health
Research Office of the Western Cape Department of Health and the
selected healthcare facilities in Cape Town, South Africa granted
permission for the recruitment of participants. Written informed
consent was obtained from all participants before inclusion in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pillay-van Wyk V, Msemburi W, Laubscher R,
Dorrington RE, Groenewald P, Glass T, Nojilana B, Joubert JD,
Matzopoulos R, Prinsloo M, et al: Mortality trends and
differentials in South Africa from 1997 to 2012: Second national
burden of disease study. Lancet Glob Health. 4:e642–e653.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Statistics South Africa: Mid-year
population estimates. Statistics South Africa, Pretoria, pp1-50,
2022.
|
|
3
|
Coetzee L, Bogler L, De-Neve JW,
Bärnighausen T, Geldsetzer P and Vollme S: HIV, antiretroviral
therapy and non-communicable diseases in sub-Saharan Africa:
Empirical evidence from 44 countries over the period 2000 to 2016.
J Int AIDS Soc. 22(e25364)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Statistics South Africa: Mortality and
causes of death in South Africa: Findings from death notification.
Statistics South Africa, Pretoria, pp1-145, 2017.
|
|
5
|
Mohan J, Ghazi T and Chuturgoon AA: A
critical review of the biochemical mechanisms and epigenetic
modifications in HIV- and antiretroviral-induced metabolic
syndrome. Int J Mol Sci. 22(12020)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Masuku SKS, Tsoka-Gwegweni J and Sartorius
B: HIV and antiretroviral therapy-induced metabolic syndrome in
people living with HIV and its implications for care: A critical
review. J Diabetol. 10:41–47. 2019.
|
|
7
|
Levitt NS, Peer N, Steyn K, Lombard C,
Maartens G, Lambert EV and Dave JA: Increased risk of dysglycaemia
in South Africans with HIV; especially those on protease
inhibitors. Diabetes Res Clin Pract. 119:41–47. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Murakami M and Kudo I: Phospholipase A2. J
Biochem. 131:285–292. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dennis EA, Cao J, Hsu YH, Magrioti V and
Kokotos G: Phospholipase A2 enzymes: Physical structure, biological
function, disease implication, chemical inhibition, and therapeutic
intervention. Chem Rev. 111:6130–6185. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Khan SA and Ilies MA: The phospholipase A2
superfamily: Structure, isozymes, catalysis, physiologic and
pathologic roles. Int J Mol Sci. 24(1353)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Massaad C, Paradon M, Jacques C, Salvat C,
Bereziat G, Berenbaum F and Olivier JL: Induction of secreted type
IIA phospholipase A2 gene transcription by interleukin-1beta. Role
of C/EBP factors. J Biol Chem. 275:22686–22694. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee C, Park DW, Lee J, Lee TI, Kim YJ, Lee
YS and Baek SH: Secretory phospholipase A2 induces apoptosis
through TNF-alpha and cytochrome c-mediated caspase cascade in
murine macrophage RAW 264.7 cells. Eur J Pharmacol. 536:47–53.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Iyer A, Lim J, Poudyal H, Reid RC, Suen
JY, Webster J, Prins JB, Whitehead JP, Fairlie DP and Brown L: An
inhibitor of phospholipase A2 group IIA modulates adipocyte
signaling and protects against diet-induced metabolic syndrome in
rats. Diabetes. 61:2320–2329. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kuefner MS, Pham K, Redd JR, Stephenson
EJ, Harvey I, Deng X, Bridges D, Boilard E, Elam MB and Park EA:
Secretory phospholipase A2 group IIA modulates insulin
sensitivity and metabo. J Lipid Res. 58:1822–1833. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kuefner MS, Deng X, Stephenson EJ, Pham K
and Park EA: Secretory phospholipase A2 group IIA
enhances the metabolic rate and increases glucose utilization in
response to thyroid hormone. FASEB J. 33:738–749. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sato H, Taketomi Y, Ushida A, Isogai Y,
Kojima T, Hirabayashi T, Miki Y, Yamamoto K, Nishito Y, Kobayashi
T, et al: The adipocyte-inducible secreted phospholipases PLA2G5
and PLA2G2E play distinct roles in obesity. Cell Metab. 20:119–132.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Guijas C, Rodríguez JP, Rubio JM, Balboa
MA and Balsinde J: Phospholipase A2 regulation of lipid droplet
formation. Biochim Biophys Acta. 1841:1661–1671. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Peña L, Meana C, Astudillo AM, Lordén G,
Valdearcos M, Sato H, Murakami M, Balsinde J and Balboa MA:
Critical role for cytosolic group IVA phospholipase A2 in early
adipocyte differentiation and obesity. Biochim Biophys Acta.
1861:1083–1095. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khan MI and Hariprasad G: Human secretary
phospholipase A2 mutations and their clinical implications. J
Inflamm Res. 13:551–561. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Breitling LP, Koenig W, Fischer M, Mallat
Z, Hengstenberg C, Rothenbacher D and Brenner H: Type II secretory
phospholipase A2 and prognosis in patients with stable coronary
heart disease: Mendelian randomization study. PLoS One.
6(e22318)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
World health Organization: Noncommunicable
Disease Surveillance, Monitoring and Reporting: STEPwise approach
to NCD risk factor surveillance (STEPS). https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps.
Accessed January 13, 2014.
|
|
22
|
Ngwa NE, Peer N, Matsha TE, de-Villiers A,
Sobngwi E and Kengne AP: Associations of leucocyte telomere length
with cardio-metabolic risk profile in a South African HIV-infected
population. Medicine (Baltimore). 101(5e28642)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
National Kidney Foundation. K/DOQI
clinical practice guidelines for chronic kidney disease:
Evaluation, classification, and stratification. Am J Kidney Dis. 39
(2 Suppl 1):S1–S266. 2002.PubMed/NCBI
|
|
24
|
Allain CC, Poon LS, Chan CS, Richmond W
and Fu PC: Enzymatic determination of total serum cholesterol. Clin
Chem. 20:470–475. 1974.PubMed/NCBI
|
|
25
|
Jabbar J, Siddique I and Qaiser R:
Comparison of two methods (precipitation manual and fully automated
enzymatic) for the analysis of HDL and LDL cholesterol. J Pak Med
Assoc. 56:59–61. 2006.PubMed/NCBI
|
|
26
|
Mcgowan MW, Artiss JD, Strandbergh DR and
Zak BA: Peroxidase-coupled method for the colorimetric
determination of serum triglycerides. Clin Chem. 29:538–542.
1983.PubMed/NCBI
|
|
27
|
Friedewald WT, Levy RI and Fredrickson DS:
Estimation of the concentration of low-density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 18:499–502. 1972.PubMed/NCBI
|
|
28
|
Miller SA, Dykes DD and Polesky HF: A
simple salting out procedure for extracting DNA from human
nucleated cells. Nucleic Acids Res. 16(1215)1988.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Holmes MV, Simon T, Exeter HJ, Folkersen
L, Asselbergs FW, Guardiola M, Cooper JA, Palmen J, Hubacek JA,
Carruthers KF, et al: Secretory phospholipase A2-IIA and
cardiovascular disease: A Mendelian randomization study. J Am Coll
Cardiol. 62:1966–1976. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Akinkuolie AO, Lawler PR, Chu AY,
Caulfield M, Mu J, Ding B, Nyberg F, Glynn RJ, Ridker PM,
Hurt-Camejo E, et al: Group IIA secretory phospholipase
A2, vascular inflammation, and incident cardiovascular
disease. Arterioscler Thromb Vasc Biol. 39:1182–1190.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
No authors listed. Obesity: preventing and
managing the global epidemic. Report of a WHO consultation. World
Health Organ Tech Rep Ser. 894:i–xii, 1-253. 2000.PubMed/NCBI
|
|
32
|
World Health Organization (WHO). Waist
Circumference and Waist-Hip Ratio: Report of a WHO Expert
Consultation, Geneva, 8-11 December 2008. WHO, Geneva, 2011.
|
|
33
|
Williams B, Mancia G, Spiering W, Rosei
EA, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak
A, et al: 2018 ESC/ESH guidelines for the management of arterial
hypertension. Eur Heart J. 39:3021–3104. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Diagnosis management and prevention of the
common dyslipidaemias in South Africa-clinical guideline, 2000.
South African medical association and lipid and atherosclerosis
society of Southern Africa working group. S Afr Med J. 90:164–174,
176-178. 2000.PubMed/NCBI
|
|
35
|
World Health Organization (WHO):
Definition, diagnosis and classification of diabetes mellitus and
its complications. Report of a WHO Consultation. WHO, Geneva,
1999.
|
|
36
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Alberti KGMM, Eckel RH, Grundy SM, Zimmet
PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith
SC Jr, et al: Harmonizing the metabolic syndrome: A joint interim
statement of the international diabetes federation task force on
epidemiology and prevention; national heart, lung, and blood
institute; American heart association; world heart federation;
international atherosclerosis society; and international
association for the study of obesity. Circulation. 120:1640–1645.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Statistics South Africa: Mid-year
population estimates. Statistics South Africa, Pretoria, pp1-20,
2015.
|
|
39
|
Xu L, Zhou J, Huang S, Huang Y, Le Y,
Jiang D, Wang F, Yang X, Xu W, Huang X, et al: An association study
between genetic polymorphisms related to lipoprotein-associated
phospholipase A(2) and coronary heart disease. Exp Ther Med.
5:742–750. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wootton PT, Drenos F, Cooper JA, Thompson
SR, Stephens JW, Hurt-Camejo E, Wiklund O, Humphries SE and Talmud
PJ: Tagging-SNP haplotype analysis of the secretory PLA2IIa gene
PLA2G2A shows strong association with serum levels of sPLA2IIa:
Results from the UDACS study. Hum Mol Genet. 15:355–361.
2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Simon T, Mallat Z, Kotti-Tounsi S,
Benessiano J, Lambeau G, Steg G, Allard-Latour G, Normand JP,
Bourlard P, Tedgui A, et al: Abstract 5908: Impact of Secretory
PLA2-IIa Gene Polymorphims on sPLA2 Activity and Cardiovascular
Events Following an AMI: Results From the French Registry of Acute
ST Elevation or Non-ST-elevation Myocardial Infarction (FAST-MI)
Registry. Circulation. 120 (Suppl 18)(S1174)2009.
|
|
42
|
Leinonen E, Hurt-Camejo E, Wiklund O,
Hultén LM, Hiukka A and Taskinen MR: Insulin resistance and
adiposity correlate with acute-phase reaction and soluble cell
adhesion molecules in type 2 diabetes. Atherosclerosis.
166:387–394. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Leinonen ES, Hiukka A, Hurt-Camejo E,
Wiklund O, Sarna SS, Mattson Hultén L, Westerbacka J, Salonen RM,
Salonen JT and Taskinen MR: Low-grade inflammation, endothelial
activation and carotid intima-media thickness in type 2 diabetes. J
Intern Med. 256:119–127. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Huggins KW, Boileau AC and Hui DY:
Protection against diet-induced obesity and obesity-related insulin
resistance in group 1B PLA2-deficient mice. Am J Physiol Endocrinol
Metab. 283:E994–E1001. 2002.PubMed/NCBI View Article : Google Scholar
|