|
1
|
Heidenreich PA, Bozkurt B, Aguilar D,
Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM,
Evers LR, et al: 2022 AHA/ACC/HFSA guideline for the management of
heart failure: A report of the American College of
Cardiology/American heart association joint committee on clinical
practice guidelines. Circulation. 145:e895–e1032. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kemp CD and Conte JV: The pathophysiology
of heart failure. Cardiovasc Pathol. 21:365–371. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Savarese G, Becher PM, Lund LH, Seferovic
P, Rosano GMC and Coats AJS: Global burden of heart failure: A
comprehensive and updated review of epidemiology. Cardiovasc Res.
118:3272–3287. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Farré N, Vela E, Clèries M, Bustins M,
Cainzos-Achirica M, Enjuanes C, Moliner P, Ruiz S, Verdú-Rotellar
JM and Comín-Colet J: Real world heart failure epidemiology and
outcome: A population-based analysis of 88,195 patients. PLoS One.
12(e0172745)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heidenreich PA: Healthy lifestyles and
personal responsibility. J Am Coll Cardiol. 64:1786–1788.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McDonagh TA, Metra M, Adamo M, Gardner RS,
Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et
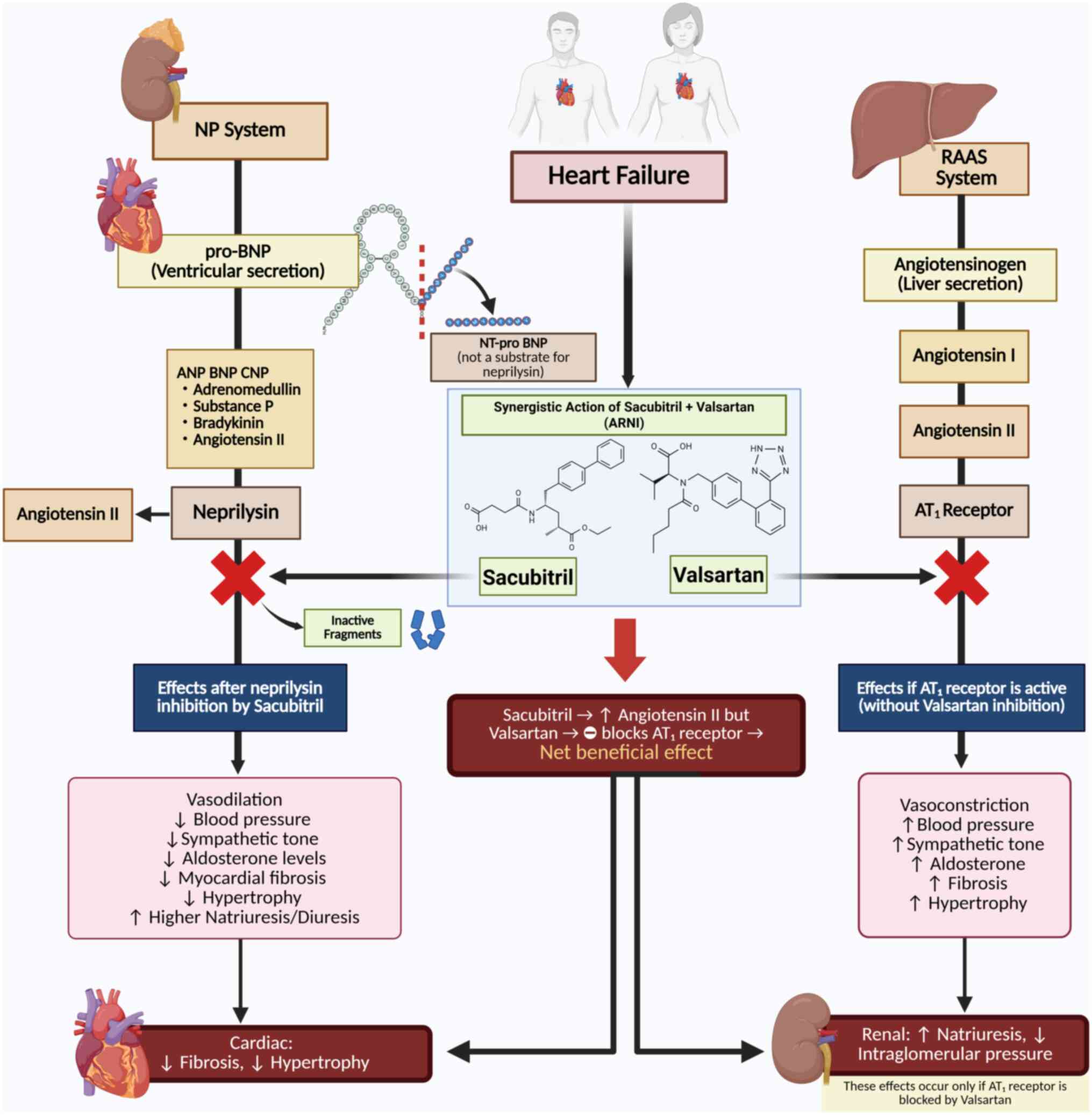
al: 2021 ESC Guidelines for the diagnosis and treatment of acute
and chronic heart failure. Eur Heart J. 42:3599–3726.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure. Kardiol Pol.
74:1037–1147. 2016.PubMed/NCBI View Article : Google Scholar : (In Polish).
|
|
8
|
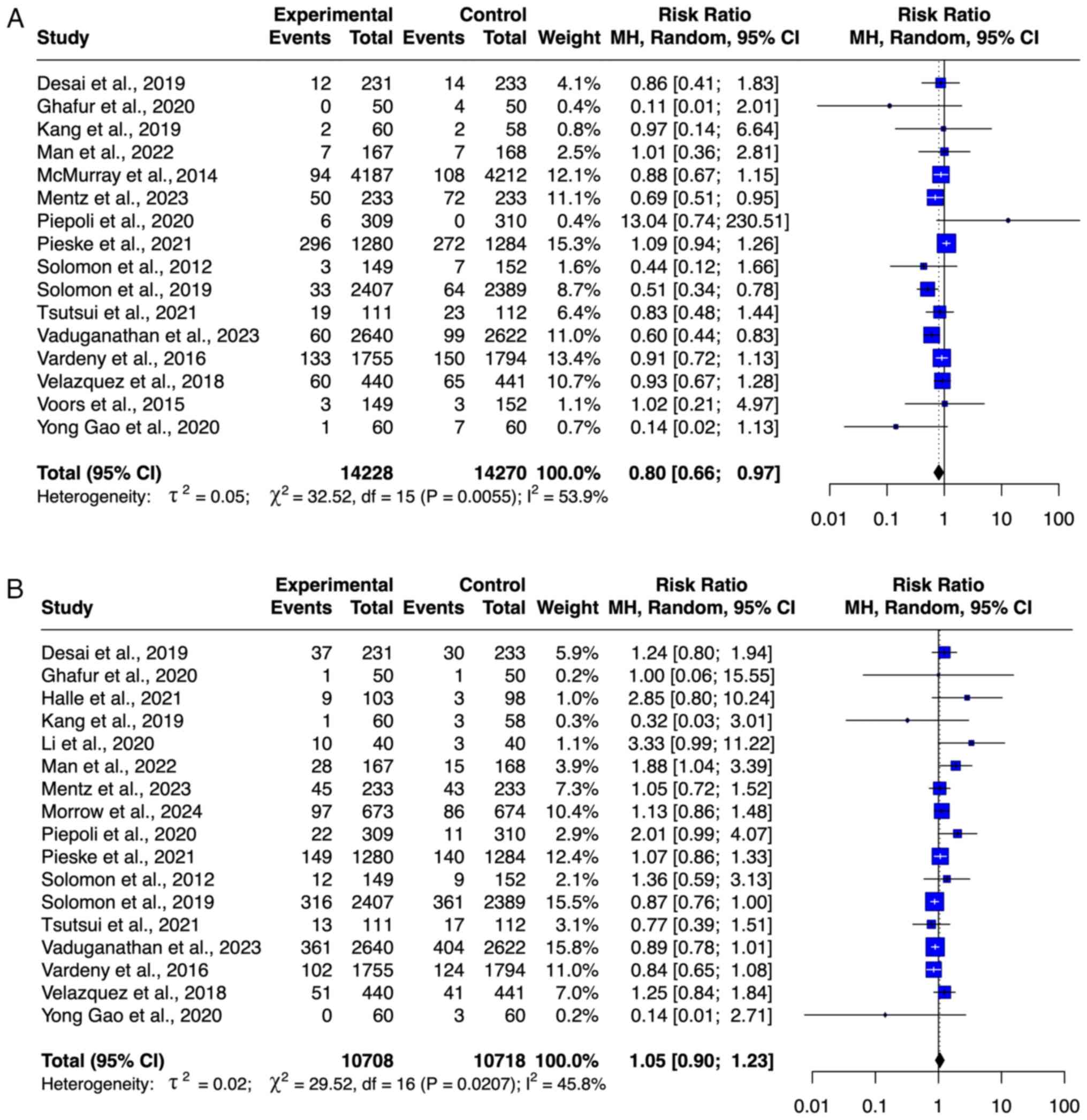
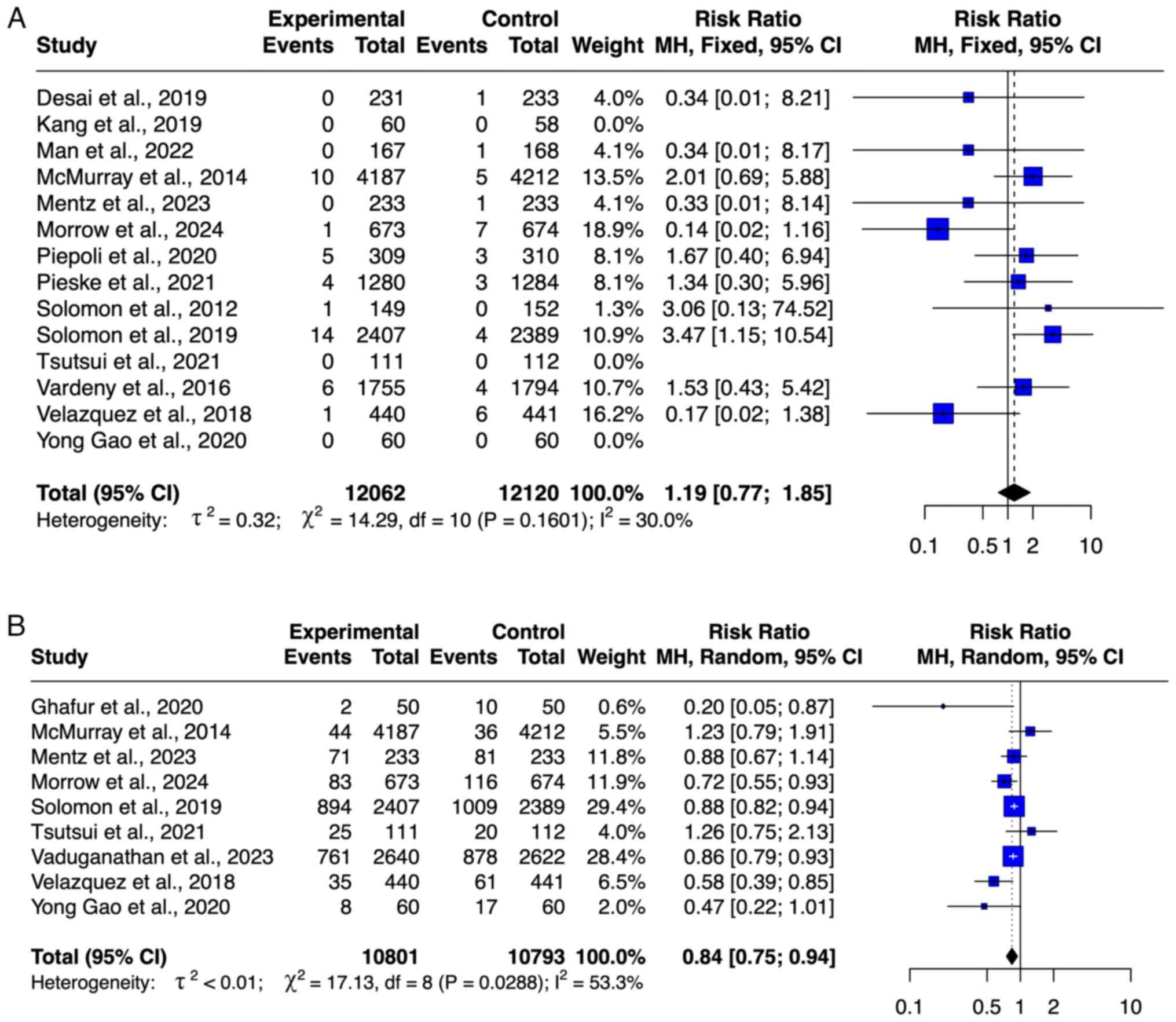
McMurray JJ, Packer M, Desai AS, Gong J,
Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg
K, et al: Angiotensin-neprilysin inhibition versus enalapril in
heart failure. N Engl J Med. 371:993–1004. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Solomon SD, McMurray JJV, Anand IS, Ge J,
Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B,
et al: Angiotensin-neprilysin inhibition in heart failure with
preserved ejection fraction. N Engl J Med. 381:1609–1620.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Duta TF, Zulfa PO, Alina M, Henira N,
Tsurayya G, Fakri F and Acharya Y: Efficacy of acetazolamide and
loop diuretics combinatorial therapy in congestive heart failure: A
meta-analysis. Narra X. 2(e124)2024.
|
|
11
|
Villaschi A, Pellegrino M, Condorelli G
and Chiarito M: Diuretic combination therapy in acute heart
failure: An updated review. Curr Pharm Des. 30:2597–2605.
2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gogikar A, Nanda A, Janga LSN, Sambe HG,
Yasir M, Man RK and Mohammed L: Combination diuretic therapy with
thiazides: A systematic review on the beneficial approach to
overcome refractory fluid overload in heart failure. Cureus.
15(e44624)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park DY, An S, Attanasio S, Jolly N,
Malhotra S, Doukky R, Samsky MD, Sen S, Ahmad T, Nanna MG and Vij
A: Network meta-analysis comparing angiotensin receptor-neprilysin
inhibitors, angiotensin receptor blockers, and
angiotensin-converting enzyme inhibitors in heart failure with
reduced ejection fraction. Am J Cardiol. 187:84–92. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang H, Huang T, Shen W, Xu X, Yang P,
Zhu D, Fang H, Wan H, Wu T, Wu Y and Wu Q: Efficacy and safety of
sacubitril-valsartan in heart failure: A meta-analysis of
randomized controlled trials. ESC Hear Fail. 7:3841–3850.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
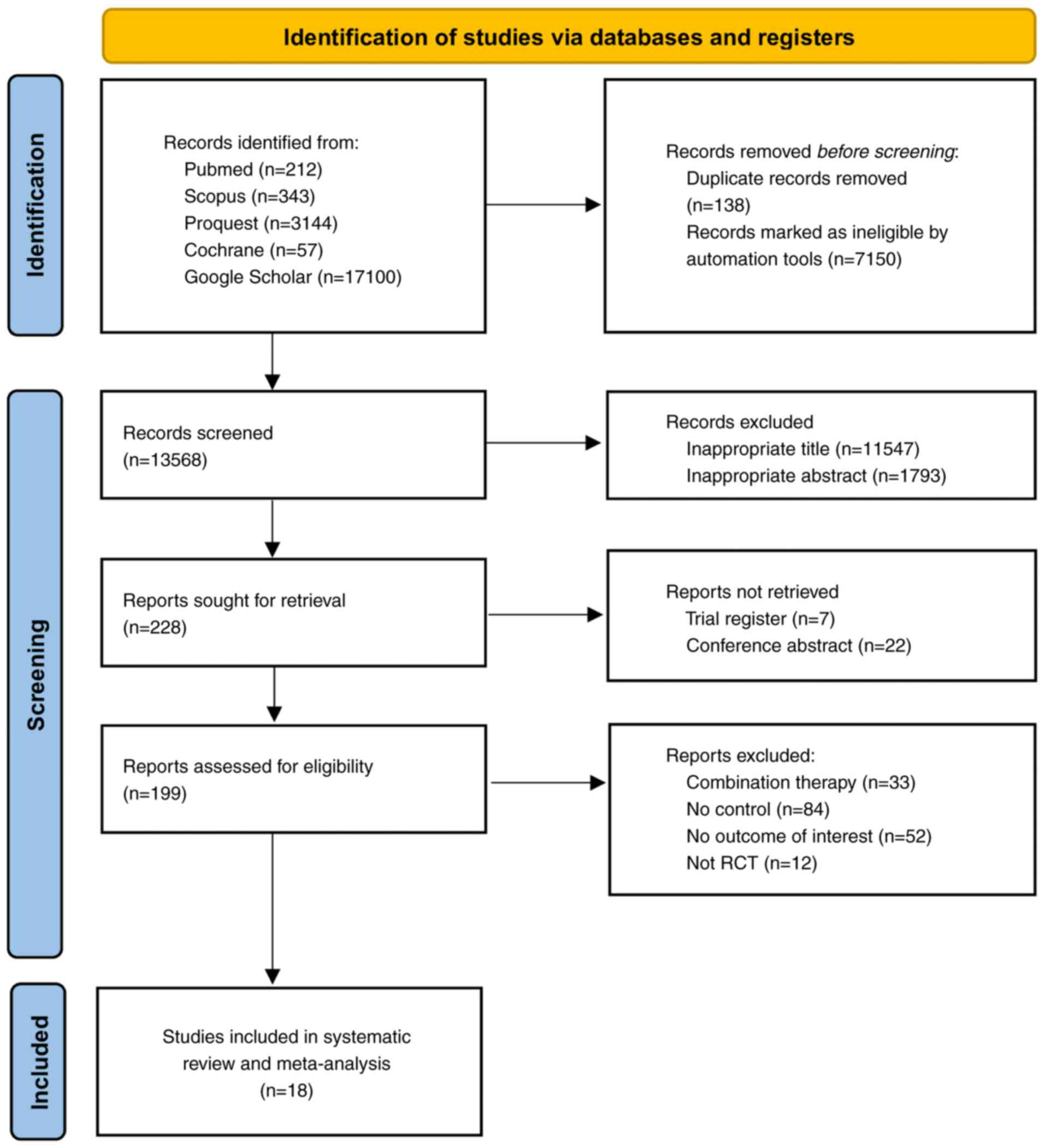
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
The National Cancer Institute (NCI) of the
National Institute of Health: Common terminology criteria for
adverse events (CTCAE) version 5.0. US Dep Heal Hum Serv, 2017.
|
|
17
|
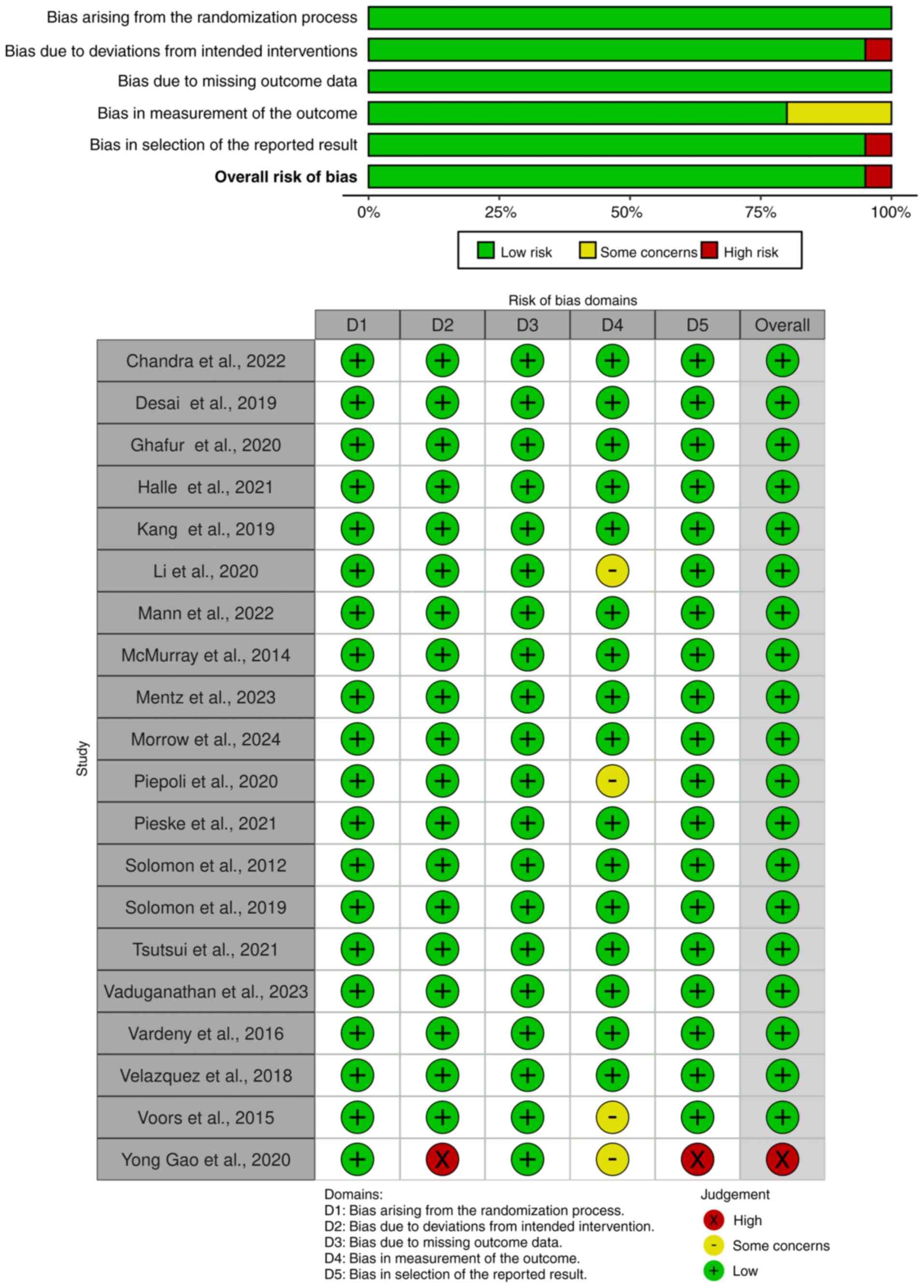
Jørgensen L, Paludan-Müller AS, Laursen
DR, Savović J, Boutron I, Sterne JA, Higgins JP and Hróbjartsson A:
Evaluation of the Cochrane tool for assessing risk of bias in
randomized clinical trials: overview of published comments and
analysis of user practice in Cochrane and non-Cochrane reviews.
Syst Rev. 5(80)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vardeny O, Miller R and Solomon SD:
Combined neprilysin and renin-angiotensin system inhibition for the
treatment of heart failure. JACC Heart Fail. 2:663–670.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chandra A, Polanczyk CA, Claggett BL,
Vaduganathan M, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC,
Schwende H, et al: Health-related quality of life outcomes in
PARAGON-HF. Eur J Heart Fail. 24:2264–2274. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Desai AS, Solomon SD, Shah AM, Claggett
BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R and Mitchell GF:
EVALUATE-HF Investigators. Effect of sacubitril-valsartan vs
enalapril on aortic stiffness in patients with heart failure and
reduced ejection fraction: A Randomized clinical trial. JAMA.
322:1077–1084. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao Y, Xing C, Hao W, Zhao H, Wang L, Luan
B and Hou A: The impact of sacrubitril/valsartan on clinical
treatment and hs-cTnT and NT-ProBNP serum levels and the left
ventricular function in patients with chronic heart failure. Int
Heart J. 61:1–6. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ghafur S, Zahid M, Sarkar H, Barman R,
Al-Mahmud A, Rahman M and Islam H: Effect of angiotensin
receptor-neprilysin inhibitor versus valsartan on cardiac status in
patients with chronic heart failure with reduced ejection fraction:
A Randomized clinical trial in Rangpur Medical College Hospital,
Bangladesh. Open J Intern Med. 10:21–34. 2020.
|
|
24
|
Halle M, Schöbel C, Winzer EB, Bernhardt
P, Mueller S, Sieder C and Lecker LSM: A randomized clinical trial
on the short-term effects of 12-week sacubitril/valsartan vs.
enalapril on peak oxygen consumption in patients with heart failure
with reduced ejection fraction: Results from the ACTIVITY-HF study.
Eur J Heart Fail. 23:2073–2082. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kang DH, Park SJ, Shin SH, Hong GR, Lee S,
Kim MS, Yun SC, Song JM, Park SW and Kim JJ: Angiotensin receptor
neprilysin inhibitor for functional mitral regurgitation.
Circulation. 139:1354–1365. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li BH, Fang KF, Lin PH, Zhang YH, Huang YX
and Jie H: Effect of sacubitril valsartan on cardiac function and
endothelial function in patients with chronic heart failure with
reduced ejection fraction. Clin Hemorheol Microcirc. 77:425–433.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mann DL, Givertz MM, Vader JM, Starling
RC, Shah P, McNulty SE, Anstrom KJ, Margulies KB, Kiernan MS, Mahr
C, et al: Effect of treatment with sacubitril/valsartan in patients
with advanced heart failure and reduced ejection fraction: A
Randomized clinical trial. JAMA Cardiol. 7:17–25. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mentz RJ, Ward JH, Hernandez AF, Lepage S,
Morrow DA, Sarwat S, Sharma K, Starling RC, Velazquez EJ,
Williamson KM, et al: Angiotensin-neprilysin inhibition in patients
with mildly reduced or preserved ejection fraction and worsening
heart failure. J Am Coll Cardiol. 82:1–12. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Piepoli MF, Hussain RI, Comin-Colet J,
Dosantos R, Ferber P, Jaarsma T and Edelmann F: OUTSTEP-HF:
Randomised controlled trial comparing short-term effects of
sacubitril/valsartan versus enalapril on daily physical activity in
patients with chronic heart failure with reduced ejection fraction.
Eur J Heart Fail. 23:127–135. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pieske B, Wachter R, Shah SJ, Baldridge A,
Szeczoedy P, Ibram G, Shi V, Zhao Z and Cowie MR: PARALLAX
Investigators and Committee members. Effect of sacubitril/valsartan
vs standard medical therapies on plasma NT-proBNP concentration and
submaximal exercise capacity in patients with heart failure and
preserved ejection fraction: The PARALLAX Randomized clinical
trial. JAMA. 326:1919–1929. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Solomon SD, Zile M, Pieske B, Voors A,
Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J,
et al: The angiotensin receptor neprilysin inhibitor LCZ696 in
heart failure with preserved ejection fraction: A phase 2
double-blind randomised controlled trial. Lancet. 380:1387–1395.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tsutsui H, Momomura SI, Saito Y, Ito H,
Yamamoto K, Sakata Y, Desai AS, Ohishi T, Iimori T, Kitamura T, et
al: Efficacy and safety of sacubitril/valsartan in japanese
patients with chronic heart failure and reduced ejection
fraction-results from the PARALLEL-HF study. Circ J. 85:584–594.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Velazquez EJ, Morrow DA, DeVore AD, Duffy
CI, Ambrosy AP, McCague K, Rocha R and Braunwald E: PIONEER-HF
Investigators. Angiotensin-neprilysin inhibition in acute
decompensated heart failure. N Engl J Med. 380:539–548.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Voors AA, Gori M, Liu LC, Claggett B, Zile
MR, Pieske B, McMurray JJ, Packer M, Shi V, Lefkowitz MP, et al:
Renal effects of the angiotensin receptor neprilysin inhibitor
LCZ696 in patients with heart failure and preserved ejection
fraction. Eur J Heart Fail. 17:510–517. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nasrallah D, Abdelhamid A, Tluli O,
Al-Haneedi Y, Dakik H and Eid AH: Angiotensin receptor
blocker-neprilysin inhibitor for heart failure with reduced
ejection fraction. Pharmacol Res. 204(107210)2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nielsen EE, Feinberg JB, Bu FL, Hecht
Olsen M, Raymond I, Steensgaard-Hansen F and Jakobsen JC:
Beneficial and harmful effects of sacubitril/valsartan in patients
with heart failure: A systematic review of randomised clinical
trials with meta-analysis and trial sequential analysis. Open Hear.
7(e001294)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hishida E and Nagata D: Angiotensin
receptor-neprilysin inhibitor for chronic kidney disease:
Strategies for renal protection. Kidney Blood Press Res.
49:916–932. 2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang Y, Zhou R, Lu C, Chen Q, Xu T and Li
D: Effects of the angiotensin-receptor neprilysin inhibitor on
cardiac reverse remodeling: Meta-analysis. J Am Heart Assoc.
8(e012272)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tromp J, Ouwerkerk W, van Veldhuisen DJ,
Hillege HL, Richards AM, van der Meer P, Anand IS, Lam CSP and
Voors AA: A systematic review and network meta-analysis of
pharmacological treatment of heart failure with reduced ejection
fraction. JACC Heart Fail. 10:73–84. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
van Essen BJ, Ceelen DCH, Ouwerkerk W,
Teng TK, Tharshana GN, Hew FM, Butler J, Zannad F, Lam CS,
Ezekowitz J, et al: Pharmacologic treatment of heart failure with
reduced ejection fraction: An updated systematic review and network
meta-analysis. J Am Coll Cardiol: Aug 30, 2025 (Epub ahead of
print).
|
|
41
|
Khan NA, Ma I, Thompson CR, Humphries K,
Salem DN, Sarnak MJ and Levin A: Kidney function and mortality
among patients with left ventricular systolic dysfunction. J Am Soc
Nephrol. 17:244–253. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Banerjee D, Winocour P, Chowdhury TA, De
P, Wahba M, Montero R, Fogarty D, Frankel AH, Karalliedde J, Mark
PB, et al: Management of hypertension and
renin-angiotensin-aldosterone system blockade in adults with
diabetic kidney disease: Association of British clinical
diabetologists and the renal association UK guideline update 2021.
BMC Nephrol. 23(9)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Murphy E: Estrogen signaling and
cardiovascular disease. Circ Res. 109:687–696. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rudolf H, Mügge A, Trampisch HJ, Scharnagl
H, März W and Kara K: NT-proBNP for risk prediction of
cardiovascular events and all-cause mortality: The getABI-study.
Int J Cardiol Hear Vasc. 29(100553)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Greenberg B: Angiotensin
receptor-neprilysin inhibition (ARNI) in heart failure. Int J Hear
Fail. 2:73–90. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bayes-Genis A, Barallat J and Richards AM:
A test in context: Neprilysin: Function, inhibition, and biomarker.
J Am Coll Cardiol. 68:639–653. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sauer AJ, Cole R, Jensen BC, Pal J, Sharma
N, Yehya A and Vader J: Practical guidance on the use of
sacubitril/valsartan for heart failure. Heart Fail Rev. 24:167–176.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chandra A, Lewis EF, Claggett BL, Desai
AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz
MP, et al: Effects of sacubitril/valsartan on physical and social
activity limitations in patients with heart failure: A secondary
analysis of the PARADIGM-HF trial. JAMA Cardiol. 3:498–505.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Park SK, Hong SH, Kim H, Kim S and Lee EK:
Cost-Utility analysis of sacubitril/valsartan use compared with
standard care in chronic heart failure patients with reduced
ejection fraction in South Korea. Clin Ther. 41:1066–1079.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gan L, Lyu X, Yang X, Zhao Z, Tang Y, Chen
Y, Yao Y, Hong F, Xu Z, Chen J, et al: Application of angiotensin
receptor-neprilysin inhibitor in chronic kidney disease patients:
Chinese expert consensus. Front Med (Lausanne).
9(877237)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Nagata D, Hishida E and Masuda T:
Practical strategy for treating chronic kidney disease
(CKD)-Associated with hypertension. Int J Nephrol Renovasc Dis.
13:171–178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nakano Y, Suzuki Y, Onishi T, Ando H,
Matsuo Y, Suzuki W, Kuno S, Ohashi H, Waseda K, Takahashi H, et al:
Predictors of hypotension after angiotensin receptor-neprilysin
inhibitor administration in patients with heart failure. Int Heart
J. 65:658–666. 2024.PubMed/NCBI View Article : Google Scholar
|