Introduction
Heart failure (HF), a progressive clinical syndrome,
is characterized by impaired cardiac output and/or elevated
intracardiac pressure, resulting in systemic hypoperfusion and
congestion (1). It encompasses a
broad spectrum of hemodynamic and structural abnormalities,
frequently associated with the dysregulation of neurohormonal
pathways, particularly the renin-angiotensin-aldosterone system
(RAAS) and the sympathetic nervous system (2). The incidence of HF is increasing,
causing an escalating burden on healthcare systems (3). In a population-based study from Spain
in 2012, 30.8% of patients with heart failure were hospitalized
within one year of follow-up, and the all-cause mortality rate
during that period was 14.3% (4).
In the United States, it is one of the most costly medical
disorders, with a total annual cost of $30.7 billion (5).
The classification of HF based on ejection fraction
(EF) has advanced to support more precise therapeutic decisions. HF
is classified on the basis of the left ventricular EF (LVEF) into
reduced (HFrEF, ≤40%), mildly reduced (HFmrEF, 41-49%), preserved
(HFpEF, ≥50%), and improved ejection fraction (HFimpEF), defined as
a baseline LVEF of <40% with an increase of ≥10 percentage
points to >40% on follow-up (6).
This classification based on LVEF, is currently endorsed by the
2021 European Society of Cardiology (ESC) Guidelines on Heart
Failure. This is a positive change compared with the 2016 ESC
Guidelines, which reassigned patients with an exact LVEF of 40% to
HFrEF rather than HFmrEF, and improves alignment between management
strategies and clinical trial evidence (7).
The revised classification has informed contemporary
treatment guidelines, particularly regarding the use of angiotensin
receptor-neprilysin inhibitors (ARNIs). The 2022 the American Heart
Association/American College of Cardiology/Heart Failure Society of
America (HFSA) Guideline for the Management of HF recommend
replacing angiotensin-converting enzyme inhibitors (ACE-Is) or
angiotensin receptor blockers (ARBs) with ARNIs, including
sacubitril/valsartan, in patients with symptomatic HFrEF (New York
Heart Association classes II-III) and initiating ARNI therapy
before discharge in patients hospitalized with acute HF.
Furthermore, to decrease the risk of hospitalization, ARNIs may be
considered in select patients with HFmrEF or HFpEF (1). Previous landmark trials, including
PARADIGM-HF and PARAGON-HF, have demonstrated the superiority of
ARNIs by achieving a substantial decrease in
cardiovascular-associated mortality, HF-related hospitalization and
complication rates compared with the use of either ACE-Is or ARBs
alone (8,9).
In parallel with advancements in neurohormonal
modulation therapies such as ARNI, diuretic strategies remain a key
component in the symptomatic management of congestive HF,
particularly in patients presenting with volume overload. Recently,
interest has grown in combinatorial diuretic regimens to overcome
diuretic resistance and improve decongestive outcomes (10-12).
A meta-analysis by Duta et al (10) demonstrated that the combination of
acetazolamide with loop diuretics significantly enhances
natriuresis and fluid removal compared with loop diuretics alone.
This approach targets both proximal and distal renal tubular sites,
optimizing diuretic response and offering promising adjunctive
benefits in acute and chronic HF settings (10). Incorporating evolving strategies
into the broader HF treatment paradigm reflects the growing
recognition of the need for individualized, phenotype-based
interventions beyond guideline-directed medical therapy.
Although prior meta-analyses have demonstrated the
clinical benefits of ARNIs, particularly in decreasing mortality,
hospitalization and improving functional outcomes, these have
primarily focused on patients with rEF, with limited exploration of
its effects across the full spectrum of HF phenotypes (13,14).
The generalizability is further limited by the heterogeneity in
study populations, outcome definitions and reporting standards.
Therefore, the present systematic review and meta-analysis aimed to
evaluate the efficacy, safety and BP-associated outcomes of ARNIs
across populations with HFrEF, HFmrEF and HFpEF while accounting
for key clinical modifiers, including age, sex, anthropometric
profile, laboratory parameters, hemodynamic factors and cardiac and
renal function. By integrating a large body of evidence and
applying meta-regression to key hemodynamic and renal parameters,
the present study aimed to offer clinically relevant insights to
inform more individualized and hypertension-conscious HF
management.
Materials and methods
Study design and protocol
registration
The present systematic review and meta-analysis
followed the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) 2020 guidelines (15). The study protocol was registered in
the International Prospective Register of Systematic Reviews with
registration no. CRD42024569374 (crd.york.ac.uk/PROSPERO/view/CRD42024569374).
Search strategies
A systematic search of the relevant studies was
conducted across five databases, including PubMed (pubmed.ncbi.nlm.nih.gov/), Scopus (https://www.scopus.com/), Cochrane (https://www.cochranelibrary.com/), ProQuest
(https://www.proquest.com/) and Google
Scholar (https://scholar.google.com/).
Databases were searched from inception to July 7, 2025; data
extraction and all derived estimations were completed on July 29,
2025, after which no additional updates were incorporated. The
following main keywords, combined with Boolean operators ‘AND’ and
‘OR’, were initially established: (‘Angiotensin Receptor Neprilysin
Inhibitor’ OR ‘ARNI’ OR ‘Sacubitril/Valsartan’ OR ‘LCZ696’ OR
‘Entresto’) AND (‘Heart Failure’ OR ‘Congestive Heart Failure’ OR
‘CHF’ OR ‘Cardiac Insufficiency’ OR ‘Left Ventricular Dysfunction’)
AND (‘Reduced Ejection Fraction’ OR ‘Reduced EF’ OR ‘HfrEF’ OR
‘Heart Failure with Reduced Ejection Fraction’ OR ‘Systolic Heart
Failure’) AND (‘Preserved Ejection Fraction’ OR ‘Preserved EF’ OR
‘HfpEF’ OR ‘Heart Failure with Preserved Ejection Fraction’ OR
‘Diastolic Heart Failure’). No publication date and language
restrictions were set.
Selection of studies
The search results from each database were collected
and managed using Google Sheets (Google LLC). After removing
duplicates, the remaining articles were screened based on title and
abstract. Full-text studies that were available and published were
assessed according to the pre-specified eligibility criteria by
four investigators. Any disagreements were resolved through a group
discussion.
Exclusion criteria were as follows: i) Wrong study
design (single-arm or non-comparative reports); ii) wrong
population (not chronic HF); iii) wrong comparator (not ACE-I/ARB
or guideline-directed therapy); iv) wrong outcomes (no extractable
data for prespecified endpoints); and v) overlapping populations
(for multiple publications from the same trial, the most complete
primary report was retained). When outcome data were unavailable,
author contact was attempted; studies with unresolved missing data
were excluded from quantitative synthesis.
Eligibility criteria
Population, Intervention, Comparison, Outcome (PICO)
framework (Table SI) designed for
systematic reviews was used to establish the eligibility criteria
as follows: i) Study population consisted of patient with various
stages of EF HF (HFmEF, HFpEF, HFrEF); ii) used ARNI as
intervention; iii) used ACE-I/ARB as control therapy; iv) evaluated
efficacy [all-cause and cardiovascular-related mortality, major
adverse cardiac events (MACE) and HF hospitalization] and safety
(hypotension, hyperkalemia, angioedema and renal impairment); and
v) randomized control trial design. Exclusion criteria were as
follows: i) Title or abstract was irrelevant; ii) full-text was
irretrievable and iii) the study was a review article, case report,
case series or conference abstract. Titles or abstracts were
considered irrelevant if they did not pertain to the predefined
PICO framework.
Data extraction
A total of two investigators extracted data from
each included study. The data extracted included the first author
and year of publication, study location (country), HF type, age,
sample size, sex (% of males), type of intervention and control
(drug administration and dosing regimens), follow-up duration
(months), efficacy (all-cause and cardiovascular-related mortality,
MACEs and HF-related hospitalization), and safety (hypotension,
hyperkalemia, angioedema and renal impairment). Adverse events
(AEs) were classified using the Common Terminology Criteria for AEs
(CTCAE) developed by developed by the US National Cancer Institute
of the National Institute of Health, with severity graded from 1
(mild) to 5 (death) (16).
Quality assessment of individual
studies
To evaluate the risk of bias of each eligible study,
two investigators independently conducted a methodological quality
assessment using the Cochrane Collaboration's Risk of Bias 2 (RoB
2) tool (17). The RoB 2 is a
revised tool comprising five bias domains designed to consider the
risk of bias of randomized trials arising from the randomization
process, deviations from intended interventions, missing outcome
data, outcome measurement and selecting the reported results. The
risk of bias on each domain was rated as low or high risk, or some
concerns (unclear). A study was considered to have a low risk of
bias when all domains exhibited low risk. A study was judged to
have some concerns when at least one domain was rated unclear. A
study was considered to be at a high risk of bias when at least one
domain presented a high risk or some concerns in multiple domains
that may lower the confidence in the results.
Statistical analysis
The present study performed a meta-analysis with
fixed- or DerSimonian-Laird random effect to compute the risk ratio
(RR) for all dichotomous outcomes using Review Manager version 5.4
(The Cochrane Collaboration), STATA version 16.0 (StataCorp LLC)
and meta package in RStudio version 4.4.1 (Posit PBC). The data are
presented as RR and 95% confidence interval. A random effect was
used when heterogeneity was detected (I2>50%).
Heterogeneity was assessed using Higgins' I² statistic. To
interpret the degree of heterogeneity, I² values of 0, 1-24, 25-49,
50-74 and ≥75% were considered to indicate no, very low, low,
moderate and high heterogeneity, respectively (18). P<0.05 was considered to indicate
a statistically significant difference.
Subgroup analyses and meta-regression were performed
to explore heterogeneity. Subgroups were prespecified by LVEF ≤40%
vs. >40%, with pooled effects estimated within strata and
between-group differences assessed using an interaction P-value.
Meta-regression employed the DerSimonian-Laird random-effects
approach to evaluate continuous study-level covariates, including
mean age, proportion of female patients, N-terminal pro-B-type
natriuretic peptide (NT-proBNP), LVEF, heart rate, systolic (S)BP,
body mass index (BMI) and renal impairment as estimated by
glomerular filtration rate (eGFR). Where the same construct
appeared in both frameworks, it was modeled categorically in
subgroup analyses and continuously in meta-regression.
Funnel plots were constructed for each outcome. For
outcomes with ≥10 studies, Egger's regression test (primary) and
Begg's rank-correlation test (sensitivity) were performed, using
α=0.10 (two-sided) to flag potential asymmetry; outcomes with
<10 studies were not tested.
Results
Study selection and
identification
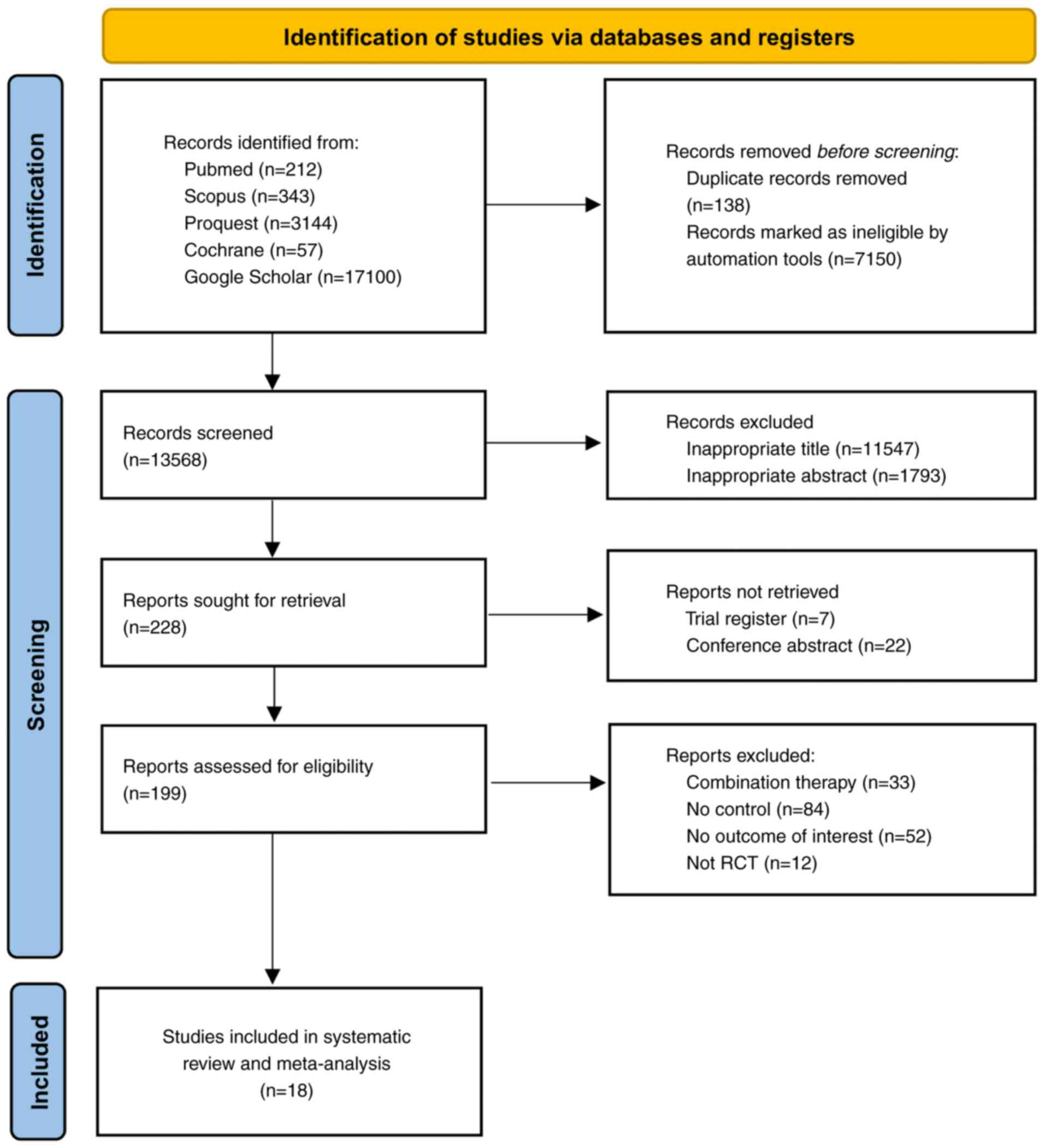
The initial database search yielded 20,856 studies.
Before screening, 7,288 articles were removed, including 138
duplicates and 7,150 identified as ineligible. Following the title
and abstract screening, 13,340 articles were excluded. A total of
228 articles were assessed for retrieval, with 29 removed. The
remaining 199 articles underwent eligibility assessment, leading to
the inclusion of 18 studies for quantitative and qualitative
analyses (8,9,19-34).
The PRISMA flowchart detailing the study selection process is
depicted in Fig. 1.
Characteristics of the included
studies
A total of 18 RCTs were eligible for inclusion in
the analysis, involving 28,001 HF patients from nine studies
conducted in the United States (9,19-21,27,28,31,33,34),
one each from the United Kingdom (8), Canada (28), Italy (29), South Korea (25), Japan (32) and Bangladesh (23) and two each from Germany (24,30)
and China (22,26) (Table
I). The mean patient age was 59.4-72.8 years, with most
participants being male (64.18%). The patient population comprised
individuals with three types of HF, categorized by LVEF: Preserved
(six studies) (9,20,28,30,31,34),
mildly reduced (one study) (30)
and reduced (12 studies) (8,19,21-27,27,32,33),.
A total of 13,876 patients received ARNI intervention, with doses
of 50, 100 and 200 mg, taken twice daily. Conversely, 14,125
patients received control treatments, consisting of ARB in 10
studies (6,704 patients) (9,20,22,23,26-28,30,31,33)
and ACE-I in eight studies (7,204 patients) (8,19,21,24,26,29,32,33).
The follow-up time ranged from 2 to 36 months. The most frequently
reported CTCAE-graded AEs were hypotension, worsening renal
function, hyperkalemia, angioedema and acute kidney injury.
 | Table ICharacteristics of studies. |
Table I
Characteristics of studies.
| | Control | Intervention | |
|---|
| First author,
year | Country | Number of
patients | Male patients
(%) | Mean age,
years | Type of HF | Type | n | Type | n | Follow-up duration,
months | CTCAE (grade) | (Refs.) |
|---|
| McMurray et
al, 2014 | United Kingdom | 8,399 | 6,567 (78.2) | 63.80±11.40 | HFrEF |
Sacubitril/valsartan, 200 mg twice
daily | 4,187 | Enalapril, 10 mg
twice daily | 4,212 | 4 | Hyperkalemia
(G1-2), worsening renal function (G2-3), symptomatic hypotension
(G2-3), angioedema (G2-3), hospitalization (G3-4),
cardiovascular-associated mortality (G5) | (8) |
| Solomon et
al, 2019 | United States of
America | 4,796 | 2,317 (48.3) | 72.75±8.40 | HFpEF |
Sacubitril/valsartan, 97/103 mg twice
daily | 2,407 | Valsartan, 160 mg
twice daily | 2,389 | 35 | Hypotension (G2-3),
worsening renal function (G2-3), hyperkalemia (G1-2), angioedema
(G3), cough (G1-2), dizziness (G1-2), acute kidney injury (G2-3),
elevated creatinine (G2-3), syncope (G2-3) | (9) |
| Vardeny et
al, 2016 | United States of
America | 3,549 | 2,785 (78.5) | 63.85±11.48 | HFrEF |
Sacubitril/valsartan, 200 mg twice
daily | 1,755 | Enalapril, 10 mg
twice daily | 1,794 | 27 | Hyperkalemia
(G1-2), hypotension (G2-3), worsening renal function (G2-3), acute
kidney injury (G2-3), angioedema (G3), cough (G1-2), dizziness
(G1-2) | (19) |
| Chandra et
al, 2022 | United States of
America | 4,476 | 2,192 (48.9) | 72.60±8.50 | HFpEF |
Sacubitril/valsartan, 97/103 mg twice
daily | 2,226 | Valsartan, 160 mg
twice daily | 2,250 | 36 | NA | (20) |
| Desai et al,
2019 | United States of
America | 464 | 355 (76.5) | 71.70±10.30 | HFrEF |
Sacubitril/valsartan, 97/103 mg twice
daily | 231 | Enalapril, 10 mg
twice daily | 233 | 3 | Hypotension (G2-3);
no significant CTCAE-graded differences in renal dysfunction
(G2-3), hyperkalemia (G1-2), or angioedema (G3) | (21) |
| Gao et al,
2020 | China | 120 | 88 (73.3) | 70.30±7.30 | HFrEF |
Sacubitril/valsartan, 50 mg twice
daily | 60 | Valsartan, 80 mg
once daily | 60 | 18 | Hyperkalemia
(G1-2), worsening renal function (G2-3), acute kidney injury
(G2-3), hypotension (G2-3), all-cause morality (G5),
cardiovascular-associated mortality (G5), discontinuation due to
AEs (G3) | (22) |
| Ghafur et
al, 2020 | Bangladesh | 100 | 67(67) | 61.40±11.90 | HFrEF |
Sacubitril/valsartan, 100 mg twice
daily | 50 | Valsartan, 80 mg
twice daily | 50 | 4 |
Cardiovascular-associated mortality (G5),
hospitalization due to HF (G3), elevated serum creatinine (G2-3),
hyperkalemia (G1-2), hyperglycemia (G1-2) | (23) |
| Halle et al,
2021 | Germany | 201 | 163 (81.1) | 66.90±10.40 | HFrEF |
Sacubitril/valsartan, 97/103 mg twice
daily | 103 | Enalapril, 10 mg
twice daily | 98 | 3 | Hyperkalemia
(G1-2), worsening renal function (G2-3), hypotension (G2-3),
angioedema (G3), acute kidney injury (G2-3) | (24) |
| Kang et al,
2019 | South Korea | 118 | 72(61) | 62.60±11.20 | HFrEF |
Sacubitril/valsartan, 97/103 mg twice
daily | 60 | Valsartan, 80 mg
once daily | 58 | 6 | Hyperkalemia
(G1-2), hypotension (G2-3), worsening renal function (G2-3),
dizziness (G1-2), cough (G1-2) | (25) |
| Li et al,
2020 | China | 80 | 47 (58.8) | 63.00±5.70 | HFrEF |
Sacubitril/valsartan, 200 mg twice
daily | 40 | Perindopril
tert-butylamine, 4 mg once daily | 40 | 3 | NA | (26) |
| Mann et al,
2022 | United States of
America | 335 | 245 (73.1) | 59.40±13.50 | HFrEF |
Sacubitril/valsartan, 97/103 mg twice
daily | 167 | Valsartan, 160 mg
twice daily | 168 | 6 | Hyperkalemia
(G1-2), worsening renal function (G2-3), hypotension (G2-3),
hospitalization (G3-4), cardiovascular-associated mortality
(G5) | (27) |
| Mentz et al,
2023 | United States of
America and Canada | 466 | 224 (48.1) | 71.40±3.10 | HFpEF |
Sacubitril/valsartan, 97/103 mg twice
daily | 233 | Valsartan, 160 mg
twice daily | 233 | 2 | Hyperkalemia
(G1-2), hypotension (G2-3), worsening renal function (G2-3), acute
kidney injury (G2-3), dizziness (G1-2), cough (G1-2), fatigue
(G1-2) | (28) |
| Piepoli et
al, 2020 | Italy | 619 | 487 (78.7) | 66.90±10.70 | HFrEF |
Sacubitril/valsartan, 97/103 mg twice
daily | 309 | Enalapril, 10 mg
twice daily | 310 | 3 | Hyperkalemia
(G1-2), hypotension (G2-3), worsening renal function (G2-3), cough
(G1-2), dizziness (G1-2) | (29) |
| Pieske et
al, 2021 | Germany | 2,572 | 1,271 (49.4) | 72.60±8.50 | HFpEF, HFmrEF |
Sacubitril/valsartan, 97/103 mg twice
daily | 1,288 | Valsartan, 160 mg
twice daily | 1,284 | 6 | Hypotension (G2-3),
albuminuria (G1-2), hyperkalemia (G1-2) | (30) |
| Solomon et
al, 2012 | United States of
America | 301 | 131 (43.5) | 71.00±9.10 | HFpEF |
Sacubitril/valsartan, 200 mg twice
daily | 149 | Valsartan, 160 mg
twice daily | 152 | 26 | Hypotension (G2-3),
albuminuria (G1-2), hyperkalemia (G1-2), hypotension (G2-3),
elevated serum creatinine (G2-3), hyperkalemia (G1-2), angioedema
(G3), liver-related AEs (G1-2). | (31) |
| Tsutsui et
al, 2021 | Japan | 223 | 192 (86.1) | 67.85±10.36 | HFrEF |
Sacubitril/valsartan, 100 mg twice
daily | 111 | Enalapril, 10 mg
twice daily | 112 | 12 | Hypotension (G2-3),
hyperkalemia (G1-2), worsening renal function (G2-3), cough (G1-2),
dizziness (G1-2), angioedema (G3), acute kidney injury (G2-3) | (32) |
| Velazquez et
al, 2018 | United States of
America | 881 | 635 (72.1) | 62.00±3.42 | HFrEF |
Sacubitril/valsartan, 97/103 mg twice
daily | 440 | Enalapril, 5 mg
twice daily | 441 | 2 | Hypotension (G2-3),
hyperkalemia (G1-2), worsening renal function (G2-3), acute kidney
injury (G2-3), angioedema (G3), cough (G1-2), dizziness (G1-2),
elevated creatinine (G2-3) | (33) |
| Voors et al,
2015 | United States of
America | 301 | 131 (43.5) | 70.73±9.01 | HFpEF |
Sacubitril/valsartan, 50 mg twice
daily | 60 | Valsartan, 80 mg
once daily | 60 | 8.3 | Hypotension (G2-3),
hyperkalemia (G1-2), worsening renal function (G2-3), acute kidney
injury (G2-3), cough (G1-2), dizziness (G1-2), angioedema (G3) | (34) |
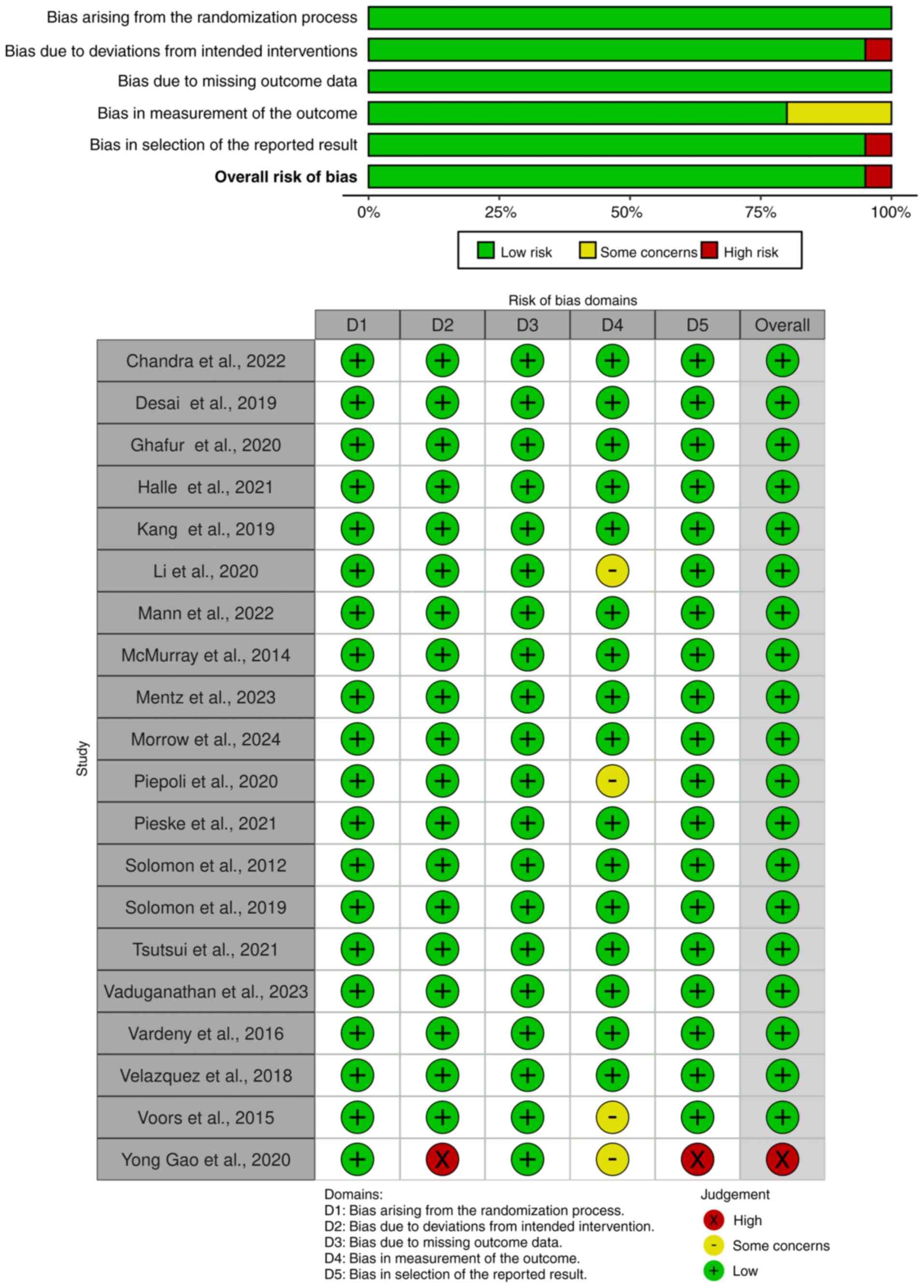
Quality assessment of studies
Studies were assessed for bias using the Cochrane
RoB 2.0 tool (Fig. 2). A total of
two studies (26,34) were rated as having some concerns,
while one study (22) was judged to
have a high risk of bias. Gao et al (22) was rated high risk in the domains of
deviation from intended interventions and selection of the reported
result, primarily due to the absence of blinding procedures and
lack of trial registration. This omission raises concern about
selective outcome reporting, particularly in the context of
exploratory or post hoc analyses. Additionally, outcome measurement
in the aforementioned study was graded as some concerns due to
unblinded assessment of endpoints. Li et al (26) demonstrated some concerns in the
domains of randomization and outcome measurement, as the allocation
concealment process was not clearly reported and there was
insufficient detail regarding blinding of outcome assessors. Voors
et al (34) was also rated
as having some concerns in the domain of missing outcome data, due
to incomplete reporting of renal endpoints from a post hoc
analysis. Despite these exceptions, the overall risk of bias across
trials was low, indicating generally high methodological
quality.
Efficacy of ARNI
The clinical outcomes regarding the efficacy and
safety of ARNI compared with the control group are summarized in
Table II. The analysis of the
included studies highlighted favorable results associated with ARNI
use (Figs. S1 and S2).
 | Table IIEffectiveness and safety of ARNI vs.
control. |
Table II
Effectiveness and safety of ARNI vs.
control.
| A,
Effectiveness |
|---|
| Outcome | ARNI (n/total) | Control
(n/total) | RR (95%CI) | I2
value,% | Favours ARNI | Favours
control | Significant |
|---|
| All-cause
mortality | 1,100/8,630 | 1,226/8,646 | 0.67
(0.83-0.97) | 25.1 | Yes | No | Yes |
|
Cardiovascular-associated mortality | 800/7,165 | 952/7,164 | 0.84
(0.77-0.92) | 41 | Yes | No | Yes |
| HF
hospitalization | 1,079/7,488 | 1,234/7,497 | 0.87
(0.81-0.93) | 60 | Yes | No | Yes |
| MACE | 1,981/8,413 | 2,241/8,471 | 0.89
(0.85-0.94) | 57 | Yes | No | Yes |
| B, Safety |
| Outcome | ARNI (n/total) | Control
(n/total) | RR (95%CI) | I2
value,% | Favours ARNI | Favours
control | Significant |
| Renal
impairment | 719/11,588 | 798/11,648 | 0.91
(0.83-1.00) | 46.4 | Yes | No | No |
| Hyperkalaemia | 796/7,395 | 804/7,422 | 0.99
(0.90-1.09) | 47 | Yes | No | No |
| Angioedema | 41/11,389 | 28/11,446 | 1.44
(0.90-2.29) | 4.9 | No | Yes | No |
| Symptomatic
hypotension | 1,759/11,492 | 1,150/11,544 | 1.54
(1.43-1.65) | 54.2 | No | Yes | Yes |
All-cause mortality
A total of six studies (8,9,27,30,31,33)
with 17,276 patients with HF, including 8,630 patients who received
ARNI and 8,646 patients who received control, were included in the
meta-analysis of all-cause mortality (Fig. 3). ARNI in patients with HF
significantly decreased all-cause mortality (RR=0.67; 95%
CI=0.83-0.97). No outlier was detected based on the funnel plot
(Fig. S1). The level of
heterogeneity was low (I2=25.1%).
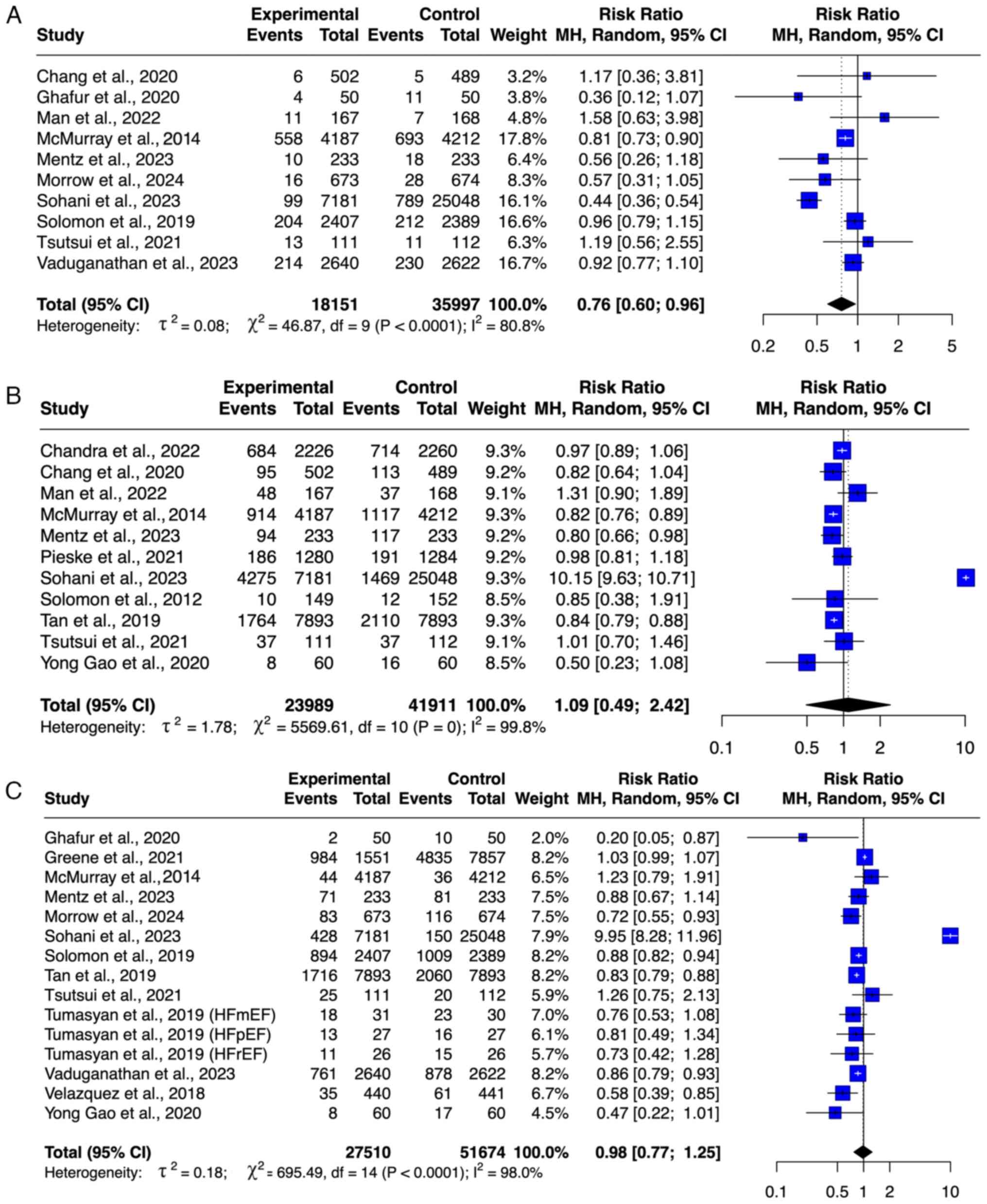
Cardiovascular-associated mortality. A
cumulative total of 14,319 participants, including 7,155
participants treated with ARNI and 7,164 participants in the
control group from six studies (8,9,23,27,28,32),
were included in the meta-analysis of cardiovascular-related
mortality (Fig. 4A). The use of
ARNI for various types of HF was associated with a significantly
lower risk of cardiovascular-associated mortality (RR=0.84; 95%
CI=0.77-0.92). No outlier was detected based on the funnel plot
(Fig. S1). The degree of
heterogeneity was low (I2=41%).
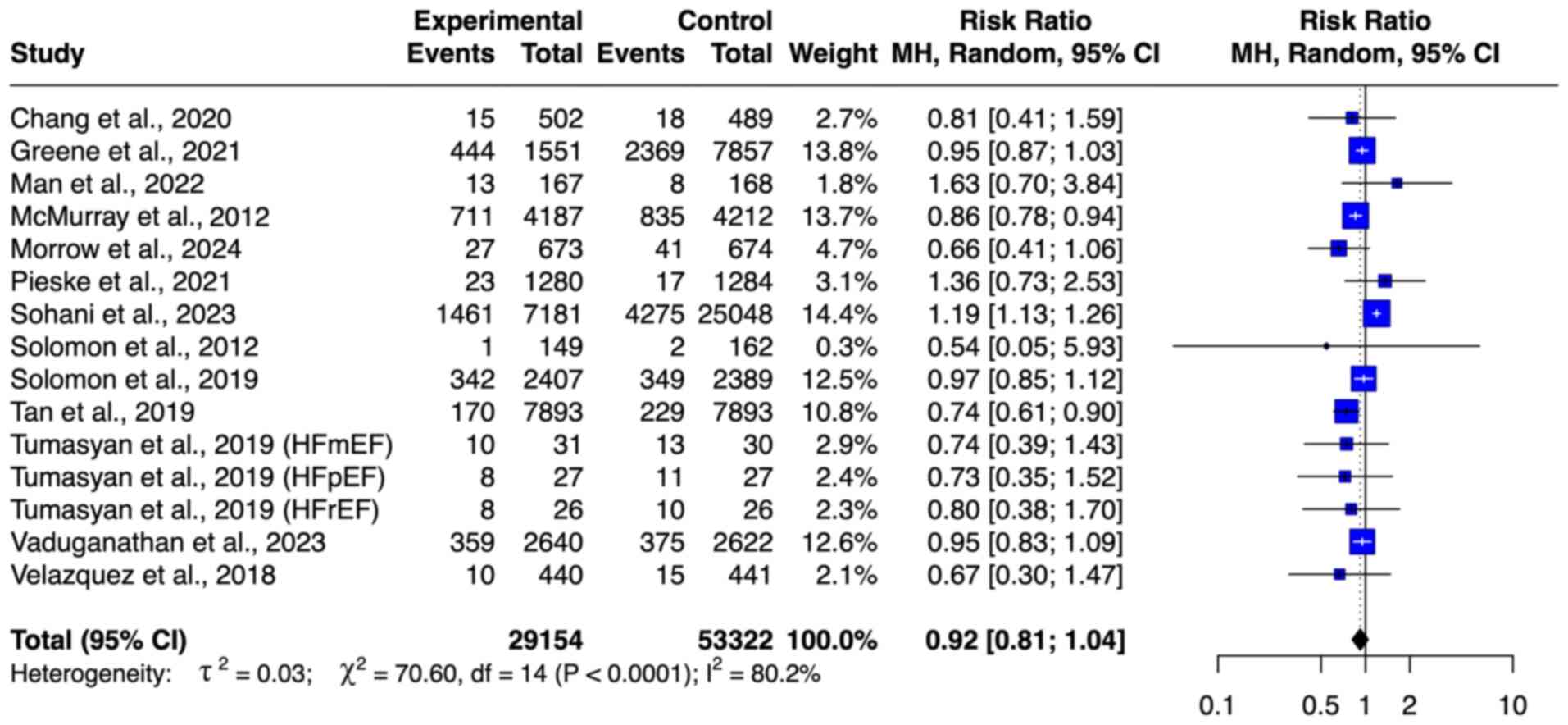
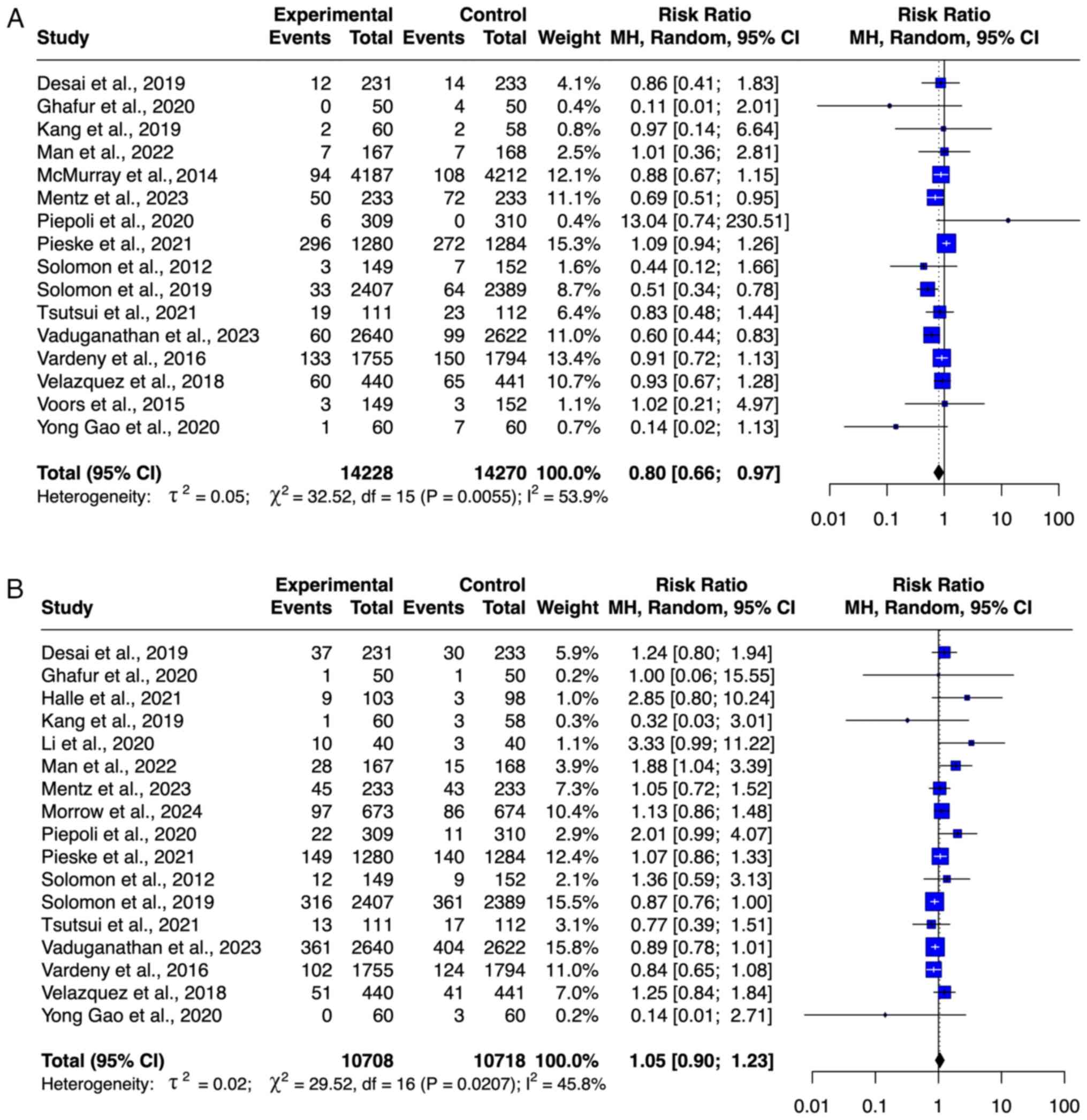
HF-associated hospitalization. A total of
seven studies (8,9,22,23,28,32,33),
with 14,985 patients, including 7,488 patients who received ARNI
and 7,497 patients in the control, were included in the
meta-analysis of HF-related hospitalization (Fig. 4B). ARNI resulted in a significant
decrease in the risk of HF-related hospitalization (RR=0.87; 95%
CI=0.81-0.93). No outlier was detected on the basis of the funnel
plot (Fig. S1). The level of
heterogeneity was moderate (I2=60%).
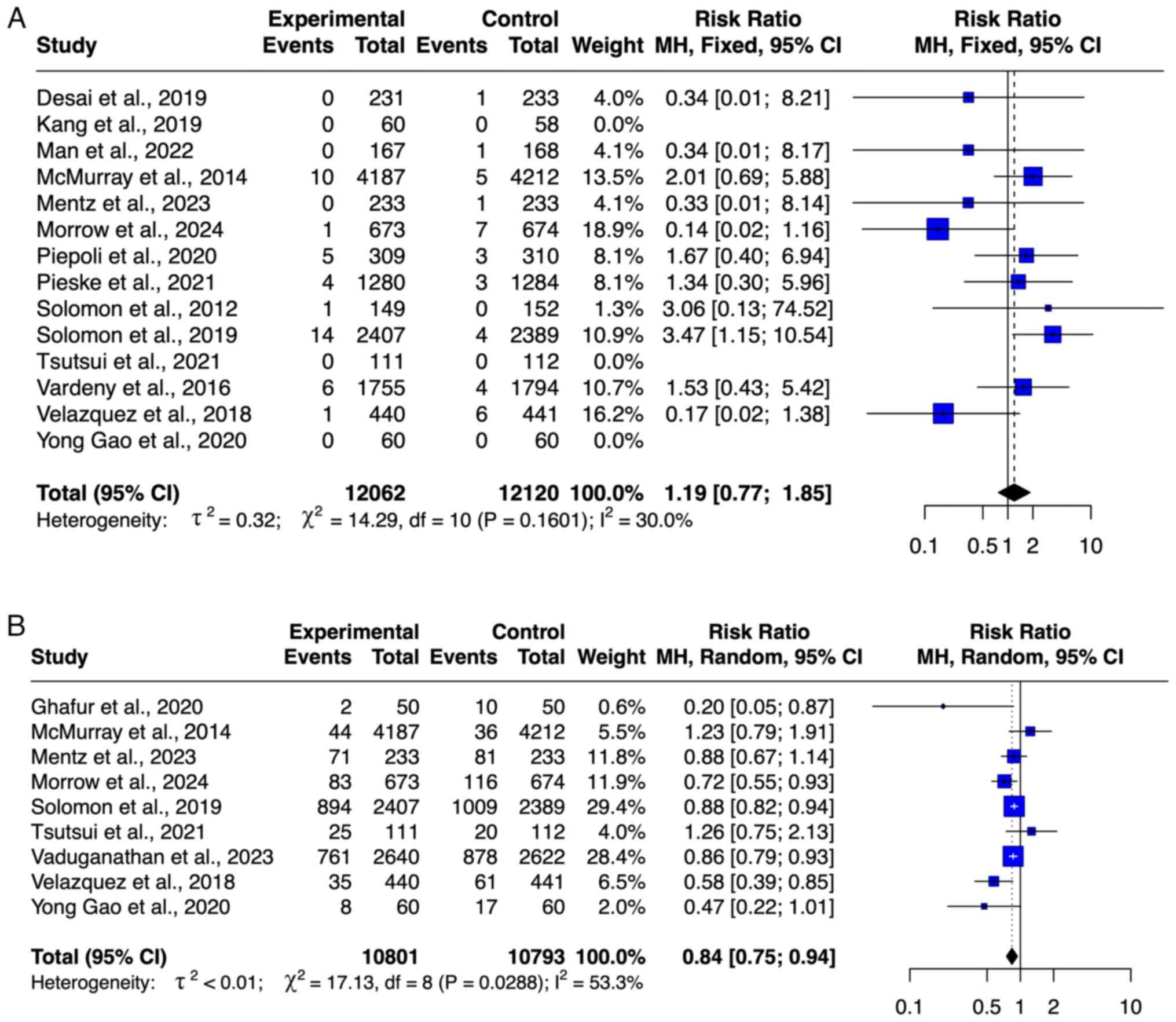
MACEs. A total of eight studies (8,20,22,27,28,30-32)
with 16,884 patients, including 8,413 patients who received ARNI
and 8,471 patients in the control group, were included in the
meta-analysis of MACE (Fig. 4C).
Treatment with ARNI significantly decreased the risk of MACEs in
the pooled analysis (RR=0.89; 95% CI=0.85-0.94). No outlier was
detected on the basis of the funnel plot (Fig. S1). The level of heterogeneity was
moderate (I2=57%).
Safety outcome of ARNI. Renal
impairment
A total of 23,236 participants from 15 studies
(8,9,19,21-23,25,27-34)
were included in the meta-analysis of renal impairment outcomes,
comprising 11,588 participants in the ARNI group and 11,648
participants in the control group (Fig.
5A). ARNI use was associated with decreased risk of renal
impairment (RR=0.91; 95% CI: 0.83-1.00), although this was not
significant. No publication bias was observed based on the funnel
plot, and heterogeneity was low (I²=46.4%).
Hyperkalemia
A total of 14,817 participants, consisting of 7,395
participants treated with ARNI and 7,422 participants in the
control group from 15 studies (9,19,21-33),
were included in the meta-analysis of hyperkalaemia (Fig. 5B). The use of ARNI in HF
interventions showed a lower risk of hyperkalaemia but this was not
significant (RR=0.99; 95% CI=0.90-1.09). No outlier was detected
based on the funnel plot (Fig.
S2). The degree of heterogeneity was low
(I2=47%).
Angioedema
A total of 13 studies (8,9,19,21,22,25,27,28-33)
with a total sample of 22,835 patients, consisting of 11,389
patients who received ARNI and 11,446 patients who received
control, were included in the meta-analysis of angioedema (Fig. 6A). The meta-analysis showed no
significant difference between the ARNI and the control groups in
decreasing the risk of angioedema (RR=1.44; 95% CI=0.90-2.29). No
outlier was detected based on the funnel plot (Fig. S2). The level of heterogeneity was
very low (I2=4.9%).
Symptomatic hypotension
A total of 23,036 participants, comprising 11,492
participants treated with ARNI and 11,544 participants in the
control group from 14 studies (8,9,19,21,22,24,25,27-33)
was included in the meta-analysis of symptomatic hypotension
(Fig. 6B). ARNI was associated with
a significantly increased risk of symptomatic hypotension (RR=1.54;
95% CI=1.43-1.65). No outlier was detected based on the funnel plot
(Fig. S2). The degree of
heterogeneity was moderate (I2=54.2%).
Subgroup analysis
Subgroup analyses stratified by LVEF (≤40 vs.
>40%) demonstrated no significant interaction between LVEF
category and the effect of ARNI across all assessed outcomes
(Table III). The decrease in
all-cause and cardiovascular-related mortality, MACEs and HF
hospitalization was consistent between subgroups. Hypotension risk
was increased in both LVEF subgroups, whereas hyperkalemia risk
showed a non-significant trend towards elevation in LVEF ≤40% but
not in LVEF >40%. Notably, angioedema risk was higher in the
LVEF >40% subgroup, though the difference was not statistically
significant. The incidence of renal impairment was lower in the
LVEF >40% subgroup; however, no significant subgroup effect was
detected. Heterogeneity across most comparisons was minimal, except
for hyperkalemia and angioedema, which showed moderate
variability.
 | Table IIISubgroup analysis. |
Table III
Subgroup analysis.
| Outcome | LVEF, % | Number of
studies | RR | 95% CI | P-value | I2
value, % | (Refs.) |
|---|
| All-cause
mortality | ≤40 | 3 | 0.89 | 0.67-1.19 | 0.52 | 0.0 | (8,27,33) |
| | >40 | 3 | 0.99 | 0.86-1.13 | | | (9,30,31) |
|
Cardiovascular-associated mortality | ≤40 | 4 | 0.88 | 0.58-1.32 | 0.86 | 0.0 | (8,23,27,32) |
| | >40 | 2 | 0.83 | 0.52-1.32 | | | (9,28) |
| MACE | ≤40 | 4 | 0.92 | 0.70-1.21 | 0.85 | 0.0 | (8,22,27,32) |
| | >40 | 4 | 0.95 | 0.88-1.02 | | | (20,28,30,31) |
| Hypotension | ≤40 | 11 | 1.48 | 1.25-1.75 | 0.73 | 0.0 | (8,19,21-25,27,29,32,33) |
| | >40 | 4 | 1.62 | 1.16-2.28 | | | (9,28,30,31) |
|
Hospitalization | ≤40 | 5 | 0.73 | 0.44-1.21 | 0.48 | 0.0 | (8,22,23,32,33) |
| | >40 | 2 | 0.88 | 0.82-1.94 | | | (9,28) |
| Hyperkalemia | ≤40 | 11 | 1.10 | 0.94-1.30 | 0.11 | 60.0 | (17,21-27,29,32,33) |
| | >40 | 4 | 0.94 | 0.84-1.05 | | | (9,28,30,31) |
| Angioedema | ≤40 | 9 | 1.10 | 0.61-1.99 | 0.16 | 48.2 | (8,19,21,22,25,27,29,32,33) |
| | >40 | 4 | 2.22 | 1.01-4.87 | | | (9,28,29,31) |
| Renal
impairment | ≤40 | 10 | 0.89 | 0.77-1.03 | 0.23 | 31.4 | (8,19,21-23,25,27,29,32,33) |
| | >40 | 5 | 0.67 | 0.44-1.04 | | | (9,28,30,31,34) |
Meta-regression analysis
Meta-regression analysis revealed significant
study-level predictors across outcomes (Table IV). Higher eGFR was associated with
a lower risk of all-cause mortality [standard error (SE)=0.06].
Higher NT-proBNP increased risk (SE=0.06), whereas higher BMI
decreased risk of cardiovascular-associated mortality (SE=0.05).
Hypotension was less common in females (SE=0.15) but more likely
with higher eGFR (SE=1.07). Female sex was the only factor
associated with a decreased risk of HF hospitalization (SE=0.15).
Hyperkalemia risk was greater with older age (SE=0.84), female sex
(SE=0.11) and lower LVEF (SE=0.29). Angioedema risk increased with
higher heart rate (SE=1.46), diastolic BP (SE=2.40) and NT-proBNP
(SE=0.77). Higher SBP was the only significant predictor of renal
impairment (SE=1.60). All other covariates were not significantly
associated with these outcomes.
 | Table IVRandom-effects univariate regression
analysis of ARNI therapeutic outcomes by sociodemographic and
clinical characteristics. |
Table IV
Random-effects univariate regression
analysis of ARNI therapeutic outcomes by sociodemographic and
clinical characteristics.
| A, All-cause
mortality |
|---|
| Covariate | Estimate | Standard error | P-value |
|---|
| Age | -0.05 | 0.15 | 0.719 |
| Female | -0.18 | 0.27 | 0.511 |
| NT-proBNP | -0.01 | 0.08 | 0.857 |
| LVEF | -0.03 | 0.08 | 0.693 |
| HR | -0.12 | 0.08 | 0.116 |
| SBP | 0.52 | 0.47 | 0.266 |
| BMI | -0.04 | 0.25 | 0.868 |
| eGFR | -0.21 | 0.06 | 0.001 |
| sCr | -0.10 | 0.06 | 0.102 |
| B,
Cardiovascular-associated mortality |
| Covariate | Estimate | Standard error | P-value |
| Age | 0.46 | 0.56 | 0.416 |
| Female | -0.30 | 0.24 | 0.211 |
| NT-proBNP | -0.14 | 0.06 | 0.015 |
| LVEF | -0.07 | 0.18 | 0.687 |
| HR | -1.18 | 0.62 | 0.059 |
| SBP | 0.46 | 0.48 | 0.335 |
| BMI | -0.20 | 0.05 | <0.001 |
| eGFR | -1.01 | 0.55 | 0.065 |
| sCr | -0.17 | 0.10 | 0.081 |
| C, Major adverse
cardiovascular event |
| Covariate | Estimate | Standard error | P-value |
| Age | 0.36 | 1.40 | 0.799 |
| Female | -0.06 | 0.64 | 0.925 |
| NT-proBNP | -0.04 | 0.06 | 0.427 |
| LVEF | -0.15 | 0.20 | 0.460 |
| HR | -0.05 | 0.30 | 0.877 |
| SBP | 0.36 | 0.29 | 0.212 |
| BMI | -0.03 | 0.24 | 0.918 |
| eGFR | -0.40 | 0.29 | 0.163 |
| sCr | -1.98 | 2.47 | 0.423 |
| D, Hypotension |
| Covariate | Estimate | Standard error | P-value |
| Age | 0.84 | 0.55 | 0.127 |
| Female | -0.38 | 0.15 | 0.009 |
| NT-proBNP | -0.02 | 0.10 | 0.839 |
| LVEF | 0.05 | 0.37 | 0.897 |
| HR | 0.59 | 1.77 | 0.738 |
| SBP | -0.12 | 0.17 | 0.469 |
| DBP | -0.04 | 0.06 | 0.486 |
| BMI | 0.75 | 1.36 | 0.581 |
| eGFR | -2.29 | 1.07 | 0.033 |
| sCr | -2.76 | 0.99 | 0.242 |
| E,
Hospitalization |
| Covariate | Estimate | Standard error | P-value |
| Age | -0.68 | 0.26 | 0.948 |
| Female | -0.49 | 0.15 | 0.001 |
| NT-proBNP | 0.22 | 0.47 | 0.635 |
| LVEF | -0.21 | 0.52 | 0.683 |
| HR | -0.43 | 1.61 | 0.948 |
| SBP | 0.17 | 0.57 | 0.971 |
| BMI | -1.08 | 2.03 | 0.596 |
| eGFR | -0.97 | 0.88 | 0.273 |
| sCr | 2.74 | 0.19 | 0.375 |
| F,
Hyperkalemia |
| Covariate | Estimate | Standard error | P-value |
| Age | 2.61 | 0.84 | 0.002 |
| Female | 0.35 | 0.11 | 0.001 |
| NT-proBNP | -0.14 | 0.18 | 0.453 |
| LVEF | 0.81 | 0.29 | 0.005 |
| HR | -2.15 | 3.05 | 0.481 |
| SBP | 4.55 | 1.63 | 0.084 |
| BMI | 0.58 | 2.52 | 0.818 |
| Egfr | -0.52 | 1.40 | 0.710 |
| sCr | -2.71 | 1.24 | 0.028 |
| G, Angioedema |
| Covariate | Estimate | Standard error | P-value |
| Age | -8.16 | 2.33 | 0.126 |
| Female | -0.57 | 0.43 | 0.188 |
| NT-proBNP | 1.67 | 0.77 | 0.031 |
| LVEF | -0.36 | 0.92 | 0.695 |
| HR | 5.59 | 1.46 | 0.016 |
| SBP | -10.02 | 1.71 | 0.349 |
| DBP | 11.94 | 2.40 | 0.007 |
| BMI | 4.18 | 2.03 | 0.077 |
| eGFR | -5.44 | 1.84 | 0.488 |
| sCr | 11.94 | 1.40 | 0.007 |
| H, Renal
impairment |
| Covariate | Estimate | Standard error | P-value |
| Age | 1.81 | 1.51 | 0.230 |
| Female | -0.16 | 0.14 | 0.271 |
| NT-proBNP | -0.23 | 0.18 | 0.193 |
| LVEF | 0.30 | 0.20 | 0.122 |
| HR | -0.64 | 1.80 | 0.723 |
| SBP | 5.22 | 1.60 | 0.001 |
| DBP | 8.05 | 2.83 | 0.239 |
| BMI | 1.39 | 0.89 | 0.121 |
| eGFR | -0.84 | 1.29 | 0.516 |
| sCr | -0.61 | 1.43 | 0.670 |
Discussion
The present meta-analysis confirmed the superior
efficacy of ARNI therapy in reducing all-cause and cardiovascular
mortality, HF-associated hospitalization and MACE across HF
phenotypes. The magnitude of risk reduction observed in
cardiovascular outcomes supports the pharmacological rationale of
neprilysin-angiotensin receptor pathway dual inhibition (33). These findings align with those of
earlier trials, including PARADIGM-HF, and meta-analyses, but
include a broader range of HF subtypes and integrated
meta-regression analyses to elucidate outcome modifiers (13,14,36).
The present findings support previous studies that
showed the efficacy of ARNI in HF (8,13,34,35).
Nielsen et al (36)
demonstrated that sacubitril/valsartan significantly decreased
all-cause mortality and serious AEs in HFrEF compared with
ACE-Is/ARBs. Similarly, Park et al (13) reported a reduction in all-cause and
cardiac-associated mortality and MACEs with ARNI therapy. However,
both the aforementioned studies reported an increased risk of
hypotension. The present analysis emphasizes the robust mortality
benefit of ARNIs and confirms its association with hypotension,
potentially due to enhanced NP activity, RAAS suppression and
sympathetic inhibition, which promote vasodilation and natriuresis
(8). Despite this hypotension risk,
ARNI also substantially mitigated renal impairment, reinforcing its
net clinical benefit. Its superior antihypertensive effect arises
from early sodium diuresis and sustained vasodilation, making it
more effective than traditional RAS inhibitors, even as natriuretic
effects wane with prolonged use (37).
The present meta-analysis provides a broader
evaluation of ARNI therapy across the full spectrum of HF
phenotypes, including HFrEF, HFmrEF and HfpEF, which have been
underrepresented or inconsistently analyzed in earlier reviews
(13,38). While landmark trials such as
PARADIGM-HF and PARAGON-HF have established the basis for ARNIs in
HF management, previous meta-analyses have largely centered on
patients with rEF, limiting their relevance to more diverse
clinical presentations (17,18).
By contrast, the present study integrated findings from a wider
range of HF subtypes and incorporated meta-regression techniques to
explore how key clinical and hemodynamic variables such as blood
pressure, renal function and NT-proBNP levels modify ARNI effects.
This approach not only enhances the generalizability of findings
but also allows for more personalized, phenotype-specific
interpretations of efficacy and safety. The present study
complements recent comprehensive reviews, such as Tromp et
al (39) and van Essen et
al (40), and focused analysis
on BP-associated outcomes and renal parameters, which are relevant
to daily clinical decision-making. The present analysis support
more precise and hypertension-conscious use of ARNIs in routine
practice, aligning with the growing emphasis on individualized
treatment in HF care.
Meta-regression analysis revealed that a lower eGFR
was associated with an increased risk of all-cause mortality,
supporting the findings of Khan et al (41), which linked a rapid decrease in eGFR
(>15 ml/min/year) with a higher mortality rate. Consistent with
the results of a previous study, the present analysis demonstrated
that higher SBP was associated with worsening renal impairment
(42). RAAS upregulation, increased
sympathetic nervous system activity, elevated pro-inflammatory
factors and left ventricular hypertrophy progression are potential
mechanisms underlying these findings, all contributing to
difficulties in volume handling, pump failure and mortality
(41). Additionally, female sex was
associated with a lower risk of HF-associated hospitalization,
potentially due to the effect of estrogen on vascular function,
inflammatory response, metabolism, cardiac myocytes and the
development of hypertrophy yielding better outcomes. Lastly,
elevated NT-proBNP levels were associated with
cardiovascular-associated mortality; previous findings showed that
NT-proBNP indicates increased cardiac stress (43,44).
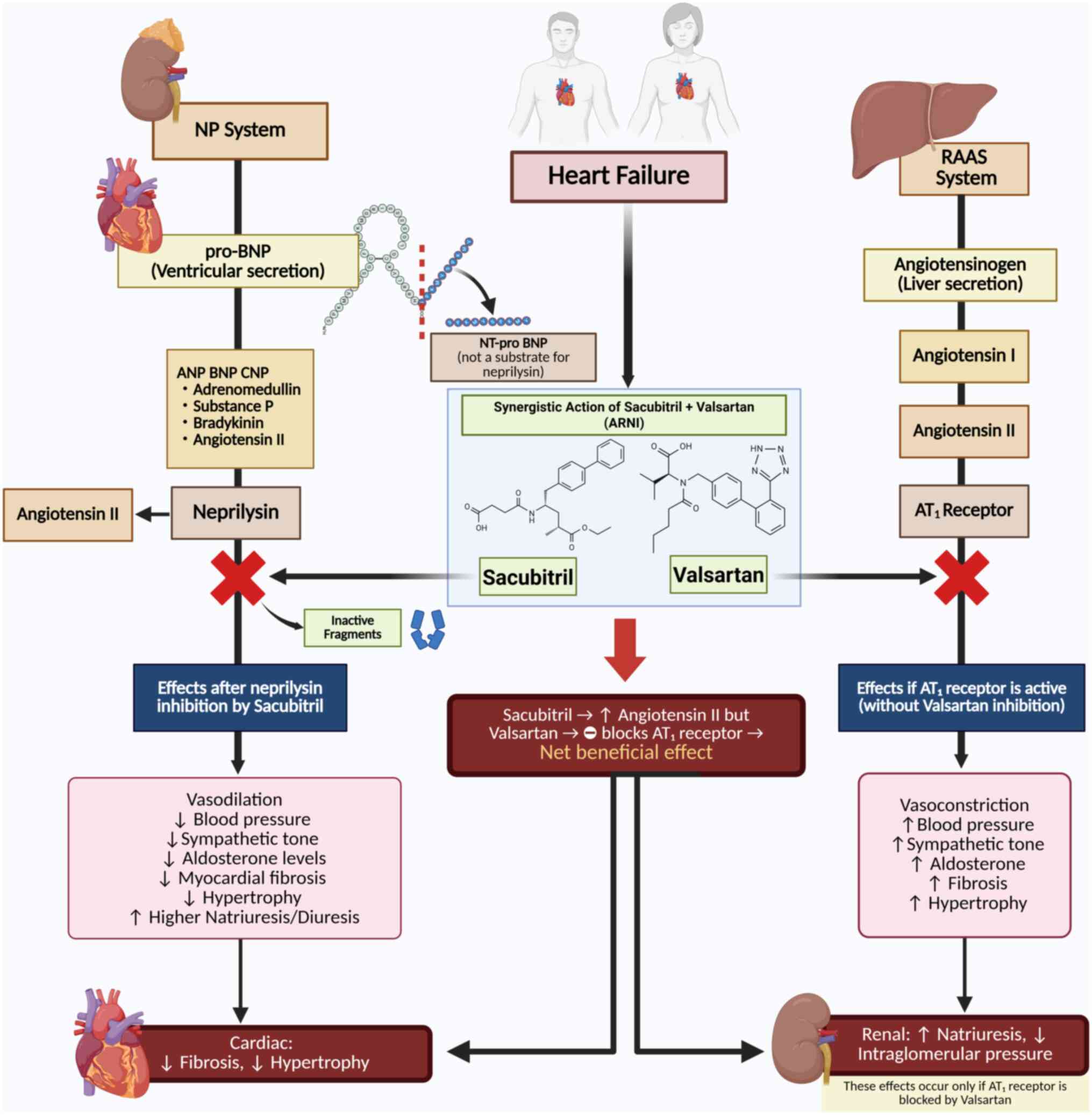
ARNI exerts its therapeutic effect in HF through a
dual mechanism that enhances beneficial pathways and suppresses
maladaptive neurohormonal activation (Fig. 7). Sacubitril inhibits neprilysin,
preventing the degradation of NPs (atrial, brain and C-type),
causing vasodilation, natriuresis, diuresis and attenuation of
myocardial fibrosis and hypertrophy. Simultaneously, valsartan
blocks the angiotensin 1 receptor, counteracting the effects of
angiotensin II and decreasing vasoconstriction, BP, aldosterone
secretion and cardiac remodeling (17).
The mechanisms of ARNI vary across different HF
stages with distinct EF profiles. In HfrEF (LVEF <40%), ARNI
improves cardiac output by decreasing preload and afterload and
improving ventricular contractility. In HfmrEF (LVEF, 40-49%), ARNI
targets both systolic and diastolic dysfunction, stabilizing LVEF
and decreasing vascular resistance. In HFpEF (LVEF ≥50%), ARNI
mitigates diastolic dysfunction by lowering left ventricular
filling pressure and relieving systemic congestion, with potential
antifibrotic effects that improve myocardial compliance (45). These phenotype-specific actions
highlight the versatility of ARNI across the HF spectrum.
ARNI therapy has become a transformative option in
HF management, particularly for patients with HFrEF who remain
symptomatic despite optimal treatment. By combining angiotensin II
receptor blockade and neprilysin inhibition, ARNI provides a dual
mechanism that attenuates the harmful effects of the RAAS and
improves the protective NP system (19,46).
This causes significant decreases in all-cause mortality,
HF-related hospitalization and improvements in the quality of life,
including enhanced exercise capacity and symptom control (8,19,44).
Additionally, the decrease in hospitalizations lessens the
healthcare system burden and mitigates costs (34). Although the benefits of ARNI in
HFpEF remain poorly understood, its potential role in this subgroup
emphasizes the need for further research to identify its influence
across all HF phenotypes.
Beyond ARNI therapy, the management of congestive HF
continues to evolve with optimizing volume control strategies. Duta
et al (10) demonstrated the
use of acetazolamide in combination with loop diuretics to enhance
decongestive therapy. This combinatorial approach not only augments
natriuresis but also addresses diuretic resistance, which is a
challenge in HF management. Although ARNI has demonstrated
beneficial effects on hemodynamics and renal function, residual
congestion remains a clinical concern in a subset of patients.
Therefore, integrating ARNI therapy with advanced decongestive
regimens may offer synergistic benefits, particularly in patients
with persistent volume overload. Future research should explore how
ARNI and adjunctive diuretic strategies can be co-optimized to
maximize clinical outcomes while minimizing renal and hemodynamic
complications.
The effective use of ARNI necessitates precise
dosing and careful patient monitoring to optimize outcomes and
minimize risks. Initial therapy typically starts with 49/51 mg
sacubitril/valsartan twice daily, with a lower dose of 24/26 mg
twice daily recommended for patients with significant renal
impairment or hypotension history (8,33). The
dose is subsequently titrated to a target of 97/103 mg twice daily
for 2-4 weeks, as tolerated, to optimize therapeutic benefits and
minimize AEs, including hypotension, hyperkalemia or renal
dysfunction (8,9). Regular BP, serum electrolyte and renal
function monitoring is crucial during titration and throughout
therapy, with dose adjustments tailored to patient needs,
particularly in the presence of comorbidities (47,48).
Park et al (49) reported
that compared with enalapril and ARBs, sacubitril/valsartan is a
cost-effective treatment for HFrEF. This supports its use as a
clinically valuable and economically sustainablealternative for
cardiologists and decision-makers in selecting therapeutic
approaches (49).
Multiple clinical factors predict hypotension risk
during ARNI therapy, informing targeted treatment decisions. To
minimize this risk, sacubitril/valsartan should be initiated at a
low dose (25-50 mg once daily), with close BP monitoring and
titrated every 2-4 weeks to a target of 100-150 mg twice daily as
tolerated (50). Caution is
required in older adult patients, those with arteriosclerosis or
those with advanced renal impairment, where reduced renal perfusion
increases susceptibility to hypotension and GFR decline (51). To avoid excessively low BP and
protect renal function, the use of other antihypertensive drugs,
including calcium channel blockers, diuretics and α- and
β-blockers, should be decreased. These approaches optimize the
renal benefits of ARNI through NP action. Furthermore, multivariate
analysis has identified baseline atrial fibrillation, a higher
blood urea nitrogen/creatinine (BUN/Cr) ratio and lower SBP as
significant independent predictors of hypotension following ARNI
administration (52). Recognizing
these risk factors allows clinicians to more safely and effectively
tailor therapy.
The present study benefits from a large sample size
(>20,000 patients), adherence to the PRISMA guidelines and
rigorous risk-of-bias assessment, enhancing the reliability and
generalizability of the findings. It confirms the efficacy and
safety of ARNI in decreasing cardiovascular-associated and
all-cause mortality, hospitalization and renal impairment across HF
spectrums. Subgroup analyses were conducted to explore potential
heterogeneity. Additionally, meta-regression analysis was performed
to decrease bias by analyzing the influence of variables, including
age, sex, NT-proBNP levels, LVEF, HR, SBP, BMI, eGFR and sCr
levels, on outcomes, facilitating more accurate identification of
potential confounding factors. However, the present study had
limitations. Heterogeneity in the follow-up durations across trials
may affect the comparability of the outcomes. The
underrepresentation of populations with HFmrEF limits the
robustness of the conclusions for this subgroup. The absence of
meta-regression accounting for patient comorbidities, including
diabetes, chronic kidney disease or atrial fibrillation, prevents a
more nuanced interpretation of treatment effects. Fourth, although
the overall bias was low, three studies demonstrated some concerns
and one exhibited high risk of bias, which may influence the pooled
estimates. The predominance of studies conducted in high-income
Western countries may limit the generalizability to diverse global
populations with differing clinical practices and healthcare
infrastructures. A further limitation is the predominant reliance
on eGFR to assess renal outcomes. While several studies reported
additional parameters, including serum Cr (28,33,34)
and urinary albumin-to-creatinine ratio (31), BUN was not consistently included as
a diagnostic endpoint across trials. Future research should
implement standardized renal outcome measures that incorporate a
broader range of biomarkers to facilitate more comprehensive and
clinically relevant assessment of renal function.
ARNIs significantly improve clinical outcomes in
patients with HF across the spectrum of EF phenotypes, particularly
in reducing all-cause and cardiovascular-associated mortality,
HF-associated hospitalization and renal impairment. However, the
increased risk of hypotension necessitates close monitoring and
targeted dose adjustment. The present findings support the broader
adoption of ARNI in HF management, focusing on patient-specific
characteristics, including renal function, sex and baseline BP.
Future large-scale trials in diverse populations, including
underrepresented HF phenotypes and resource-limited settings, are
warranted to validate and extend these findings.
Supplementary Material
Funnel plot of the efficacy of
angiotensin receptor-neprilysin inhibitor compared with control
treatment for heart failure management. (A) Cardiovascular-related
and (B) all-cause mortality. (C) Major adverse cardiovascular
events. (D) Heart failure hospitalization.
Safety of angiotensin
receptor-neprilysin inhibitor compared with control treatment for
heart failure management. (A) Hyperkalemia. (B) Symptomatic
hypotension. (C) Angioedema. (D) Renal impairment.
PICO framework.
Acknowledgements
The authors would like to thank Ms Rahmati Putri
Yaniafari (Nanyang Technological University, Singapore) for helping
with literature retrieval.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are
included in the figures and/or tables of this article.
Authors' contribution
DDCHR, SCS, KCT, INK, BI, AI and MAE designed the
study. DDCHR, SCS, KCT, JAJMNL, INK and MAE conceived the study and
performed the literature review. DDCHR assessed the risk of bias.
JAJMNL and SHR confirm the authenticity of all the raw data..
DDCHR, SCS, JAJMNL, SHR, INK, BI and AI analyzed and interpreted
data. DDCHR, SCS, INK, BI, AI, and MAE wrote the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript, and subsequently, the authors revised
and edited the content produced by the artificial intelligence
tools as necessary, taking full responsibility for the ultimate
content of the present manuscript.
References
|
1
|
Heidenreich PA, Bozkurt B, Aguilar D,
Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM,
Evers LR, et al: 2022 AHA/ACC/HFSA guideline for the management of
heart failure: A report of the American College of
Cardiology/American heart association joint committee on clinical
practice guidelines. Circulation. 145:e895–e1032. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kemp CD and Conte JV: The pathophysiology
of heart failure. Cardiovasc Pathol. 21:365–371. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Savarese G, Becher PM, Lund LH, Seferovic
P, Rosano GMC and Coats AJS: Global burden of heart failure: A
comprehensive and updated review of epidemiology. Cardiovasc Res.
118:3272–3287. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Farré N, Vela E, Clèries M, Bustins M,
Cainzos-Achirica M, Enjuanes C, Moliner P, Ruiz S, Verdú-Rotellar
JM and Comín-Colet J: Real world heart failure epidemiology and
outcome: A population-based analysis of 88,195 patients. PLoS One.
12(e0172745)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heidenreich PA: Healthy lifestyles and
personal responsibility. J Am Coll Cardiol. 64:1786–1788.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McDonagh TA, Metra M, Adamo M, Gardner RS,
Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et
al: 2021 ESC Guidelines for the diagnosis and treatment of acute
and chronic heart failure. Eur Heart J. 42:3599–3726.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure. Kardiol Pol.
74:1037–1147. 2016.PubMed/NCBI View Article : Google Scholar : (In Polish).
|
|
8
|
McMurray JJ, Packer M, Desai AS, Gong J,
Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg
K, et al: Angiotensin-neprilysin inhibition versus enalapril in
heart failure. N Engl J Med. 371:993–1004. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Solomon SD, McMurray JJV, Anand IS, Ge J,
Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B,
et al: Angiotensin-neprilysin inhibition in heart failure with
preserved ejection fraction. N Engl J Med. 381:1609–1620.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Duta TF, Zulfa PO, Alina M, Henira N,
Tsurayya G, Fakri F and Acharya Y: Efficacy of acetazolamide and
loop diuretics combinatorial therapy in congestive heart failure: A
meta-analysis. Narra X. 2(e124)2024.
|
|
11
|
Villaschi A, Pellegrino M, Condorelli G
and Chiarito M: Diuretic combination therapy in acute heart
failure: An updated review. Curr Pharm Des. 30:2597–2605.
2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gogikar A, Nanda A, Janga LSN, Sambe HG,
Yasir M, Man RK and Mohammed L: Combination diuretic therapy with
thiazides: A systematic review on the beneficial approach to
overcome refractory fluid overload in heart failure. Cureus.
15(e44624)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park DY, An S, Attanasio S, Jolly N,
Malhotra S, Doukky R, Samsky MD, Sen S, Ahmad T, Nanna MG and Vij
A: Network meta-analysis comparing angiotensin receptor-neprilysin
inhibitors, angiotensin receptor blockers, and
angiotensin-converting enzyme inhibitors in heart failure with
reduced ejection fraction. Am J Cardiol. 187:84–92. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang H, Huang T, Shen W, Xu X, Yang P,
Zhu D, Fang H, Wan H, Wu T, Wu Y and Wu Q: Efficacy and safety of
sacubitril-valsartan in heart failure: A meta-analysis of
randomized controlled trials. ESC Hear Fail. 7:3841–3850.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
The National Cancer Institute (NCI) of the
National Institute of Health: Common terminology criteria for
adverse events (CTCAE) version 5.0. US Dep Heal Hum Serv, 2017.
|
|
17
|
Jørgensen L, Paludan-Müller AS, Laursen
DR, Savović J, Boutron I, Sterne JA, Higgins JP and Hróbjartsson A:
Evaluation of the Cochrane tool for assessing risk of bias in
randomized clinical trials: overview of published comments and
analysis of user practice in Cochrane and non-Cochrane reviews.
Syst Rev. 5(80)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vardeny O, Miller R and Solomon SD:
Combined neprilysin and renin-angiotensin system inhibition for the
treatment of heart failure. JACC Heart Fail. 2:663–670.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chandra A, Polanczyk CA, Claggett BL,
Vaduganathan M, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC,
Schwende H, et al: Health-related quality of life outcomes in
PARAGON-HF. Eur J Heart Fail. 24:2264–2274. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Desai AS, Solomon SD, Shah AM, Claggett
BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R and Mitchell GF:
EVALUATE-HF Investigators. Effect of sacubitril-valsartan vs
enalapril on aortic stiffness in patients with heart failure and
reduced ejection fraction: A Randomized clinical trial. JAMA.
322:1077–1084. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao Y, Xing C, Hao W, Zhao H, Wang L, Luan
B and Hou A: The impact of sacrubitril/valsartan on clinical
treatment and hs-cTnT and NT-ProBNP serum levels and the left
ventricular function in patients with chronic heart failure. Int
Heart J. 61:1–6. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ghafur S, Zahid M, Sarkar H, Barman R,
Al-Mahmud A, Rahman M and Islam H: Effect of angiotensin
receptor-neprilysin inhibitor versus valsartan on cardiac status in
patients with chronic heart failure with reduced ejection fraction:
A Randomized clinical trial in Rangpur Medical College Hospital,
Bangladesh. Open J Intern Med. 10:21–34. 2020.
|
|
24
|
Halle M, Schöbel C, Winzer EB, Bernhardt
P, Mueller S, Sieder C and Lecker LSM: A randomized clinical trial
on the short-term effects of 12-week sacubitril/valsartan vs.
enalapril on peak oxygen consumption in patients with heart failure
with reduced ejection fraction: Results from the ACTIVITY-HF study.
Eur J Heart Fail. 23:2073–2082. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kang DH, Park SJ, Shin SH, Hong GR, Lee S,
Kim MS, Yun SC, Song JM, Park SW and Kim JJ: Angiotensin receptor
neprilysin inhibitor for functional mitral regurgitation.
Circulation. 139:1354–1365. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li BH, Fang KF, Lin PH, Zhang YH, Huang YX
and Jie H: Effect of sacubitril valsartan on cardiac function and
endothelial function in patients with chronic heart failure with
reduced ejection fraction. Clin Hemorheol Microcirc. 77:425–433.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mann DL, Givertz MM, Vader JM, Starling
RC, Shah P, McNulty SE, Anstrom KJ, Margulies KB, Kiernan MS, Mahr
C, et al: Effect of treatment with sacubitril/valsartan in patients
with advanced heart failure and reduced ejection fraction: A
Randomized clinical trial. JAMA Cardiol. 7:17–25. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mentz RJ, Ward JH, Hernandez AF, Lepage S,
Morrow DA, Sarwat S, Sharma K, Starling RC, Velazquez EJ,
Williamson KM, et al: Angiotensin-neprilysin inhibition in patients
with mildly reduced or preserved ejection fraction and worsening
heart failure. J Am Coll Cardiol. 82:1–12. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Piepoli MF, Hussain RI, Comin-Colet J,
Dosantos R, Ferber P, Jaarsma T and Edelmann F: OUTSTEP-HF:
Randomised controlled trial comparing short-term effects of
sacubitril/valsartan versus enalapril on daily physical activity in
patients with chronic heart failure with reduced ejection fraction.
Eur J Heart Fail. 23:127–135. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pieske B, Wachter R, Shah SJ, Baldridge A,
Szeczoedy P, Ibram G, Shi V, Zhao Z and Cowie MR: PARALLAX
Investigators and Committee members. Effect of sacubitril/valsartan
vs standard medical therapies on plasma NT-proBNP concentration and
submaximal exercise capacity in patients with heart failure and
preserved ejection fraction: The PARALLAX Randomized clinical
trial. JAMA. 326:1919–1929. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Solomon SD, Zile M, Pieske B, Voors A,
Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J,
et al: The angiotensin receptor neprilysin inhibitor LCZ696 in
heart failure with preserved ejection fraction: A phase 2
double-blind randomised controlled trial. Lancet. 380:1387–1395.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tsutsui H, Momomura SI, Saito Y, Ito H,
Yamamoto K, Sakata Y, Desai AS, Ohishi T, Iimori T, Kitamura T, et
al: Efficacy and safety of sacubitril/valsartan in japanese
patients with chronic heart failure and reduced ejection
fraction-results from the PARALLEL-HF study. Circ J. 85:584–594.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Velazquez EJ, Morrow DA, DeVore AD, Duffy
CI, Ambrosy AP, McCague K, Rocha R and Braunwald E: PIONEER-HF
Investigators. Angiotensin-neprilysin inhibition in acute
decompensated heart failure. N Engl J Med. 380:539–548.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Voors AA, Gori M, Liu LC, Claggett B, Zile
MR, Pieske B, McMurray JJ, Packer M, Shi V, Lefkowitz MP, et al:
Renal effects of the angiotensin receptor neprilysin inhibitor
LCZ696 in patients with heart failure and preserved ejection
fraction. Eur J Heart Fail. 17:510–517. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nasrallah D, Abdelhamid A, Tluli O,
Al-Haneedi Y, Dakik H and Eid AH: Angiotensin receptor
blocker-neprilysin inhibitor for heart failure with reduced
ejection fraction. Pharmacol Res. 204(107210)2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nielsen EE, Feinberg JB, Bu FL, Hecht
Olsen M, Raymond I, Steensgaard-Hansen F and Jakobsen JC:
Beneficial and harmful effects of sacubitril/valsartan in patients
with heart failure: A systematic review of randomised clinical
trials with meta-analysis and trial sequential analysis. Open Hear.
7(e001294)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hishida E and Nagata D: Angiotensin
receptor-neprilysin inhibitor for chronic kidney disease:
Strategies for renal protection. Kidney Blood Press Res.
49:916–932. 2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang Y, Zhou R, Lu C, Chen Q, Xu T and Li
D: Effects of the angiotensin-receptor neprilysin inhibitor on
cardiac reverse remodeling: Meta-analysis. J Am Heart Assoc.
8(e012272)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tromp J, Ouwerkerk W, van Veldhuisen DJ,
Hillege HL, Richards AM, van der Meer P, Anand IS, Lam CSP and
Voors AA: A systematic review and network meta-analysis of
pharmacological treatment of heart failure with reduced ejection
fraction. JACC Heart Fail. 10:73–84. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
van Essen BJ, Ceelen DCH, Ouwerkerk W,
Teng TK, Tharshana GN, Hew FM, Butler J, Zannad F, Lam CS,
Ezekowitz J, et al: Pharmacologic treatment of heart failure with
reduced ejection fraction: An updated systematic review and network
meta-analysis. J Am Coll Cardiol: Aug 30, 2025 (Epub ahead of
print).
|
|
41
|
Khan NA, Ma I, Thompson CR, Humphries K,
Salem DN, Sarnak MJ and Levin A: Kidney function and mortality
among patients with left ventricular systolic dysfunction. J Am Soc
Nephrol. 17:244–253. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Banerjee D, Winocour P, Chowdhury TA, De
P, Wahba M, Montero R, Fogarty D, Frankel AH, Karalliedde J, Mark
PB, et al: Management of hypertension and
renin-angiotensin-aldosterone system blockade in adults with
diabetic kidney disease: Association of British clinical
diabetologists and the renal association UK guideline update 2021.
BMC Nephrol. 23(9)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Murphy E: Estrogen signaling and
cardiovascular disease. Circ Res. 109:687–696. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rudolf H, Mügge A, Trampisch HJ, Scharnagl
H, März W and Kara K: NT-proBNP for risk prediction of
cardiovascular events and all-cause mortality: The getABI-study.
Int J Cardiol Hear Vasc. 29(100553)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Greenberg B: Angiotensin
receptor-neprilysin inhibition (ARNI) in heart failure. Int J Hear
Fail. 2:73–90. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bayes-Genis A, Barallat J and Richards AM:
A test in context: Neprilysin: Function, inhibition, and biomarker.
J Am Coll Cardiol. 68:639–653. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sauer AJ, Cole R, Jensen BC, Pal J, Sharma
N, Yehya A and Vader J: Practical guidance on the use of
sacubitril/valsartan for heart failure. Heart Fail Rev. 24:167–176.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chandra A, Lewis EF, Claggett BL, Desai
AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz
MP, et al: Effects of sacubitril/valsartan on physical and social
activity limitations in patients with heart failure: A secondary
analysis of the PARADIGM-HF trial. JAMA Cardiol. 3:498–505.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Park SK, Hong SH, Kim H, Kim S and Lee EK:
Cost-Utility analysis of sacubitril/valsartan use compared with
standard care in chronic heart failure patients with reduced
ejection fraction in South Korea. Clin Ther. 41:1066–1079.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gan L, Lyu X, Yang X, Zhao Z, Tang Y, Chen
Y, Yao Y, Hong F, Xu Z, Chen J, et al: Application of angiotensin
receptor-neprilysin inhibitor in chronic kidney disease patients:
Chinese expert consensus. Front Med (Lausanne).
9(877237)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Nagata D, Hishida E and Masuda T:
Practical strategy for treating chronic kidney disease
(CKD)-Associated with hypertension. Int J Nephrol Renovasc Dis.
13:171–178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nakano Y, Suzuki Y, Onishi T, Ando H,
Matsuo Y, Suzuki W, Kuno S, Ohashi H, Waseda K, Takahashi H, et al:
Predictors of hypotension after angiotensin receptor-neprilysin
inhibitor administration in patients with heart failure. Int Heart
J. 65:658–666. 2024.PubMed/NCBI View Article : Google Scholar
|