Introduction
Epithelial-to-mesenchymal transition (EMT) is a
critical pathological feature in fibrosis-related diseases,
including intestinal fibrosis and pulmonary fibrosis (1), and represents a highly dynamic,
multi-stage process regulated by multiple signaling pathways that
promote acquisition of the mesenchymal phenotype. In detail,
external stimulation of signaling pathways, including TGFβ/Wnt,
triggers a transcriptional program during EMT that induces the
expression of the E-cadherin transcriptional repressor SNAIL, which
promotes cell migration, invasiveness and fibrosis. The core
process of EMT is characterized by epithelial cells losing polarity
and intercellular connections to acquire a mesenchymal phenotype
with enhanced mesenchymal traits such as collagen, fibronectin
(FN), vimentin and α-smooth muscle actin (α-SMA) expression, as
well as reduced epithelial adhesion protein E-cadherin expression
(2-4).
Proton pump inhibitors (PPIs) have been shown to have anticancer
activity against several types of cancer. For instance, omeprazole
destroyed cyclic AMP response element-binding protein
(CREB)-binding protein (CBP)/p300-mediated SNAIL protein
acetylation to induce its degradation, leading to the inhibition of
EMT in cancer cells (5), while
pantoprazole was shown to inactivate the Wnt/β-catenin signaling
pathway to block the EMT process (6-8).
Moreover, rabeprazole was shown to reduce resistance to
temozolomide in glioma via EMT inhibition (9). Despite the fact that previous studies
showed that PPIs can inhibit EMT, to date, there is no direct
evidence fully describing the role of rabeprazole in fibrosis.
It is well known that SMAD family member 3 (SMAD3)
is an important mediator of TGFβ-induced fibrosis or EMT, which can
crosstalk with other pathways to modulate pathological progression.
For example, Y-box binding protein 1, a member of the
DNA/RNA-binding protein family, was reported to upregulate SMAD7
transcription or interact with SMAD3 to overcome the effect of TGFβ
stimulation (10,11). In addition, transcriptional
intermediary factor 1γ (TIF1γ; also known as TRIM33), a direct
target of CREB, interacted with SMAD3 to antagonize SMAD3-induced
fibrosis (12), which was in line
with studies reporting on the anti-EMT function of TIF1γ (13,14).
Notably, the phosphorylation of SMAD3 linker (at the ser204,
ser208, ser213 and Thr179 residues) was reported to accelerate
nuclear shuttle of cytoplasmic SMAD3, which decreased the
accessibility of non-phosphorylated SMAD3 to membrane-anchored TGFβ
receptor type I (TGFβRI), leading to inhibition of SMAD3C
phosphorylation (15,16). Moreover, TIF1γ has been shown to act
as a SMAD4 ubiquitin ligase, either dissociating SMAD2/SMAD3-SMAD4
complexes or mediating polyubiquitylation and degradation of SMAD4,
thereby promoting competitive binding of SMAD2/3 through the MH2
domain (17). In addition, TIF1γ
was reported to occupy SMAD-binding elements to prevent
SMAD2/3-mediated DNA binding (12).
Based on these findings, it is hypothesized that this TIF1γ/SMAD3
complex may have an inhibitory effect on SMAD3 phosphorylation and
nuclear translocation to ablate SMAD3-mediated gene transcription.
However, the critical phosphorylation site of SMAD3 in response to
the TIF1γ/SMAD3 complex remain unclear.
Rabeprazole, a classical PPI, was previously
recognized to be involved in the modulation of inflammation
(18,19), barrier function (20,21)
and metabolism (22). The aim of
the present study was to identify the novel biological function of
rabepraozole in fibrosis and reveal the possible relationship
between TIF1γ and SMAD3 through immunofluorescence (IF),
immunoprecipitation (IP) and luciferase assays. providing
significant implications for understanding the function of
rabeprazole.
Materials and methods
Chemical reagents
Gibco™ BASIC DMEM (cat. no. C11995500BT) and fetal
bovine serum (FBS) (Premium Plus; cat. no. A5669701) were purchased
from Thermo Fisher Scientific, Inc. Rabeprazole (cat. no. HY-B0656)
was purchased from MedChemExpress. Lipo8000™ transfection reagent
(cat. no. C0533), nuclear and cytoplasmic protein extraction kit
(cat. no. P0028) and cell lysis buffer for western blotting and
immunoprecipitation (IP; cat. no. P0013) were purchased from
Beyotime Institute of Biotechnology. shRNA-TIF1γ plasmids and TIF1γ
promoter plasmid, were constructed and obtained from Youbio Biotech
Co., Ltd. Protein A/G magnetic beads (cat. no. B23202) were
purchased from Selleck Chemicals. EZ-press RNA Purification Kit
(cat. no. B0004D), 4X EZscript Reverse Transcription Mix II (with
gDNA Remover; cat. no. EZB-RT2G) and 2X Color SYBR Green qPCR
Master Mix (cat. no. A0012) were purchased from EZBioscience. A
dual-luciferase reporter assay system (cat. no. E1910) was obtained
from Promega Corporation. Antibodies including α-SMA specific
monoclonal antibody (mAb) (cat. no. 67735-1-Ig), FN mAb (cat. no.
66042-1-Ig), vimentin polyclonal antibody (pAb) (cat. no.
10366-1-AP), collagen type I (Col1a1) mAb (cat. no. 67288-1-Ig),
SMAD3 mAb (cat. no. 66516-1-Ig), lamin A/C pAb (cat. no.
10298-1-AP) and α-tubulin mAb (cat. no. 66031-1-Ig) were purchased
from Proteintech Group, Inc. TIF1γ mouse mAb (cat. no. YM1108),
SMAD3 (phospho Ser204) rabbit pAb (cat. no. YP0363), SMAD3 (phospho
Ser213) rabbit pAb (cat. no. YP0364), SMAD3 (phospho Thr179) rabbit
pAb (cat. no. YP0745) and SMAD3 (phospho Ser208) rabbit pAb (cat.
no. YP0746) were purchased from Immunoway Biotechnology Co., Ltd.;
peroxidase affiniPure™ goat anti-rabbit IgG (H+L) (cat. no.
111-035-003) and peroxidase-conjugated affiniPure goat anti-mouse
IgG (H+L) (cat. no. 115-035-003) were obtained from Jackson
ImmunoResearch Laboratories, Inc.
Cell culture, treatment and
transfection
Gastric epithelial cells, including AGS (cat. no.
JNO-H0238) and GES-1 (cat. no. JNO-H0240), were purchased from
Guangzhou Jennio Biotech Co. Ltd. and maintained in DMEM medium
containing 10% FBS, 100 mg/ml streptomycin and 100 U/ml penicillin.
Rabeprazole was dissolved in DMSO and stored at -80˚C. The
concentration of rabeprazole used was 100 µM. For cell transfection
of each well in a 6-well plate, when cells reached 50% confluence,
2 µg plasmids were gently mixed with 4 µl Lipo8000 transfection
reagent in 125 µl Opti-MEM® medium at room temperature
according to the manufacturer's instructions. Subseqently, the mix
was added into each well for 48 h. pLVX-shRNA-PURO plasmids (cat.
no. L28550-L28552) targeting TIF1γ (also known as TRIM33) were
constructed and purchased from Youbio Biotech Co., Ltd.
Reverse transcription-quantitative
(RT-q)PCR analysis
Following treatment, total RNA was extracted from
106 cells per group with the EZ-press RNA purification
kit according to the manufacturer's instructions. cDNA was
synthesized using 4X EZscript reverse transcription mix II (with
gDNA Remover) to analyze the indicated gene expression using the 2X
color SYBR Green qPCR Master Mix. The following primer pairs were
used for qPCR: FN forward, 5'-TCAGCTTCCTGGCACTTCTG-3' and reverse,
5'-TCTTGTCCTACATTCGGCGG-3'; vimentin forward,
5'-GGACCAGCTAACCAACGACA-3' and reverse, 5'-AAGGTCAAGACGTGCCAGAG-3';
COl1a1 forward, 5'-TCGGAGGAGAGTCAGGAAGG-3' and reverse,
5'-CCCGGTGACACATCAAGACA-3'; α-SMA forward,
5'-CTATGAGGGCTATGCCTTGCC-3' and reverse,
5'-GCTCAGCAGTAGTAACGAAGGA-3'; TIF1γ forward,
5'-AGCACTACTATACAGCAAGCGA-3' and reverse,
5'-CAGAAGGTGGGATCACAATGG-3'; β-actin forward,
5'-CTTCGCGGGCGACGAT-3' and reverse,
5'-CCACATAGGAATCCTTCTGACC-3'.
Immunoblotting analysis
As described in Niu et al (23), after treatment, cells were harvested
to extract the total protein using cell lysis buffer for western
blotting and IP. Briefly, 15 µg total protein was separated by 10%
SDS-PAGE and transferred into polyvinylidene fluoride (PVDF)
membranes. The protein bands were blocked with PBST (0.5% Tween-20)
(cat. no. BL345A) containing 2% skim milk (cat. no. BS102) plus 3%
BSA (cat. no. BS114; all from Biosharp) for 1 h at room
temperature. After washing with PBST (0.5% Tween-20), the membranes
were incubated with the primary antibodies with a dilution of
1:2,000 overnight at 4˚C. Following incubation with primary
antibodies, the membranes were incubated with the secondary
antibodies at 1:2,000 for 1 h at room temperature. Finally, after
washing with PBST, the bands were visualized and captured using
Chemiluminescence imaging instrument (MiniChemi 610, Sinsage) after
incubation with an ECL chemiluminescent substrate (cat. no.
NEL105001EA; Revvity, Inc.). the density was quantified by Image J
software (version: 1.8.0_351; National Institutes of Health) and
normalized to internal control.
Subcellular isolation
According to the manufacturer's instructions, after
serum starvation and when the cells reached 80% confluence, cells
were treated with or without rabeprazole for 1 h at 37˚C and the
cytosolic and nuclear fractions were extracted using the nuclear
and cytoplasmic protein extraction kit aforementioned. Western
blotting was performed to analyze the protein expression.
IP
As described in Li et al (24), when cells reached 80% confluence in
a 6-cm dish, the medium was replaced with serum-free DMEM for 18 h.
The cells were incubated with or without rabeprazole for 1 h, after
which the medium was removed and the cells were washed with
ice-cold PBS. The cells were then incubated with cell lysis buffer
for western blotting and immunoprecipitation for 10 min on ice,
scraped, and transferred into 1.5-ml tubes for an additional 30 min
of lysis on a rotator at 4˚C. The lysates were centrifuged at
12,000 x g for 2 min at 4˚C to collect the supernatant. A total of
10 µl of anti-SMAD3 antibody was added to the supernatant and
incubated overnight at 4˚C. Subsequently, 20 µl of protein A/G
magnetic beads (cat. no. B23202; Selleck Chemicals) were added and
incubated for 1 h at 4˚C to pull down the immune complexes. After
three washes with cell lysis buffer for western blotting and IP
(cat. no. P0013), the complexes were eluted with SDS loading buffer
and boiled at 100˚C for 10 min for immunoblotting analysis.
Immunofluorescence staining
Cells were digested and reseeded into an 8-well
slide overnight and incubated at 37˚C with or without rabeprazole
for 1 h. After fixation with 3% PFA (cat. no. BL3786A) for 10 min
at room temperature and permeabilization with 0.3% Triton X-100
(cat. no. BL935B; both from Biosharp; Beijing Labgic Technology
Co., Ltd.) for 5 min at 4˚C, cells were blocked with 3% milk at
room temperature for 1 h. The incubation with anti-SMAD3 at a
dilution of 1:400 was conducted overnight at 4˚C. Subsequently,
further incubation was performed for another 1 h at room
temperature with goat anti-mouse IgG (H+L) (AbFluor 594) (cat. no.
RS3608; Immunoway Biotechnology Co., Ltd.) at a dilution of 1:300.
After washing and staining the nuclei with DAPI buffer (cat. no.
C1006; Beyotime Insitute of Biotechnology) for 5 min at room
temperature, the coverslips were covered with glass slides. Stained
cells were visualized using a laser scanning fluorescent microscope
(Leica Microsystems GmbH).
Dual luciferase reporter analysis
Briefly, the reporter plasmid containing 0.5 µg
TIF1γ promoter in PGL3-Basic (cat. no. 56089) and 10 ng pRL-TK
Renilla luciferase plasmid (cat. no. V1079; both from Youbio
Biotech Co., Ltd.) at a ratio of 50:1 were co-transfected into
cells in a 24-well plate at room temperature. Cells were maintained
for 24 h with complete medium in a cell incubator using Lipo8000
transfection reagent (C0533; Beyotime Institute of Biotechnology),
followed by incubation with 100 µM rabeprazole for another 24 h in
serum-free medium at 37˚C. An equal precentage of DMSO was used as
the vehicle control. Each group consisted of three replicates. The
cells were lysed with 100 µl Passive Lysis Buffer, and samples were
collected and measured using a plate-reading luminometer to
calculate relative light units determined by the ratio of
firely/Renilla to reflect TIF1γ transactivation, using the
dual-luciferase reporter assay system (cat. no. E1910; Promega
Corporation) according to the manufacturer's instructions.
Statistical analysis
Data analysis was performed with GraphPad Prism 10.0
(Dotmatics). Statistical comparisons between two groups were
performed using the unpaired Student's t-test. One-sample t-test
was conducted to analyze the RT-q PCR results. Statistical
comparisons among three groups were performed using one-way ANOVA
followed by the Dunnett's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Rabeprazole suppresses fibrosis in
gastric epithelial cells
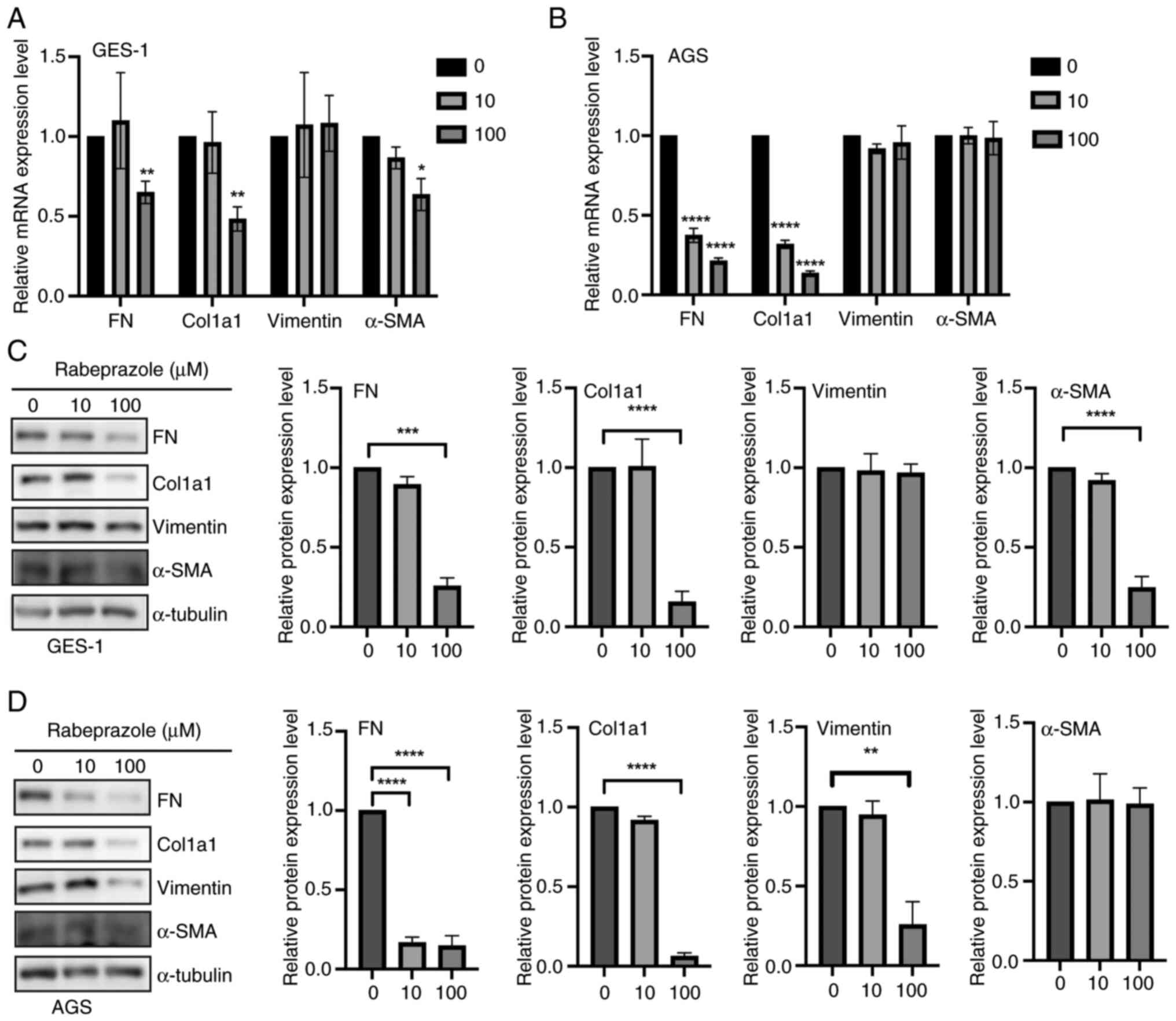
AGS and GES-1 gastric epithelial cells were employed
as in vitro models to assess the impact of rabeprazole on
fibrotic processes. The expression levels of genes involved in
fibrosis were analyzed in gastric epithelial cells after incubation
for 24 h with rabeprazole at 10 and 100 µM. Compared with the
control group (Fig. 1A and B), a significant fibrosis inhibition was
observed in GES-1 and AGS cells in response to 100 µM rabeprazole
stimulation as indicated by the largely decreased FN and Col1a1
mRNA levels, whereas no significant change was observed in vimentin
mRNA expression regardless of treatment with 10 and 100 µM.
Moreover, western blotting was performed to detect the indicated
protein expression levels, and quantification of the indicated
proteins revealed that rabeprazole treatment at 100 µM induced a
downregulation of FN and Col1a1 expression in GES-1 and AGS cells,
respectively, while no influence on vimentin expression was
observed with treatment at 10 µM. In addition, the expression of
α-SMA was downregulated in GES-1 cells (Fig. 1C and D). Taken together, these findings
indicated that rabeprazole has a potent suppressive effect in
inhibiting fibrosis.
TIF1γ is essential for the EMT
inhibition mediated by rabeprazole
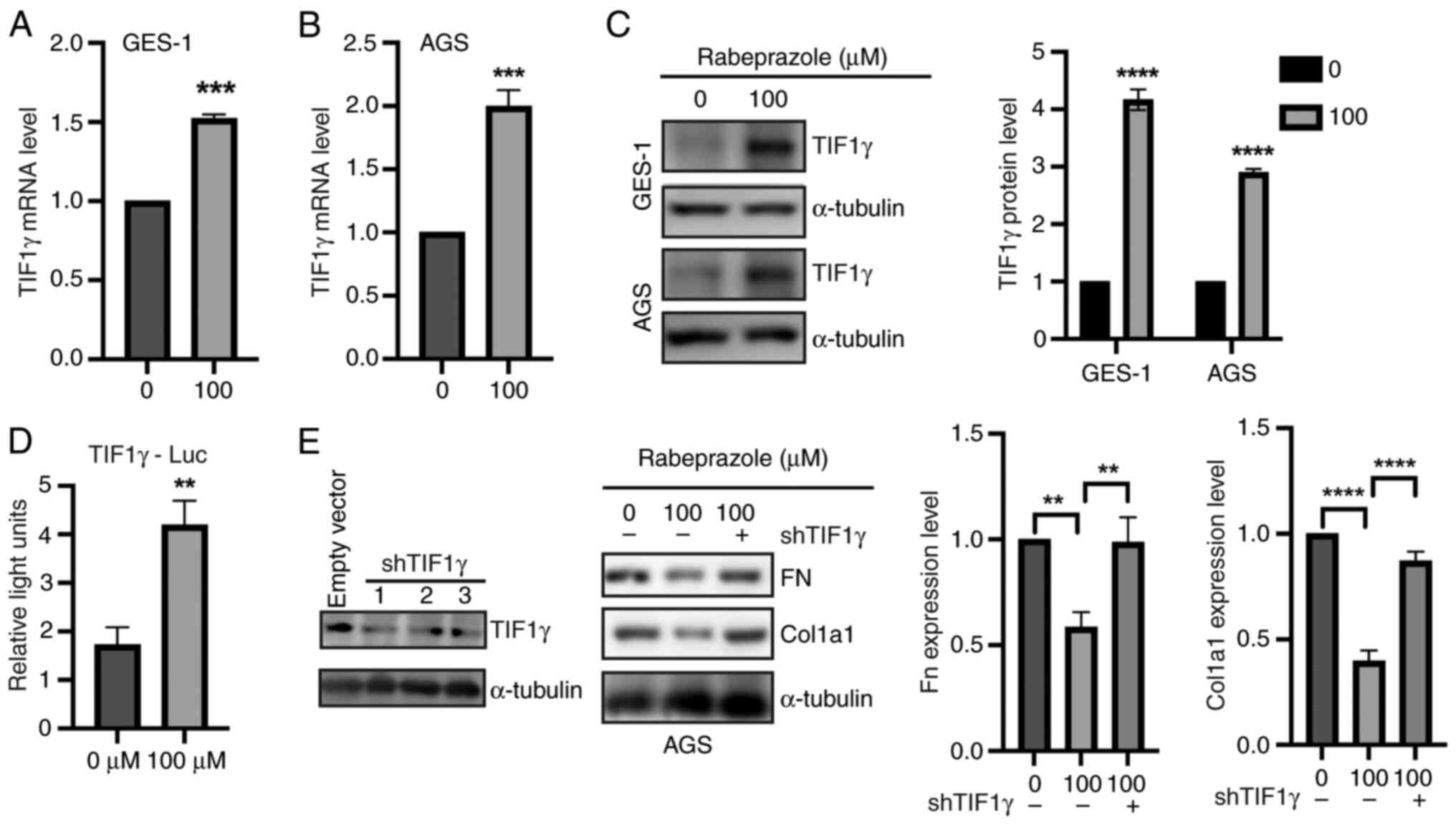
Since TIF1γ was reported to suppress extracellular
matrix (ECM) production by inhibiting TGF-β1 transcriptional
response (25-27),
the present study focused on investigating the possible effect of
rabeprazole on TIF1γ expression in gastric epithelial cells. Based
on the previous results, treatment with 100 µM rabeprazole was
selected for further experiments. Compared with the control group
(Fig. 2A and B), the mRNA levels of TIF1γ were
significantly increased in AGS and GES-1 cells in response to
rabeprazole treatment. In addition, the protein expression level of
TIF1γ was significantly enhanced by rabeprazole treatment (Fig. 2C). Moreover, dual luciferase
reporter experiments further revealed that TIF1γ transactivation
was significantly increased in response to rabeprazole treatment
(Fig. 2D). Most importantly,
depletion of TIF1γ expression by shRNA plasmid-targeted TIF1γ could
rescue the effect of rabeprazole on FN and Col1a1 protein
expression (Fig. 2E). These results
indicated that rabeprazole inhibited the ECM through upregulation
of TIF1γ expression.
Rabeprazole inhibits SMAD3
phosphorylation and nuclear translocation
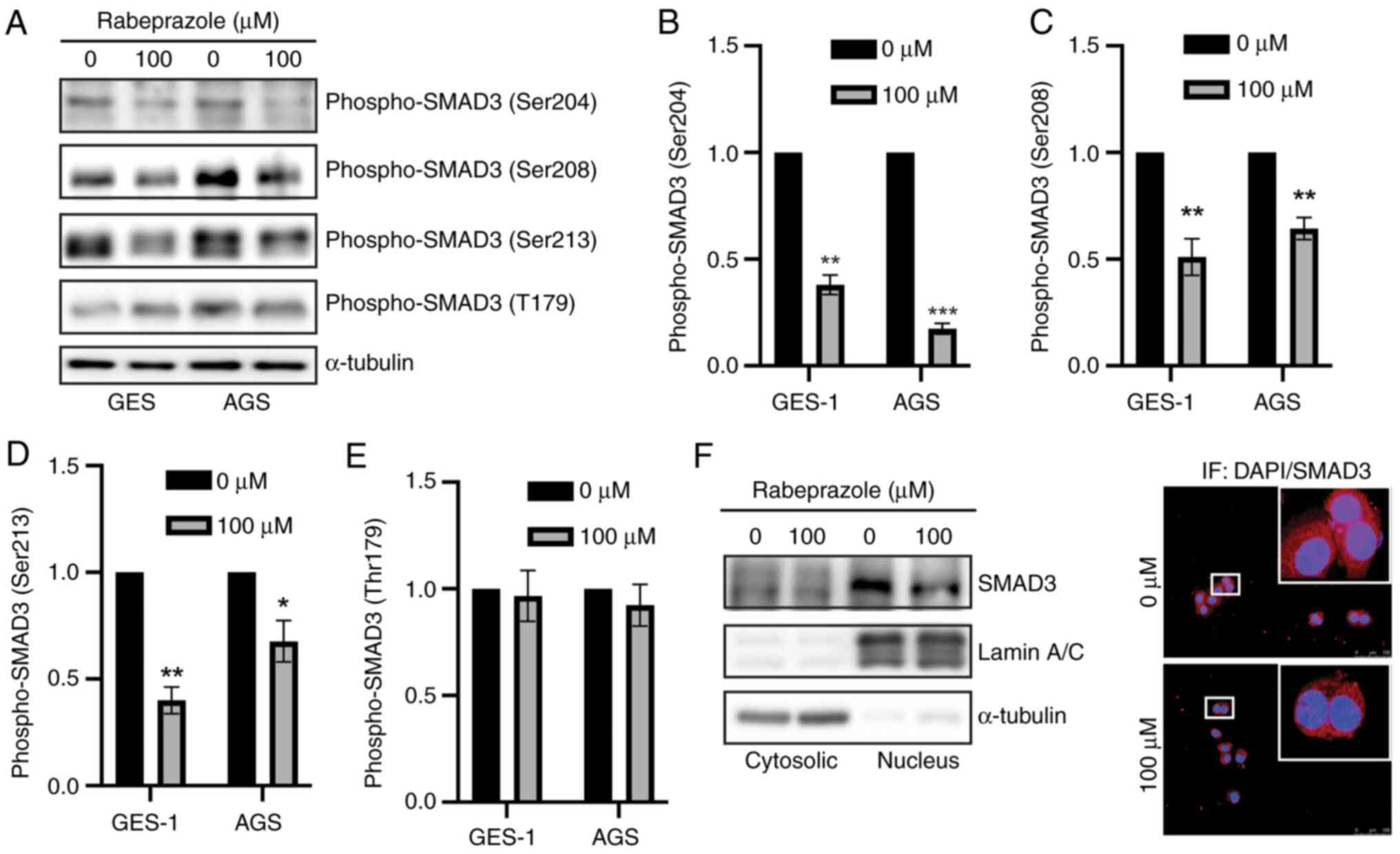
It is well known that SMAD3 phosphorylation, whether
in the linker domain or at the C-terminus, promotes SMAD3 nuclear
translocation to initiate gene transcription (16). Notably, a reciprocal inhibition
existed between phosphorylation of the SMAD3 linker domain and
C-terminus, which was attributed to the fact that SMAD3
phosphorylation at the linker domain could induce SMAD3 nuclear
shuttling, thereby inhibiting the accessibility of
non-phosphorylated SMAD3 to membrane-anchored TGFβRI (28).
The present results demonstrated that rabeprazole
attenuated fibrosis, prompting an exploration of the influence of
rabeprazole on SMAD3 activation. Through immunoblotting
quantification, it was found that, in gastric epithelial cells,
rabeprazole treatment led to a downregulation of SMAD3
phosphorylation at Ser204, Ser208 and Ser213, while no significant
changes were observed at Thr179 of the SMAD3 linker domain
(Fig. 3A-E). Moreover, subcellular
fraction combined with immunofluorescence analysis further showed
that the nuclear SMAD3 level was reduced in response to rabeprazole
(Fig. 3F). Overall, rabeprazole
inhibited SMAD3 phosphorylation and nuclear translocation.
Rabeprazole disrupts the interaction
between SMAD3 and TIF1γ
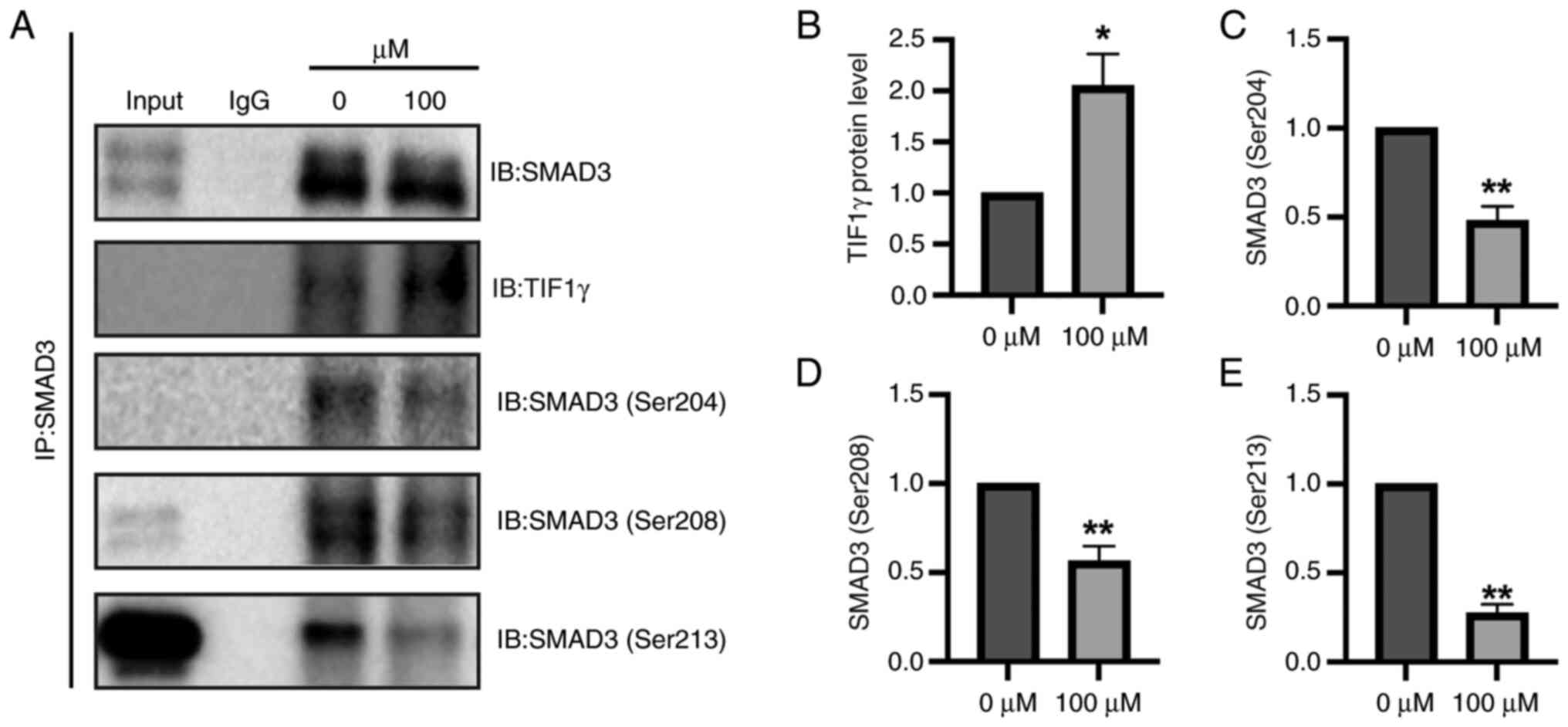
The present study aimed to elucidate the mechanism
by which rabeprazole influences fibrotic processes. SMAD3 was
identified as a key transcriptional regulator of Col1a1,
contributing to fibrosis via TGFβ-dependent signaling pathways
(29). Notably, TIF1γ was shown to
mitigate ECM accumulation by interacting with SMAD3, thereby
attenuating fibrotic responses (12). These findings suggest that SMAD3 may
play a pivotal role in the antifibrotic effects mediated by
rabeprazole-induced TIF1γ activity. On this basis, the present
study aimed to explore the possible relationship between SMAD3 and
TIF1γ. As shown in Fig. 4A,
endogenous SMAD3 interacted with TIF1γ, and this interaction was
enhanced in response to rabeprazole. In line with this,
immunoprecipitation assays further revealed that rabeprazole
treatment led to a significantly enhanced SMAD3/TIF1γ complex,
which in turn further inhibited SMAD3 linker phosphorylation,
including Ser204, Ser208 and Ser213 (Fig. 4A-E), consistent with our other
research work showing that overexpression of TIF1γ led to
inhibition of SMAD3 phosphorylation (Li et al unpublished
data). These results indicated that rabeprazole treatment led to an
antifibrotic TIF1γ/SMAD3 complex.
Discussion
Fibrosis is a common pathological change in various
diseases, including chronic hepatitis B-related liver diseases
(30) and gastrointestinal-related
diseases (31). Previous studies
demonstrated that rabeprazole has various biological functions
(9,29,32,33),
however, the present study further revealed a novel mechanism of
rabeprazole in fibrosis. The present study found that rabeprazole
exerted an inhibitory effect on fibrosis, which was attributed to
enhanced TIF1γ expression, while enhanced TIF1γ expression could
inhibit SMAD3 linker phosphorylation. Further analysis showed that
TIF1γ interacted with SMAD3, leading to suppression of SMAD3
signaling characterized by decreased phosphorylation of SMAD3
linker at Ser213, Ser204 and Ser208, and rabeprazole treatment
enhanced this interaction between TIF1γ and SMAD3 to aggravate
SMAD3 linker phosphorylation inhibition to alleviate fibrosis. This
finding not only enriched the knowledge on the biological function
of rabeprazole, but also the provided a possible therapeutic
strategy for fibrosis.
Rabeprazole, a well-known PPI, was reported to
induce M2-type adipose tissue macrophages, alleviating chronic
inflammation (18), and to improve
the survival rate and ameliorate pathological damage in
Clostridium perfringens or perfringolysin O (PFO)-treated
Galleria mellonella (34).
Moreover, rabeprazole was shown to have anti-Trypanosoma
cruzi activity by targeting cellular triosephosphate isomerase
(35). Rabeprazole has been
demonstrated to destroy gastric epithelial barrier function in
vivo and in vitro (20,36),
while exhibiting nephroprotective effects through inhibition of
organic cation transporter 2(37)
as well as promoting vascular impairment through hypoxia-inducible
factor 1-α (38). The present study
further investigated the antifibrotic function of rabeprazole. In
previous studies, rabeprazole was demonstrated to induce enhanced
TIF1γ expression, leading to suppression of TGFβ signaling.
Notably, phosphorylation of TIF1γ at Tyr-524, Tyr-610, and Tyr-1048
was found to destroy the complex between TIF1γ and SMAD3, which
could enhance TGFβ signaling (17,39).
Therefore, further investigation was required to reveal the
mechanism through which rabeprazole regulated TIF1γ expression.
However, the influence of rabeprazole on TIF1γ phosphorylation
remains unclear and will require further investigations. In
addition, the present study lacked in vivo experiments
confirming the possible role of rabeprazole in fibrosis.
Furthermore, phosphorylation of SMAD3, at both the linker domain
and C-terminus, is a critical event for the initiation of fibrosis
or EMT (40). Phosphorylation of
SMAD3 at Ser204, Ser208, and Ser213 was observed to be
significantly reduced in response to rabeprazole stimulation, and
this phenomenon was attributed to the formation of an enhanced
complex between TIF1γ and SMAD3 caused by rabeprazole. However, the
specific domain and phosphorylation status of TIF1γ that facilitate
its interaction with SMAD3, particularly under rabeprazole
treatment, remain unclear. In addition, considering that the
disease and tumor microenvironment are enriched with diverse
inflammatory and immune cells, including macrophages and
fibroblasts, our future work will employ progressively complex
in vitro systems, ranging from spheroid and organoid-based
co-cultures to air-liquid interface organoids and microfluidic
organoid-on-a-chip platforms. These models will be used to
investigate whether rabeprazole plays a role in remodeling the
cellular microenvironment (41,42).
In summary, the present study revealed a novel
antifibrotic role of rabeprazole in vitro, mediated through
the formation of a TIF1γ-SMAD3 complex. These findings offer novel
insights into the biological functions of rabeprazole and suggest
its potential as an alternative therapeutic strategy for
fibrosis-related diseases.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Project in the Field of Social Development of Zhuhai
(grant. no. 2220004000296).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
LL, XT and JC designed the experiments. LL, ZL and
LF performed the experiments and data collection. LL, YC and XT
analyzed the data and generated the figures. LL, XT and JC drafted
the manuscript and revised the manuscript. LL and XT confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luo L, Zhang W, You S, Cui X, Tu H, Yi Q,
Wu J and Liu O: The role of epithelial cells in fibrosis:
Mechanisms and treatment. Pharmacol Res. 202(107144)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Li Y, Ren BX, Li HM, Lu T, Fu R and Wu ZQ:
Omeprazole suppresses aggressive cancer growth and metastasis in
mice through promoting Snail degradation. Acta Pharmacol Sin.
43:1816–1828. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang B, Ling T, Zhaxi P, Cao Y, Qian L,
Zhao D, Kang W, Zhang W, Wang L, Xu G and Zou X: Proton pump
inhibitor pantoprazole inhibits gastric cancer metastasis via
suppression of telomerase reverse transcriptase gene expression.
Cancer Lett. 452:23–30. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Feng S, Zheng Z, Feng L, Yang L, Chen Z,
Lin Y, Gao Y and Chen Y: Proton pump inhibitor pantoprazole
inhibits the proliferation, self-renewal and chemoresistance of
gastric cancer stem cells via the EMT/β-catenin pathways. Oncol
Rep. 36:3207–3214. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang B, Yang Y, Shi X, Liao W, Chen M,
Cheng AS, Yan H, Fang C, Zhang S, Xu G, et al: Proton pump
inhibitor pantoprazole abrogates adriamycin-resistant gastric
cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin
signaling and Epithelial-mesenchymal transition. Cancer Lett.
356:704–712. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Babu D, Mudiraj A, Yadav N, Y B V K C,
Panigrahi M and Prakash Babu P: Rabeprazole has efficacy per se and
reduces resistance to temozolomide in glioma via EMT inhibition.
Cell Oncol (Dordr). 44:889–905. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dooley S, Said HM, Gressner AM, Floege J,
En-Nia A and Mertens PR: Y-box protein-1 is the crucial mediator of
antifibrotic interferon-gamma effects. J Biol Chem. 281:1784–1795.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Higashi K, Inagaki Y, Fujimori K, Nakao A,
Kaneko H and Nakatsuka I: Interferon-gamma interferes with
transforming growth factor-beta signaling through direct
interaction of YB-1 with Smad3. J Biol Chem. 278:43470–43479.
2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee EJ, Hwang I, Lee JY, Park JN, Kim KC,
Kim I, Moon D, Park H, Lee SY, Kim HS, et al: Hepatic stellate
cell-specific knockout of transcriptional intermediary factor 1γ
aggravates liver fibrosis. J Exp Med. 217(e20190402)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ikeuchi Y, Dadakhujaev S, Chandhoke AS,
Huynh MA, Oldenborg A, Ikeuchi M, Deng L, Bennett EJ, Harper JW,
Bonni A and Bonni S: TIF1γ protein regulates epithelial-mesenchymal
transition by operating as a small ubiquitin-like modifier (SUMO)
E3 ligase for the transcriptional regulator SnoN1. J Biol Chem.
289:25067–25078. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Su Z, Sun Z, Wang Z, Wang S, Wang Y, Jin
E, Li C, Zhao J, Liu Z, Zhou Z, et al: TIF1γ inhibits lung
adenocarcinoma EMT and metastasis by interacting with the TAF15/TBP
complex. Cell Rep. 41(111513)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Matsuzaki K: Smad3 phosphoisoform-mediated
signaling during sporadic human colorectal carcinogenesis. Histol
Histopathol. 21:645–662. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ooshima A, Park J and Kim SJ:
Phosphorylation status at Smad3 linker region modulates
transforming growth factor-β-induced Epithelial-mesenchymal
transition and cancer progression. Cancer Sci. 110:481–488.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
He W, Dorn DC, Erdjument-Bromage H, Tempst
P, Moore MA and Massague J: Hematopoiesis controlled by distinct
TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell.
125:929–941. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li Y, Hao J, Kong X, Yuan W, Shen Y, Hui Z
and Lu X: Rabeprazole mitigates obesity-induced chronic
inflammation and insulin resistance associated with increased
M2-type macrophage polarization. Biochim Biophys Acta Mol Basis
Dis. 1870(167142)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen SQ, Hu BF, Yang YR, He Y, Yue L, Guo
D, Wu TN, Feng XW, Li Q, Zhang W and Wen JG: The protective effect
of rabeprazole on cisplatin-induced apoptosis and necroptosis of
renal proximal tubular cells. Biochem Biophys Res Commun.
612:91–98. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang F, Li L, Zhou Y, Pan W, Liang X,
Huang L, Huang J, Cheng Y, Geng L, Xu W and Gong S: Rabeprazole
destroyed gastric epithelial barrier function through
FOXF1/STAT3-mediated ZO-1 expression. Clin Exp Pharmacol Physiol.
50:516–526. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Son M, Park IS, Kim S, Ma HW, Kim JH, Kim
TI, Kim WH, Han J, Kim SW and Cheon JH: Novel Potassium-competitive
acid blocker, tegoprazan, protects against colitis by improving gut
barrier function. Front Immunol. 13(870817)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou Y, Chen S, Yang F, Zhang Y, Xiong L,
Zhao J, Huang L, Chen P, Ren L, Li H, et al: Rabeprazole suppresses
cell proliferation in gastric epithelial cells by targeting
STAT3-mediated glycolysis. Biochem Pharmacol.
188(114525)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Niu R, Lan J, Liang D, Xiang L, Wu J,
Zhang X, Li Z, Chen H, Geng L, Xu W, et al: GZMA suppressed
GPX4-mediated ferroptosis to improve intestinal mucosal barrier
function in inflammatory bowel disease. Cell Commun Signal.
22(474)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li P, Wu Y, Deng Z, Samad A, Xi Y, Song J,
Zhang Y, Li J, Zhou YA, Xiong Q and Wu C: Two novel SH3TC2
mutations predispose to Charcot-Marie-Tooth disease type 4C by
mistargeting away from TFRC. Cell Signal.
130(111669)2025.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hesling C, Fattet L, Teyre G, Jury D,
Gonzalo P, Lopez J, Vanbelle C, Morel AP, Gillet G, Mikaelian I and
Rimokh R: Antagonistic regulation of EMT by TIF1γ and Smad4 in
mammary epithelial cells. EMBO Rep. 12:665–672. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qi G, Lu G, Yu J, Zhao Y, Wang C, Zhang H
and Xia Q: Up-regulation of TIF1γ by valproic acid inhibits the
epithelial mesenchymal transition in prostate carcinoma through
TGF-β/Smad signaling pathway. Eur J Pharmacol.
860(172551)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yin X, Xu C, Zheng X, Yuan H, Liu M, Qiu Y
and Chen J: SnoN suppresses TGF-β-induced epithelial-mesenchymal
transition and invasion of bladder cancer in a TIF1γ-dependent
manner. Oncol Rep. 36:1535–1541. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sun YM, Wu Y, Li GX, Liang HF, Yong TY, Li
Z, Zhang B, Chen XP, Jin GN and Ding ZY: TGF-β downstream of Smad3
and MAPK signaling antagonistically regulate the viability and
partial epithelial-mesenchymal transition of liver progenitor
cells. Aging (Albany NY). 16:6588–6612. 2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zheng M, Li H, Sun L, Cui S, Zhang W, Gao
Y and Gao R: Calcipotriol abrogates TGF-β1/pSmad3-mediated collagen
1 synthesis in pancreatic stellate cells by downregulating RUNX1.
Toxicol Appl Pharmacol. 491(117078)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xiao J, Liu H, Yao J, Yang S, Shen F, Bu
K, Wang Z, Liu F, Xia N and Yuan Q: The characterization of serum
proteomics and metabolomics across the cancer trajectory in chronic
hepatitis B-related liver diseases. View: 5, 2024.
|
|
31
|
Ito T and Kayama H: Roles of fibroblasts
in the pathogenesis of inflammatory bowel diseases and
IBD-associated fibrosis. Int Immunol. 37:377–392. 2025.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xie J, Liang X, Xie F, Huang C, Lin Z, Xie
S, Yang F, Zheng F, Geng L, Xu W, et al: Rabeprazole suppressed
gastric intestinal metaplasia through activation of GPX4-mediated
ferroptosis. Front Pharmacol. 15(1409001)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gu M, Zhang Y, Zhou X, Ma H, Yao H and Ji
F: Rabeprazole exhibits antiproliferative effects on human gastric
cancer cell lines. Oncol Lett. 8:1739–1744. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang G, Liu Y, Deng L, Liu H, Deng X, Li
Q, Feng H, Guo Z and Qiu J: Repurposing rabeprazole sodium as an
anti-Clostridium perfringens drug by inhibiting
perfringolysin O. J Appl Microbiol. 134:2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Garcia-Torres I, De la Mora-De la Mora I,
Lopez-Velazquez G, Cabrera N, Flores-López LA, Becker I,
Herrera-López J, Hernández R, Pérez-Montfort R and Enríquez-Flores
S: Repurposing of rabeprazole as an anti-Trypanosoma cruzi
drug that targets cellular triosephosphate isomerase. J Enzyme
Inhib Med Chem. 38(2231169)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Takashima S, Tanaka F, Kawaguchi Y, Usui
Y, Fujimoto K, Nadatani Y, Otani K, Hosomi S, Nagami Y, Kamata N,
et al: Proton pump inhibitors enhance intestinal permeability via
dysbiosis of gut microbiota under stressed conditions in mice.
Neurogastroenterol Motil. 32(e13841)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sharaf G, E ME and El-Sayed EK: Augmented
nephroprotective effect of liraglutide and rabeprazole via
inhibition of OCT2 transporter in cisplatin-induced nephrotoxicity
in rats. Life Sci. 321(121609)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Evans CE, Peng Y, Zhu MM, Dai Z, Zhang X
and Zhao YY: Rabeprazole promotes vascular repair and resolution of
Sepsis-induced inflammatory lung injury through HIF-1α. Cells.
11(1425)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yuki R, Tatewaki T and Yamaguchi N, Aoyama
K, Honda T, Kubota S, Morii M, Manabe I, Kuga T, Tomonaga T and
Yamaguchi N: Desuppression of TGF-β signaling via nuclear
c-Abl-mediated phosphorylation of TIF1γ/TRIM33 at Tyr-524, -610,
and -1048. Oncogene. 38:637–655. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Matsuzaki K: Smad phospho-isoforms direct
context-dependent TGF-β signaling. Cytokine Growth Factor Rev.
24:385–399. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhou PZ, Gao L, Wang LW, Zhang YF, Song WL
and Hao YX: Clinical observation of magnesium aluminum carbonate
combined with rabeprazole-based triple therapy in the treatment of
helicobacter pylori-positive gastric ulcer associated with
hemorrhage. Pak J Med Sci. 38:1271–1277. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yuan K, Du X, Dong L, Pan J and Xue W:
Modelling the tumor microenvironment in vitro in prostate cancer:
Current and future perspectives. VIEW:. 5(20240074)2024.
|