Introduction
Metabolic dysfunction-associated steatotic liver
disease (MASLD), formerly known as non-alcoholic fatty liver
disease, has become a global health concern (1), particularly in Asia, 41% of cases
occur in people with BMI <25 kg/m2, and 19% in those
with BMI <23 kg/m2, with no significant difference in
liver histology compared with higher-BMI groups (2). In Taiwan, the prevalence of metabolic
syndrome (MS), a condition associated with MASLD, increased from
11.5% during 2003-2004 (3,4) to more than one-third of adults in
2017-2020, increasing with age (5).
Numerous studies have explored established factors, including being
overweight, hyperglycemia, insulin resistance, energy imbalance,
metabolic inflammation, an unhealthy diet and a sedentary
lifestyle, that contribute to these conditions (1,3).
However, reliable biomarkers for MASLD, particularly for early
detection or risk prediction in asymptomatic individuals, remain
elusive, as emphasized in the latest Asian Pacific Association for
the Study of the Liver guidelines (2). This critical gap underscores the need
to explore novel pathogenic mechanisms. The role of
gastroenteropancreatic hormones (GEPHs) in the early pathogenesis
of MASLD, especially among seemingly healthy females, a key target
for prevention, is poorly understood. The present study aimed to
investigate GEPH profiles in this population.
GEPHs serve a crucial role in the regulation of
appetite, body weight and metabolic homeostasis, acting as key
mediators between the central nervous system and peripheral tissue
(6,7). Ghrelin exists in two forms: Acyl
ghrelin stimulates appetite, while des-acyl ghrelin, previously
thought to be inactive, suppresses appetite (8,9).
Des-acyl ghrelin possesses broad metabolic protective properties
beyond appetite suppression, including anti-inflammatory,
anti-fibrotic and insulin-sensitizing effects in multiple tissues
(10,11). In addition to ghrelin, other GEPHs,
regulate satiety, energy balance and metabolic dysfunction
(12-14),
including obestatin, nesfatin-1, cholecystokinin (CCK), gastric
inhibitory peptide (GIP), glucagon-like peptide 1 (GLP-1),
oxyntomodulin (OXM), peptide YY (PYY), pancreatic polypeptide (PP)
and amylin. Understanding the interactions between these hormones
and their metabolic effects is key for designing preventive and
targeted therapies for MASLD and metabolic disorder. However,
comprehensive studies simultaneously evaluating multiple GEPHs to
identify the most salient predictor of MASLD risk within a specific
ethnic population are currently lacking.
The present study employed correlation, regression
and path analysis to explore the connections and potential causal
association between a broad panel of 11 GEPHs and MASLD in
seemingly healthy females. The primary objective was to identify
the most influential biomarkers predicting MASLD.
Materials and methods
Study design and data collection
The present study adopted a hospital-based
prospective observational design, in accordance with the Helsinki
Declaration, approved by the Institutional Review Board, Cheng Hsin
General Hospital, Taipei, Taiwan [approval nos. (665) 107A-37,
(732) 108A-48 and (736) 108A-52] and was performed according to the
Association for the Accreditation of Human Research Protection
Program guidelines (aahrpp.org/).
Subjects were randomly selected from individuals undergoing
voluntary health check-ups at the Cheng Hsin Healthy Management
Center, Taipei, Taiwan between June 2019 and July 2020. Initial
recruitment included 150 participants, comprising 139 females and
11 males. The objective was to identify the most influential
biomarkers predicting MASLD in the apparently healthy population.
However, the small number of male participants precluded meaningful
sex-stratified analysis or reliable adjustment for sex as a
potential confounder. Therefore, the present study investigated
only the female patients aged 18-65 years old. The inclusion
criteria were age ≥18 years and complete anthropometric measurement
and laboratory data; exclusion criterion was a history of alcohol
consumption.
‘Apparently healthy’ was operationally defined as:
i) No prior diagnosis of chronic diseases requiring medication; ii)
no hospitalization in the past 6 months and iii) voluntarily
participating in health check-ups. A standardized questionnaire
collected basic information, such as sex, age and medical history.
Height, weight, waist circumference (WC) and blood pressure were
measured. The following data were obtained from the database of the
health examination center: Fasting blood sugar (FBS), triglyceride
(TG), total cholesterol (TC), low-density lipoprotein cholesterol
(LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine
aminotransferase (ALT) and laboratory tests for acyl ghrelin,
des-acyl ghrelin, obestatin, nesfatin-1, CCK, GIP, GLP-1, OXM, PP,
PYY and amylin. Body mass index (BMI), an indicator used to
identify obese individuals, was calculated as body weight
(kg)/[body height (m)]2 (15). The TG/HDL-C ratio is indicative of
insulin resistance (16,17). The diagnosis of MS criteria was made
when at least three of the five following risk determinants were
present: WC ≥80 cm; blood pressure >130/85 mmHg or use of
antihypertensive medications; HDL-C <50 mg/dl; FBS ≥100 mg/dl or
regular treatment for diabetes mellitus and TG ≥150 mg/dl (5). Lipid accumulation product (LAP) was
employed as a blood-based surrogate marker for MASLD, serving as a
non-invasive alternative to imaging or liver biopsy. LAP was
calculated as: [(WC (cm)-58) x TG (mmol/l)] (18,19).
WC values ≤58 cm were adjusted to 59 cm to ensure a non-positive
LAP value. Abnormal metabolic indicators were defined as FBS ≥126
mg/dl, TG ≥150 mg/dl and HDL-C <50 mg/dl.
Measurement of serum GEPHs
Enzyme immunoassays for hormones were performed
according to the manufacturers' protocols in a single, blinded
batch using commercial kits. These included acyl ghrelin, des-acyl
ghrelin (A05106, A05119; all Bertin Pharma), obestatin, CCK, GIP,
GLP-1, OXM, PYY, amylin (S-1284, S-1205, S-1273, S-1359, S-1272,
S-1187; all Bachem), nesfatin-1and OXM (EK-003-26, EK-028-22;
bothPhoenix Pharmaceuticals, Inc) (19).
Statistical analysis
The analysis was conducted using Social Sciences
Statistics Package version 20.0 (IBM Corp.). Data are presented as
mean ± SD for continuous variables. Bivariate correlation analysis
was performed using Spearman's correlation to assess the
association between age, GEPHs (including acyl ghrelin, des-acyl
ghrelin, obestatin, nesfatin-1, CCK, GIP, GLP-1, OXM, PYY, PP and
amylin) and metabolic parameters (BMI, TG/HDL-C ratio, LAP and
ALT). SAS 9.4 (SAS Institute, Inc.) software was applied for
regression and path analysis. For multivariable analysis, separate
forward-selected regression models were constructed for each
metabolic outcome, including BMI, WC, FBS, TG, TC, LDL-C and HDL-C.
A total of 12 candidate predictors, comprising age and 11 GEPHs,
were evaluated. Variables were retained in the final model only if
they met the criteria of P<0.05, ΔR² >3% and variance
inflation factor (VIF) <5 to ensure both statistical and
explanatory robustness while minimizing multicollinearity. Notably,
predictors showing significant bivariate correlations were excluded
from the final multivariate models if they no longer contributed
independently after adjusting for other covariates or if they
exhibited excessive shared variance (VIF ≥5). Forward regression
was employed to identify significant predictor variables for
inclusion in the subsequent path analysis. Model fit was evaluated
to determine how well the proposed structure aligned with the
observed data (20,21).
GEPHs retained in the final model (des-acyl ghrelin,
and obestatin) and age underwent exploratory path analysis,
Mahalanobis distance-based classification was applied. P<0.05
was considered to indicate a statistically significant
difference.
Results
Characteristics of the study
subjects
The study cohort consisted of 139 female
participants with a mean age of 38.4±10.1 years, BMI of 23.1±3.8
kg/m2 and WC of 73.2±9.4 cm (Table I). Notably, 9.4% of these
participants met the Taiwanese MS criteria, despite being recruited
from the healthy management center without prior diagnosis of major
chronic disease. This finding highlights the potential
underdiagnosis of metabolic risk in apparently healthy females and
underscores the value of early metabolic screening in normal-weight
individuals (mean BMI <24 kg/m2).
 | Table ICharacteristics of the study
subjects. |
Table I
Characteristics of the study
subjects.
| Variable | All | Non-MS (n=126) | MS (n=13) | P-value |
|---|
| Age, years | 38.4±10.1 | 37.9±10.1 | 43.6±9.3 | 0.050 |
| BMI,
kg/m2 | 23.1±3.8 | 22.4±3.3 | 29.0±2.6 | <0.001 |
| WC, cm | 73.2±9.4 | 71.4±7.9 | 89.8±5.0 | <0.001 |
| FBS, mg/dl | 95.0±11.3 | 93.3±8.8 | 111.4±18.6 | <0.001 |
| TG, mg/dl | 77.0±50.7 | 71.4±46.7 | 130.7±57.8 | <0.001 |
| TC, mg/dl | 193.7±34.9 | 192.5±33.1 | 205.9±49.1 | 0.190 |
| LDL-C, mg/dl | 117.2±32.1 | 114.7±30.1 | 141.1±42.0 | 0.040 |
| HDL-C, mg/dl | 60.4±13.3 | 62.1±12.8 | 44.5±5.6 | <0.001 |
| ALT, U/l | 19.2±14.7 | 17.1±7.9 | 40.2±36.4 | <0.001 |
| TG/HDL-C ratio | 1.4±1.3 | 1.3±1.1 | 3.0±1.4 | <0.001 |
| LAP, cm mmol/l | 14.9±17.0 | 11.6±11.8 | 47.6±24.3 | <0.001 |
| Acyl ghrelin,
pg/ml | 50.3±30.0 | 51.0±29.6 | 43.5±34.5 | 0.392 |
| Des-acyl ghrelin,
pg/ml | 341.2±199.5 | 353.8±199.9 | 219.8±154.7 | 0.021 |
| Obestatin,
ng/ml | 2.6±1.3 | 2.6±1.3 | 2.4±0.8 | 0.645 |
| Nesfatin-1,
ng/ml | 1.0±6.1 | 1.0±6.4 | 0.2±0.2 | 0.630 |
| CCK, ng/ml | 2.4±5.5 | 2.4±5.8 | 2.1±1.9 | 0.824 |
| GIP, ng/ml | 0.3±0.2 | 0.3±0.2 | 0.3±0.2 | 0.843 |
| GLP-1, ng/ml | 0.05±0.05 | 0.05±0.04 | 0.08±0.11 | 0.022 |
| OXM, ng/ml | 0.9±0.3 | 0.9±0.3 | 0.9±0.2 | 0.655 |
| PYY, ng/ml | 0.6±0.3 | 0.6±0.3 | 0.6±0.2 | 0.782 |
| PP, ng/ml | 0.2±0.8 | 0.2±0.8 | 0.2±0.4 | 0.931 |
| Amylin, ng/ml | 0.8±0.7 | 0.8±0.8 | 0.7±0.6 | 0.828 |
Patients with MS were older and had significantly
higher BMI, WC, FBS, TG, LDL-C, ALT, TG/HDL-C ratio, LAP and GLP-1,
as well as lower HDL-C and des-acyl ghrelin levels, than non-MS
subjects (Table I).
Association between GEPHs and
metabolic factors
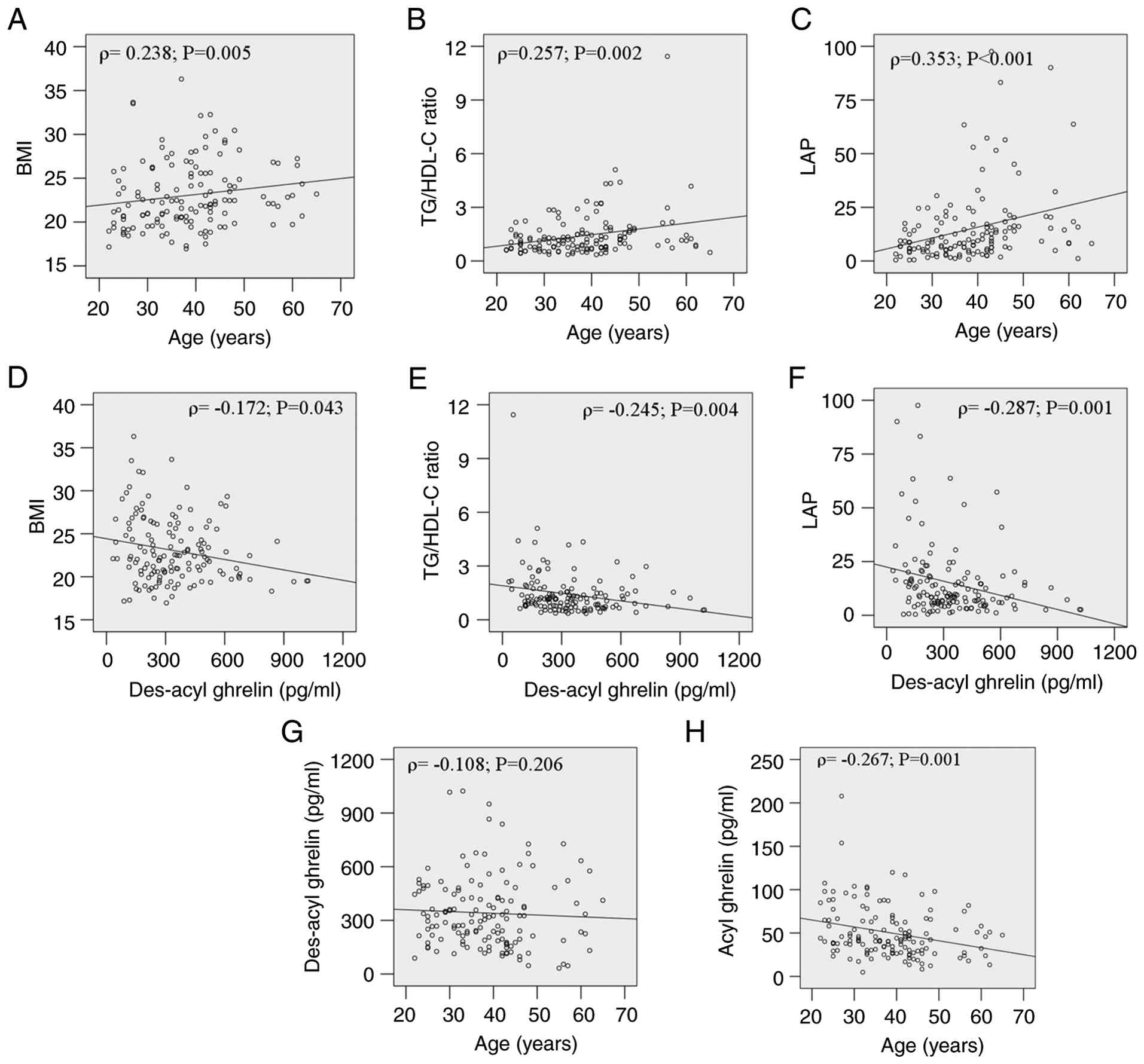
Bivariate correlation analysis revealed significant
associations between age, GEPH and metabolic outcomes (Table II; Fig.
1). Age, serum acyl ghrelin and des-acyl ghrelin correlated
positively with BMI and TG/HDL-C ratio, while age, serum acyl
ghrelin, des-acyl ghrelin and obestatin were correlated with LAP.
Age also had a significant relationship with ALT. No correlation
existed between age and des-acyl ghrelin (Fig. 1G), while acyl-ghrelin declined
significantly with age (Fig. 1H).
Despite these bivariate associations, forward-selected multivariate
regression models using age and all GEPHs as candidate variables
identified independent predictors for metabolic abnormalities
(Table III). Age positively
predicted BMI (β=0.060), WC (β=0.223), FBS, TG, TC and LDL-C.
Des-acyl ghrelin was independently associated with BMI (β=-0.004)
and WC (β=-0.014) but positively associated with HDL-C (β=0.023).
Obestatin predicted FBS (β=2.028). Notably, while significant in
the bivariate correlations (Table
II), acyl-ghrelin was not retained in the final
forward-selected model (Table
III) as it did not meet the statistical criterion for entry
after accounting for variance explained by the other variables.
Overall, age was the strongest predictor, associated with all
sevenmetabolic parameters . Among GEPHs, des-acyl ghrelin showed
the significant associations with the highest number of metabolic
parameters (3/7), while other GEPHs showed significant associations
with ≤1 parameter (Table
III).
 | Table IICorrelations between variables and
metabolic factors. |
Table II
Correlations between variables and
metabolic factors.
| | BMI | TG/HDL-C ratio | LAP | ALT |
|---|
| Factor | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value |
|---|
| Age | 0.238 | 0.005 | 0.257 | 0.002 | 0.353 | <0.001 | 0.375 | <0.001 |
| Acyl ghrelin | -0.194 | 0.022 | -0.245 | 0.004 | -0.282 | 0.001 | -0.049 | 0.567 |
| Des-acyl
ghrelin | -0.172 | 0.043 | -0.245 | 0.004 | -0.287 | 0.001 | -0.046 | 0.595 |
| Obestatin | -0.128 | 0.134 | -0.162 | 0.057 | -0.235 | 0.005 | -0.035 | 0.685 |
| Nesfatin-1 | 0.066 | 0.438 | 0.064 | 0.454 | 0.112 | 0.189 | 0.022 | 0.801 |
| CCK | -0.052 | 0.547 | -0.119 | 0.162 | -0.129 | 0.131 | -0.008 | 0.927 |
| GIP | -0.058 | 0.497 | -0.029 | 0.733 | -0.082 | 0.339 | -0.041 | 0.633 |
| GLP-1 | -0.126 | 0.138 | -0.152 | 0.074 | -0.155 | 0.068 | -0.143 | 0.092 |
| OXM | -0.025 | 0.769 | 0.059 | 0.487 | -0.011 | 0.895 | 0.105 | 0.217 |
| PYY | -0.063 | 0.462 | 0.023 | 0.791 | -0.010 | 0.905 | 0.079 | 0.354 |
| PP | -0.002 | 0.977 | 0.071 | 0.404 | 0.055 | 0.519 | -0.103 | 0.229 |
| Amylin | -0.118 | 0.165 | 0.039 | 0.648 | -0.049 | 0.567 | -0.093 | 0.278 |
 | Table IIIVariables significantly associated
with age and gastroenteropancreatic hormones using forward-selected
regression model. |
Table III
Variables significantly associated
with age and gastroenteropancreatic hormones using forward-selected
regression model.
| | Age | Des-acyl
ghrelin | Obestatin |
|---|
| Variable | Estimated β | P-value | Estimated β | P-value | Estimated β | P-value |
|---|
| BMI | 0.060 | 0.050 | -0.004 | 0.015 | - | - |
| WC | 0.223 | 0.003 | -0.014 | <0.001 | - | - |
| FBS | 0.519 | <0.001 | - | - | 2.028 | 0.042 |
| TG | 1.440 | <0.001 | - | - | - | - |
| TC | 0.858 | 0.024 | - | - | - | - |
| LDL-C | 0.601 | 0.013 | - | - | - | - |
| HDL-C | - | - | 0.023 | <0.001 | - | - |
Potential mechanism of metabolic
abnormalities
Path analysis was performed to determine whether
age, des-acyl ghrelin and obestatin influence metabolic
abnormalities and contribute to the onset of MASLD through
mediators such as BMI and WC. The discriminating criteria for
metabolic abnormalities in female patients are BMI ≥24
kg/m2, WC ≥80 cm, FBS ≥126 mg/dl, TG ≥150 mg/dl, TC ≥200
mg/dl, LDL-C ≥100 mg/dl and HDL-C <50 mg/dl (4,5). Serum
des-acyl ghrelin levels were not significantly affected by age
(β=-0.003, P=0.560; Table IV). The
analyses for general metabolic abnormalities (Table IV) highlight the pervasive
influence of age, which was a significant predictor in 4 out of 7
final models (for WC, FBS, TG, and TC). In comparison, des-acyl
ghrelin was a significant predictor in 3 models (for BMI, WC, and
HDL-C). These findings were derived from a systematically
comparison of parallel models using either BMI or WC as the primary
adiposity measure. This approach revealed that adiposity measures
often served as both significant predictors and potential
mediators.
 | Table IVIdentification of metabolic
abnormality pathways using path analysis. |
Table IV
Identification of metabolic
abnormality pathways using path analysis.
| A, Age |
|---|
| Model | Variable | Standardized β | P-value | Model fitting
P-value |
|---|
| 1 | Des-acyl
ghrelin | -0.003 | 0.560 | 0.560 |
| 2 | Obestatin | -1.685 | 0.011 | 0.011 |
| 3 | Des-acyl
ghrelin | -0.001 | 0.737 | 0.038 |
| | Obestatin | -1.663 | 0.013 | |
| B, BMI |
| Model | Variable | Standardized β | P-value | Model fitting
P-value |
| 1 | Des-acyl
ghrelin | -0.004 | 0.012 | 0.012 |
| 2 | Obestatin | -0.233 | 0.352 | 0.352 |
| 3 | Age | 0.061 | 0.056 | 0.056 |
| 4 | Age | 0.055 | 0.088 | 0.022 |
| | Des-acyl
ghrelin | -0.004 | 0.017 | |
| | Obestatin | -0.081 | 0.748 | |
| C, WC |
| Model | Variable | Standardized β | P-value | Model fitting
P-value |
| 1 | Des-acyl
ghrelin | -0.014 | <0.001 | <0.001 |
| 2 | Obestatin | -1.159 | 0.061 | 0.061 |
| 3 | Age | 0.241 | 0.002 | 0.002 |
| 4 | Age | 0.212 | 0.006 | <0.001 |
| | Des-acyl
ghrelin | -0.013 | <0.001 | |
| | Obestatin | -0.592 | 0.314 | |
| D, FBS |
| Model | Variable | Standardized β | P-value | Model fitting
P-value |
| 1 | Age | 0.458 | <0.001 | <0.001 |
| 2 | BMI | 1.203 | <0.001 | <0.001 |
| 3 | WC | 0.481 | <0.001 | <0.001 |
| 4 | Obestatin | 0.950 | 0.025 | 0.025 |
| 5 | Age | 0.444 | <0.001 | <0.001 |
| | BMI | 1.064 | <0.001 | |
| | Obestatin | 1.956 | 0.003 | |
| 6 | Age | 0.414 | <0.001 | <0.001 |
| | WC | 0.412 | <0.001 | |
| | Obestatin | 2.134 | 0.001 | |
| E, TG |
| Model | Variable | Standardized β | P-value | Model fitting
P-value |
| 1 | Age | 1.424 | 0.007 | 0.007 |
| 2 | BMI | 3.710 | 0.001 | 0.001 |
| 3 | WC | 1.761 | <0.001 | <0.001 |
| 4 | Des-acyl
ghrelin | -0.042 | 0.056 | 0.056 |
| 5 | Age | 1.223 | 0.003 | <0.001 |
| | BMI | 2.882 | 0.010 | |
| | Des-acyl
ghrelin | -0.027 | 0.201 | |
| 6 | Age | 1.084 | 0.009 | <0.001 |
| | WC | 1.33 | 0.005 | |
| | Des-acyl
ghrelin | -0.021 | 0.334 | |
| F, TC |
| Model | Variable | Standardized β | P-value | Model fitting
P-value |
| 1 | Age | 0.840 | 0.004 | 0.004 |
| 2 | BMI | 1.547 | 0.056 | 0.056 |
| 3 | WC | 0.454 | 0.155 | 0.155 |
| 4 | Age | 0.767 | 0.009 | 0.005 |
| | BMI | 1.215 | 0.118 | |
| 5 | Age | 0.784 | 0.009 | 0.012 |
| | WC | 0.233 | 0.469 | |
| G, LDL-C |
| Model | Variable | Standardized β | P-value | Model fitting
P-value |
| 1 | Age | 0.840 | 0.004 | 0.004 |
| 2 | BMI | 1.547 | 0.056 | 0.056 |
| 3 | WC | 0.454 | 0.155 | 0.155 |
| 4 | Age | 0.516 | 0.057 | <0.001 |
| | BMI | 2.518 | <0.001 | |
| 5 | Age | 0.480 | 0.076 | 0.0013 |
| | WC | 0.783 | 0.008 | |
| H, HDL-C |
| Model | Variable | Standardized β | P-value | Model fitting
P-value |
| 1 | BMI | -1.376 | <0.001 | <0.001 |
| 2 | WC | -0.624 | <0.001 | <0.001 |
| 3 | Des-acyl
ghrelin | 0.025 | <0.001 | <0.001 |
| 4 | BMI | -1.204 | 0.004 | <0.001 |
| | Des-acyl
ghrelin | 0.015 | <0.001 | |
| 5 | WC | -0.480 | 0.018 | <0.001 |
| | Des-acyl
ghrelin | 0.023 | <0.001 | |
HDL-C (Table IV),
both BMI (β=-1.204) and WC (β=-0.480) were significant predictors
alongside des-acyl ghrelin. Furthermore, in the case of TG
(Table IV-E), the effect of age
was partially mediated by WC, as evidenced by the attenuation of
the direct effect co-efficient from β=1.424 to β=1.084 after
including WC.
A consistent finding across all these analyses was
that both the BMI-based and WC-based models demonstrated a poor
overall goodness-of-fit, indicating that the simple linear
structures proposed do not fully capture the complexity of the
metabolic interrelationships.
Potential mechanism of MASLD
To investigate the potential mechanism of MASLD,
path analyses were conducted for its key indicators, LAP and ALT,
systematically comparing parallel models that incorporated either
BMI or WC as the primary adiposity measure (Table V). The analyses for LAP revealed
clear patterns of full mediation (Table
V). The significant total effect of age on LAP (β=0.517) became
non-significant in the final model that included WC as a mediator.
Similarly, the significant total effect of des-acyl ghrelin on LAP
(β=-0.024) became non-significant in the final model that included
BMI as its mediator. For ALT (Table
V-B), LAP itself emerged as the most significant direct
predictor (β=0.338) and also functioned as a key mediator. The
initial significant association between age and ALT (β=0.311)
became non-significant after accounting for LAP. This finding was
consistent in both the BMI-based and WC-based models, where LAP
maintained a strong, direct path to ALT (final model β=0.338, ),
suggesting the effect of age on liver enzymes is transmitted
through liver fat accumulation. In summary, these analyses
elucidate the key role of age and serum des-acyl ghrelin in the
progression of MASLD. Their influence, however, appears to be
largely indirect. Adiposity measures like BMI and WC act as crucial
mediators, connecting these initial factors to downstream LAP,
which in turn impacts ALT. This highlights a potential cause for
MASLD progression (Fig. 2).
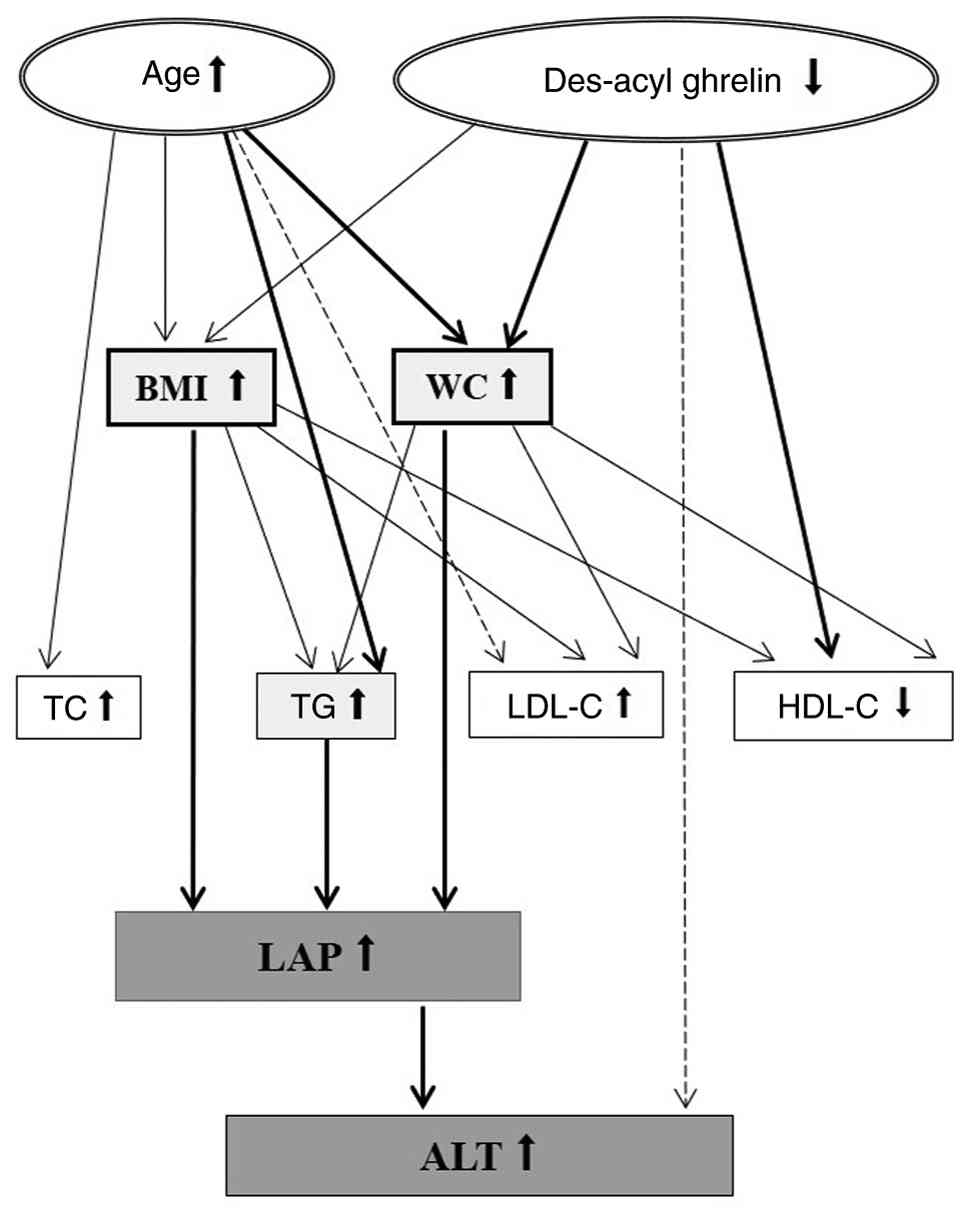 | Figure 2Pathways of MASLD development.
Upstream factors such as age and des-acyl ghrelin on BMI and WC are
associated with metabolic markers such as TG, LDL-C and HDL-C. BMI,
WC and TG promote LAP (an indicator for MASLD), which impacts ALT
levels and is an essential indicator of liver health. Thick lines
represent the high correlation (R2>0.6). The solid
and dotted lines indicate significant (P<0.05) and borderline
significant (0.05<P<0.06) results, respectively. BMI, body
mass index; WC, waist circumference; TG, triglyceride; TC, total
cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C,
high-density lipoprotein cholesterol; LAP, lipid accumulation
product; ALT, alanine aminotransferase; MASLD, metabolic
dysfunction-associated steatotic liver disease. |
 | Table VProposed physiological pathways
linking LAP and ALT identified through path analysis modeling. |
Table V
Proposed physiological pathways
linking LAP and ALT identified through path analysis modeling.
| A, LAP |
|---|
| Model | Variable | Standardized β | P-value | Model fitting
P-value |
|---|
| 1 | Age | 0.517 | <0.001 | <0.001 |
| 2 | Des-acyl
ghrelin | -0.024 | 0.003 | 0.003 |
| 3 | TG | 0.269 | <0.001 | <0.001 |
| 4 | TC | 0.152 | <0.001 | <0.001 |
| 5 | LDL-C | 0.176 | <0.001 | <0.001 |
| 6 | HDL-C | -0.572 | <0.001 | <0.001 |
| 7 | BMI | 2.931 | <0.001 | <0.001 |
| 8 | WC | 1.390 | <0.001 | <0.001 |
| 9 | Age | 0.076 | 0.235 | <0.001 |
| | TG | 0.218 | <0.001 | |
| | TC | -0.008 | 0.916 | |
| | LDL-C | 0.014 | 0.845 | |
| | HDL-C | -0.031 | 0.745 | |
| | BMI | 1.980 | <0.001 | |
| | Des-acyl
ghrelin | -0.003 | 0.296 | |
| 10 | Age | -0.035 | 0.452 | <0.001 |
| | TG | 0.214 | <0.001 | |
| | TC | -0.029 | 0.591 | |
| | HDL-C | 0.056 | 0.423 | |
| | LDL-C | 0.043 | 0.409 | |
| | WC | 1.038 | <0.001 | |
| | Des-acyl
ghrelin | 0.001 | 0.604 | |
| B, ALT |
| Model | Variable | Standardized β | P-value | Model fitting
P-value |
| 1 | Age | 0.311 | 0.012 | 0.012 |
| 2 | BMI | 1.562 | <0.001 | <0.001 |
| 3 | WC | 0.574 | <0.001 | <0.001 |
| 4 | Des-acyl
ghrelin | -0.013 | 0.013 | 0.014 |
| 5 | LAP | 0.389 | <0.001 | <0.001 |
| 6 | Age | 0.1328 | 0.2543 | <0.001 |
| | BMI | 0.7436 | 0.050 | |
| | Des-acyl
ghrelin | -0.0065 | 0.094 | |
| | LAP | 0.2570 | 0.004 | |
| 7 | Age | 0.125 | 0.308 | <0.001 |
| | WC | 0.0641 | 0.734 | |
| | Des-acyl
ghrelin | -0.0016 | 0.059 | |
| | LAP | 0.3378 | 0.001 | |
Prediction of MASLD using age and
serum des-acyl ghrelin
Mahalanobis distance analysis was performed to
determine whether age and serum des-acyl ghrelin were key
predictors of MASLD. LAP, a powerful tool for diagnosing metabolic
disorders, was used with a threshold of 31.6 for MASLD (18). Sensitivity and specificity were
71.43 and 71.20%, respectively, for individuals with LAP
>31.6.
Discussion
MASLD and metabolic abnormalities are increasingly
recognized as notable health concerns, particularly in aging and
hormonal regulation (10,12-15,17,22-24).
To assess MASLD, the present study used the LAP, a simple and
non-invasive measure that is associated with liver steatosis and
fibrosis (18). Although liver
biopsy is more accurate, its invasive nature limits its suitability
for initial screening and monitoring. Therefore, LAP provides a
practical and patient-friendly alternative for evaluating the risk
and severity of MASLD. Based on the screening results, subsequent
analysis should prioritize hormones demonstrating the strongest
statistical associations. The present GEPH survey of apparently
healthy female patients demonstrated des-acyl ghrelin as the
strongest and most consistent predictor of the risk of MASLD, along
with age. While extensive literature implicates multiple GEPHs
(GLP-1, PYY and CCK) in the pathogenesis of metabolic disorders and
MASLD, predominantly in populations with higher body weight,
diabetes or differing ethnic backgrounds (12-14),
none showed predictive strength comparable with des-acyl ghrelin in
the present cohort. This divergence may arises from two key
factors: First, unlike earlier studies (12-14)
that typically assess one or two hormones in isolation, the present
approach integrated forward regression and path analysis to
evaluate multiple GEPHs, capturing their interactive effects.
Second, the key role of des-acyl ghrelin in the present cohort may
reflect population-specific hormonal drivers associated with early
MASLD development, consistent with its reported metabolic
protective functions (9,10). Together, age and des-acyl ghrelin
constitute key upstream factors determinants of MASLD and metabolic
disturbance, primarily mediated through increased WC and BMI, and
demonstrated utility in stratifying MASLD risk within this group.
Therefore, monitoring des-acyl ghrelin levels in combination with
age, even in individuals who are neither obese nor of normal
weight, may offer a biologically grounded approach for screening
early MASLD risk in East Asian female populations. This strategy
may help identify people at higher risk for MASLD before symptoms
appear, supporting the value of tracking body weight and WC for
early prevention.
Age was a fundamental upstream driver of metabolic
abnormalities and MASLD in the present cohort. Age showed a
significant association with key metabolic indicators (BMI,
TG/HDL-C ratio, LAP and ALT), suggesting as patients get older,
they become more susceptible to metabolic dysfunction, such as
MASLD. Consistently, Alexander et al (15) demonstrated that aging, independent
of BMI, worsens metabolic dysregulation. Path analysis confirmed
that age substantially increased MASLD risk via promoting central
adiposity (elevated WC). This extends findings by Yuan et al
(24) of aging as an independent
MASLD risk factor in Chinese adults by delineating a central
mechanism mediated by visceral adiposity. The present results are
also consistent with Bilson et al (22), which demonstrated that age-related
visceral fat dysfunction contributes to hepatic steatosis, even in
patients who seem healthy.
Equally pivotal as a modifiable upstream factor,
des-acyl ghrelin emerged with age as a core regulator within the
network of metabolic dysregulation. Des-acyl ghrelin, once
considered an inactive metabolite, serves distinct physiological
roles compared with its acylated counterpart (25), particularly in anti-aging and
anti-inflammatory processes (10,19,26-30).
Unlike studies in populations with obesity (12,13),
such as Ahmad et al (31),
who investigated dysregulated fasting GLP1/ghrelin (using patrial
correlation/logistic regression on five GEPHs, without
distinguishing acyl/des-acyl isoforms), the present study targeted
apparently healthy female patients and performed forward regression
and path analysis on 11 GEPHs (including ghrelin isoforms) to
delineate causal pathways. The present study revealed des-acyl
ghrelin, not acyl ghrelin, as the primary upstream modulator of
MASLD within a comprehensive GEPH framework, aligning with a
cross-ethnic observation (32). In
asymptomatic Korean men with MS, plasma des-acyl ghrelin and total
ghrelin levels are significantly lower than in controls, and both
are inversely correlated with insulin resistance (32). Transgenic models have confirmed the
protective role of des-acyl ghrelin (27,28),
attenuating age-associated wasting and enhancing muscle
performance, while its deficiency accelerates aging and muscle
wasting (28). Des-acyl ghrelin
modulates inflammatory and oxidative stress markers, which is
essential for slowing the aging process (26,28,33).
Circulating ghrelin levels decrease with aging, which may impair
endogenous ghrelin signaling (25,28,33).
The present study similarly found that ghrelin was negatively
correlated with age. In MASLD and MASH, insulin resistance leads to
the accumulation of fatty acids and visceral fat in the liver,
exacerbates hepatic insulin resistance and damages hepatocytes
(21,29,35,36).
Des-acyl ghrelin modulates blood glucose independent of growth
hormone secretagogue receptor (12,25,29,30,36-38),
and has been shown to enhance insulin sensitivity and prevent
diabetes in high-fat diet-fed mice and rats (29,30).
While the present study did not detect a direct association with
FBS, the strong inverse association between des-acyl ghrelin and
the TG/HDL-C suggested an indirect modulatory effect, potentially
via mechanisms involving adipose tissue regulation (12,25,29,37),
hormone balance or glycogen metabolism. Overexpression of des-acyl
ghrelin in mice leads to decreased body size due to decreased food
intake and delayed gastric emptying (39,40).
Des-acyl ghrelin also stimulates basal autophagy via AMPK for lipid
mobilization and counters TNF-α-induced cell damage, aiding in the
improvement of MASLD (34,41). Beyond these cellular effects, recent
evidence suggests des-acyl ghrelin may also act through a
neuroendocrine pathway (42). Lv
et al (42) identified a
novel gut-brain-liver axis by which des-acyl ghrelin alleviates
MASLD. Des-acyl ghrelin increases GLP-1 expression in the nucleus
tractus solitarius and GLP-1 receptor in the paraventricular
nucleus, leading to enhanced hepatic lipid metabolism (42). Disruption of this pathway via GLP-1R
antagonism or hepatic vagotomy significantly attenuates therapeutic
effects of des-acyl ghrelin, underscoring the relevance of this
neural circuit (42). These
findings reinforce the observation that des-acyl ghrelin is
inversely associated with LAP and TG/HDL-C ratio, highlighting its
systemic role in hepatic and lipid regulation. Obestatin, derived
from the ghrelin-obestatin precursor gene (GHRL), also
exhibited a significant negative correlation with LAP and a strong
positive association with fasting glucose. Obestatin shares
functional overlap with des-acyl ghrelin in stimulating
preadipocyte differentiation, promoting adipocyte fatty acid
uptake, and inhibiting lipolysis (41). However, its role appears limited.
Other GEPHs, such as OXM, PYY, PP and amylin, did not show any
detectable effects on metabolic abnormalities or MASLD, suggesting
the metabolic relevance is specific to ghrelin-derived peptides,
particularly des-acyl ghrelin.
Incidence of MASLD is similar in postmenopausal
females and males (19 and 22%, respectively), but lower in
premenopausal females (9%) (44),
suggesting sex hormones influence the prevalence of the disease.
Estrogen enhances insulin sensitivity, while androgens increase
insulin resistance (45).
Sex-specific fat distribution patterns also affect the risk of
metabolic disease, with males more prone to visceral fat
accumulation, while postmenopausal patients exhibit increased
central obesity and insulin resistance (46). The role of estrogen in regulating
lipid metabolism, protecting the liver from oxidative damage and
slowing the progression of liver fibrosis may provide partial
protection for premenopausal patients against MS and MASLD
(1). In females, ghrelin levels are
naturally higher than in males and fluctuate with the menstrual
cycle (47). Estrogen directly
stimulates ghrelin-secreting cells in the stomach, increasing the
expression and secretion of ghrelin (47). The interaction between ghrelin and
sex hormones, such as estrogen, serves a crucial role in regulating
appetite and energy balance. For example, the absence of ovaries
increases ghrelin-induced effects, while estrogen therapy can
counteract these (47,48). The interaction between sex hormones
and GPEHs may be a key mechanism in the development of MASLD/MASH.
The complex association between ghrelin and estrogen, especially in
terms of food reward and stress eating, is an area of active
research due to the importance of these hormonal interactions in
metabolic diseases (47,48).
The present findings offer novel insight into the
pathogenesis of MASLD and lay the groundwork for tailored
prevention and therapeutic strategies. However, the study has
limitations. First, its cross-sectional design precluded causal
inference. While path analysis explored associations among GEPHs,
metabolic parameters, and MASLD, it evaluates the statistical
coherence of a prespecified model at a single time point and cannot
establish a temporal sequence or rule out reverse causation.
Second, the modest sample size (n=139), limited statistical power
for subgroup analyses. Future longitudinal studies should include
larger, multicenter, multiethnic cohorts encompassing both sexes
and varying metabolic states (including obesity) along with
analysis of sex hormones, to validate and expand the present
findings. Third, using LAP as a surrogate for MASLD diagnosis,
though non-invasive and scalable, may not accurately reflect the
true severity of the disease compared with gold-standard methods
(biopsy/FibroScan®). Fourth, the present findings are
specific to apparently healthy females in Taiwan. Thus, caution is
warranted in generalizing the results to other populations. Fifth,
the data collection occurred in early 2020, before the global
impact of the coronavirus disease-19 pandemic. While this timing
may raise concerns about the applicability of the findings, it also
provides a valuable pre-pandemic baseline for understanding
metabolic health. As this study included only female participants,
future research should enroll male subjects to enable analysis of
sex differences. The core mechanistic pathway of age and des-acyl
ghrelin influencing adiposity and MASLD reflects stable biological
processes that are unlikely to be altered by temporal shifts. As
such, the present data offer a meaningful reference point for
studies comparing pre- and post-pandemic cohorts.
The present cross-sectional study identified
significant correlations between age, serum des-acyl ghrelin and
metabolic complications, including MASLD, in adult females in
Taiwan. The findings suggest that age and des-acyl ghrelin could
serve as a predictor of metabolic risks. Despite the limited number
of participants, the data underscore the importance of monitoring
serum des-acyl ghrelin levels in preventive medicine. Further
longitudinal studies are essential to validate these associations,
determine the precision and reliability of these predictions and
clarify the specific role of des-acyl ghrelin in MASLD to develop
more accurate predictive models and therapeutic approaches to
manage metabolic diseases.
Acknowledgements
The authors would like to thank the Clinical
Research Core Laboratory of Taipei Veterans General Hospital,
Taipei, Taiwan for providing experimental space and facilities, as
well as Dr Hsien-Hao Huang (Taipei Veterans General Hospital,
Taipei, Taiwan) for administration assistance.
Funding
Funding: The present study was supported by Taipei Veterans
General Hospital-National Yang-Ming Chiao Tung University Excellent
Physician Scientists Cultivation Program (grant no.
113-V-B-034).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CYC conceived the study. CY, PYC and CYC performed
the experiments. HHC and WCC confirm the authenticity of all the
raw data. CPL, HHC and WCC analyzed and interpreted data. CPL, WCC
and CYC wrote, reviewed and edited the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Institutional
Review Board of Cheng Hsin General Hospital [Taipei, Taiwan;
approval nos. (665) 107A-37, (732) 108A-48 and (736) 108A-52] and
conducted in compliance with the Declaration of Helsinki and
institutional ethical guidelines. The rights and interests of all
participants were fully protected by the Association for the
Accreditation of Human Research Protection Program guidelines.
Written informed consent was obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
European Association for the Study of the
Liver (EASL); European Association for the Study of Diabetes
(EASD); European Association for the Study of Obesity (EASO).
EASL-EASD-EASO clinical practice guidelines on the management of
metabolic dysfunction-associated steatotic liver disease (MASLD).
Obes Facts. 17:374–444. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eslam M, Fan JG, Yu ML, Wong VW, Cua IH,
Liu CJ, Tanwandee T, Gani R, Seto WK, Alam S, et al: The Asian
Pacific association for the study of the liver clinical practice
guidelines for the diagnosis and management of metabolic
dysfunction-associated fatty liver disease. Hepatol Int.
19:261–301. 2025.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen CH, Huang MH, Yang JC, Nien CK, Yang
CC, Yeh YH and Yueh SK: Prevalence and risk factors of nonalcoholic
fatty liver disease in an adult population of Taiwan: Metabolic
significance of nonalcoholic fatty liver disease in nonobese
adults. J Clin Gastroenterol. 40:745–752. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang WS, Wahlqvist ML, Hsu CC, Chang HY,
Chang WC and Chen CC: Age- and gender-specific population
attributable risks of metabolic disorders on all-cause and
cardiovascular mortality in Taiwan. BMC Public Health.
12(111)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Health Promotion Administration, Ministry
of Health and Welfare: Report on the Changes in National
Nutritional and Health Status 2017-2020: Survey on Changes in
National Nutritional and Health Status, 2022 (In Chinese).
https://www.hpa.gov.tw/EngPages/Detail.aspx?nodeid=3999&pid=15562.
|
|
6
|
Chen CY, Fujimiya M, Laviano A, Chang FY,
Lin HC and Lee SD: Modulation of ingestive behavior and
gastrointestinal motility by ghrelin in diabetic animals and
humans. J Chin Med Assoc. 73:225–229. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen CY and Tsai CY: From endocrine to
rheumatism: Do gut hormones play roles in rheumatoid arthritis?
Rheumatology (Oxford). 53:205–212. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen CY, Asakawa A, Fujimiya M, Lee SD and
Inui A: Ghrelin gene products and the regulation of food intake and
gut motility. Pharmacol Rev. 61:430–481. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Iwakura H, Ensho T and Ueda Y:
Desacyl-ghrelin, not just an inactive form of ghrelin? A review of
current knowledge on the biological actions of desacyl-ghrelin.
Peptides. 167(171050)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gong Y, Qiu B, Zheng H, Li X, Wang Y, Wu
M, Yan M and Gong Y: Unacylated ghrelin attenuates acute liver
injury and hyperlipidemia via its anti-inflammatory and
anti-oxidative activities. Iran J Basic Med Sci.
27(49)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim H, Ranjit R, Claflin DR, Georgescu C,
Wren JD, Brooks SV, Miller BF and Ahn B: Unacylated ghrelin
protects against age-related loss of muscle mass and contractile
dysfunction in skeletal muscle. Aging Cell.
23(e14323)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koliaki C, Liatis S, Dalamaga M and
Kokkinos A: The implication of gut hormones in the regulation of
energy homeostasis and their role in the pathophysiology of
obesity. Curr Obes Rep. 9:255–271. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tarantino G and Balsano C:
Gastrointestinal peptides and nonalcoholic fatty liver disease.
Curr Opin Endocrinol Diabetes Obes. 27:11–15. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bakar RB, Reimann F and Gribble FM: The
intestine as an endocrine organ and the role of gut hormones in
metabolic regulation. Nat Rev Gastroenterol Hepatol. 20:784–796.
2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Alexander CM, Landsman PB and Grundy SM:
The influence of age and body mass index on the metabolic syndrome
and its components. Diabetes Obes Metab. 10:246–250.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baneu P, Văcărescu C, Drăgan SR, Cirin L,
Lazăr-Höcher AI, Cozgarea A, Faur-Grigori AA, Crișan S, Gaiță D,
Luca CT and Cozm D: The Triglyceride/HDL ratio as a surrogate
biomarker for insulin resistance. Biomedicines.
12(1493)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen CY, Lee WJ, Asakawa A, Fujitsuka N,
Chong K, Chen SC, Lee SD and Inui A: Insulin secretion and
interleukin-1β dependent mechanisms in human diabetes remission
after metabolic surgery. Curr Med Chem. 20:2374–2388.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ebrahimi M, Seyedi SA, Nabipoorashrafi SA,
Rabizadeh S, Sarzaeim M, Yadegar A, Mohammadi F, Bahri RA, Pakravan
P, Shafiekhani P, et al: Lipid accumulation product (LAP) index for
the diagnosis of nonalcoholic fatty liver disease (NAFLD): A
systematic review and meta-analysis. Lipids Health Dis.
22(41)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chiang JK and Koo M: Lipid accumulation
product: A simple and accurate index for predicting metabolic
syndrome in Taiwanese people aged 50 and over. BMC Cardiovasc
Disord. 12(78)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lockie S, Lyons K, Lawrence G and Grice J:
Choosing organics: A path analysis of factors underlying the
selection of organic food among Australian consumers. Appetite.
43:135–146. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Senn TE, Espy KA and Kaufmann PM: Using
path analysis to understand executive function organization in
preschool children. Dev Neuropsychol. 26:445–464. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bilson J, Sethi JK and Byrne CD:
Non-alcoholic fatty liver disease: A multi-system disease
influenced by ageing and sex, and affected by adipose tissue and
intestinal function. Proc Nutr Soc. 81:146–161. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Muratsu J, Kamide K, Fujimoto T, Takeya Y,
Sugimoto K, Taniyama Y, Morishima A, Sakaguchi K, Matsuzawa Y and
Rakugi H: The combination of high levels of adiponectin and insulin
resistance are affected by aging in non-obese old peoples. Front
Endocrinol (Lausanne). 12(805244)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yuan Q, Wang H, Gao P, Chen W, Lv M, Bai S
and Wu J: Prevalence and risk factors of metabolic-associated fatty
liver disease among 73,566 individuals in Beijing, China. Int J
Environ Res Public Health. 19(2096)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Delhanty PJ, Neggers SJ and van der Lely
AJ: Mechanisms in endocrinology: Ghrelin: The differences between
acyl- and des-acyl ghrelin. Eur J Endocrinol. 167:601–608.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shimada T, Furuta H, Doi A, Ariyasu H,
Kawashima H, Wakasaki H, Nishi M, Sasaki H and Akamizu T: Des-acyl
ghrelin protects microvascular endothelial cells from oxidative
stress-induced apoptosis through sirtuin 1 signaling pathway.
Metabolism. 63:469–474. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cappellari GG, Zanetti M, Semolic A, Vinci
P, Ruozi G, Falcione A, Filigheddu N, Guarnieri G, Graziani A,
Giacca M and Barazzoni R: Unacylated ghrelin reduces skeletal
muscle reactive oxygen species generation and inflammation and
prevents high-fat diet-induced hyperglycemia and whole-body insulin
resistance in rodents. Diabetes. 65:874–886. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Agosti E, De Feudis M, Angelino E, Belli
R, Teixeira MA, Zaggia I, Tamiso E, Raiteri T, Scircoli A, Ronzoni
F, et al: Both ghrelin deletion and unacylated ghrelin
overexpression preserve muscles in aging mice. Aging (Albany NY).
12:13939–13957. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yuan F, Zhang Q, Dong H, Xiang X, Zhang W,
Zhang Y and Li Y: Effects of des-acyl ghrelin on insulin
sensitivity and macrophage polarization in adipose tissue. J Transl
Int Med. 9:84–97. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Alharbi S: Exogenous administration of
unacylated ghrelin attenuates hepatic steatosis in high-fat
diet-fed rats by modulating glucose homeostasis, lipogenesis,
oxidative stress, and endoplasmic reticulum stress. Biomed
Pharmacother. 151(113095)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ahmad MA, Karavetian M, Moubareck CA, Wazz
G, Mahdy T and Venema K: The association between peptide hormones
with obesity and insulin resistance markers in lean and obese
individuals in the United Arab Emirates. Nutrients.
14(1271)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cho YH, Lee SY, Jeong DW, Cho AR, Jeon JS,
Kim YJ, Lee JG, Yi YH, Tak YJ, Hwang HR, et al: Metabolic syndrome
is associated with lower plasma levels of desacyl ghrelin and total
ghrelin in asymptomatic middle-aged Korean men. J Obes Metab Syndr.
26:114–121. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Amitani M, Amitani H, Cheng KC, Kairupan
TS, Sameshima N, Shimoshikiryo I, Mizuma K, Rokot NT, Nerome Y,
Owaki T, et al: The role of ghrelin and ghrelin signaling in aging.
Int J Mol Sci. 18(1511)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ezquerro S, Mocha F, Frühbeck G,
Guzmán-Ruiz R, Valentí V, Mugueta C, Becerril S, Catalán V,
Gómez-Ambrosi J, Silva C, et al: Ghrelin reduces TNF-α-induced
human hepatocyte apoptosis, autophagy, and pyroptosis: Role in
obesity-associated NAFLD. J Clin Endocrinol Metab. 104:21–37.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ezquerro S, Méndez-Giménez L, Becerril S,
Moncada R, Valentí V, Catalán V, Gómez-Ambrosi J, Frühbeck G and
Rodríguez A: Acylated and desacyl ghrelin are associated with
hepatic lipogenesis, β-oxidation and autophagy: Role in NAFLD
amelioration after sleeve gastrectomy in obese rats. Sci Rep.
6(39942)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zang P, Yang CH, Liu J, Lei HY, Wang W,
Guo QY, Lu B and Shao JQ: Relationship between acyl and desacyl
ghrelin levels with insulin resistance and body fat mass in type 2
diabetes mellitus. Diabetes Metab Syndr Obes. 15:2763–2770.
2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Delhanty PJ, Sun Y, Visser JA, van
Kerkwijk A, Huisman M, van Ijcken WF, Swagemakers S, Smith RG,
Themmen AP and van der Lely AJ: Unacylated ghrelin rapidly
modulates lipogenic and insulin signaling pathway gene expression
in metabolically active tissues of GHSR deleted mice. PLoS One.
5(e11749)2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu X, Guo Y, Li Z and Gong Y: The role of
acylated ghrelin and unacylated ghrelin in the blood and
hypothalamus and their interaction with nonalcoholic fatty liver
disease. Iran J Basic Med Sci. 23:1191–1196. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Asakawa A, Inui A, Fujimiya M, Sakamaki R,
Shinfuku N, Ueta Y, Meguid MM and Kasuga M: Stomach regulates
energy balance via acylated ghrelin and desacyl ghrelin. Gut.
54:18–24. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ariyasu H, Takaya K, Iwakura H, Hosoda H,
Akamizu T, Arai Y, Kangawa K and Nakao K: Transgenic mice
overexpressing des-acyl ghrelin show small phenotype.
Endocrinology. 146:355–364. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rodríguez A, Gómez-Ambrosi J, Catalán V,
Rotellar F, Valentí V, Silva C, Mugueta C, Pulido MR, Vázquez R,
Salvador J, et al: The ghrelin O-acyltransferase-ghrelin system
reduces TNF-α-induced apoptosis and autophagy in human visceral
adipocytes. Diabetologia. 55:3038–3050. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lv P, Li H, Li X, Wang X, Yu J and Gong Y:
Intestinal perfusion of unacylated ghrelin alleviated metabolically
associated fatty liver disease in rats via a central glucagon-like
peptide-1 pathway. Am J Physiol Gastrointest Liver Physiol.
326:G643–G658. 2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pei XM, Yung BY, Yip SP, Chan LW, Wong CS,
Ying M and Siu PM: Protective effects of desacyl ghrelin on
diabetic cardiomyopathy. Acta Diabetol. 52:293–306. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Long MT, Pedley A, Massaro JM, Hoffmann U,
Ma J, Loomba R, Chung RT and Benjamin EJ: A simple clinical model
predicts incident hepatic steatosis in a community-based cohort:
The Framingham heart study. Liver Int. 38:1495–1503.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mumusoglu S and Yildiz BO: Metabolic
syndrome during menopause. Curr Vasc Pharmacol. 17:595–603.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
de Mutsert R, Gast K, Widya R, de Koning
E, Jazet I, Lamb H, le Cessie S, de Roos A, Smit J, Rosendaal F and
den Heijer M: Associations of abdominal subcutaneous and visceral
fat with insulin resistance and secretion differ between men and
women: The Netherlands epidemiology of obesity study. Metab Syndr
Relat Disord. 16:54–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Smith A, Woodside B and Abizaid A: Ghrelin
and the control of energy balance in females. Front Endocrinol
(Lausanne). 13(904754)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
de Souza GO, Wasinski F and Donato J Jr:
Characterization of the metabolic differences between male and
female C57BL/6 mice. Life Sci. 301(120636)2022.PubMed/NCBI View Article : Google Scholar
|