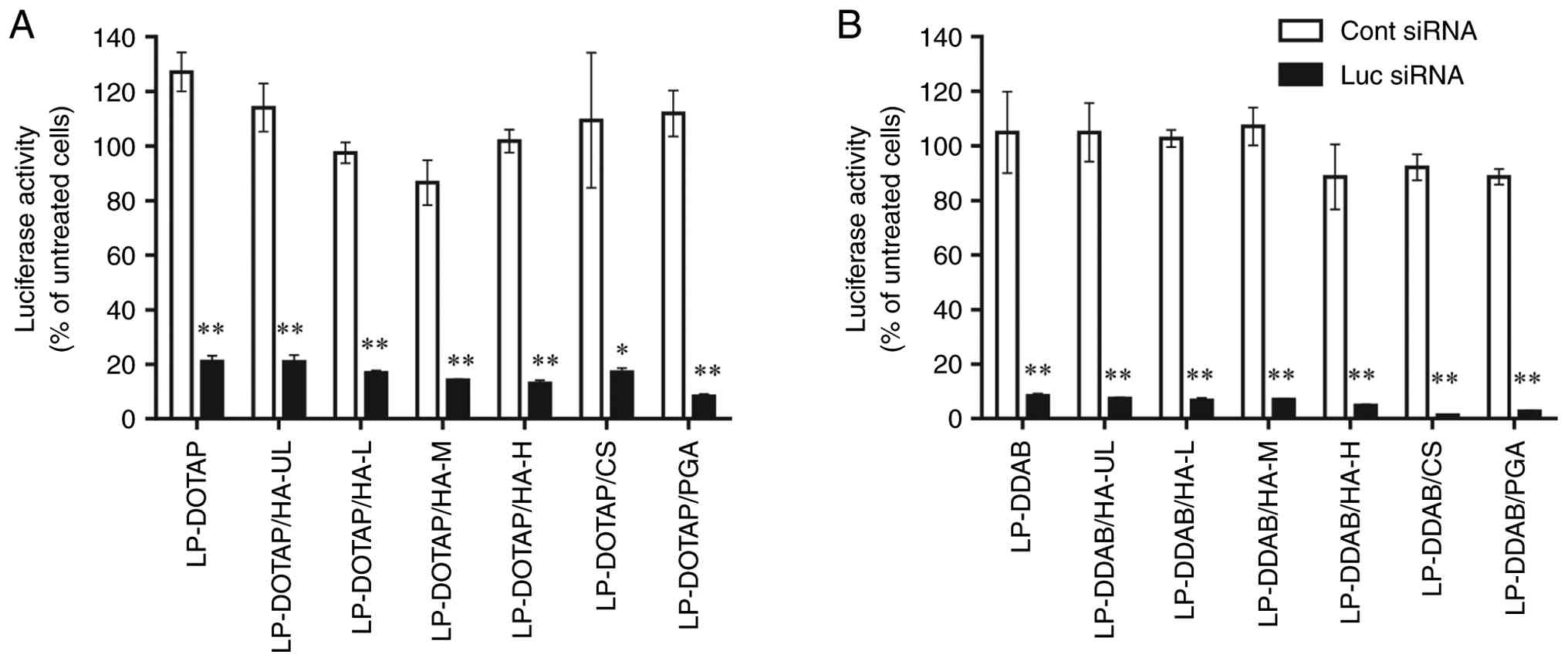
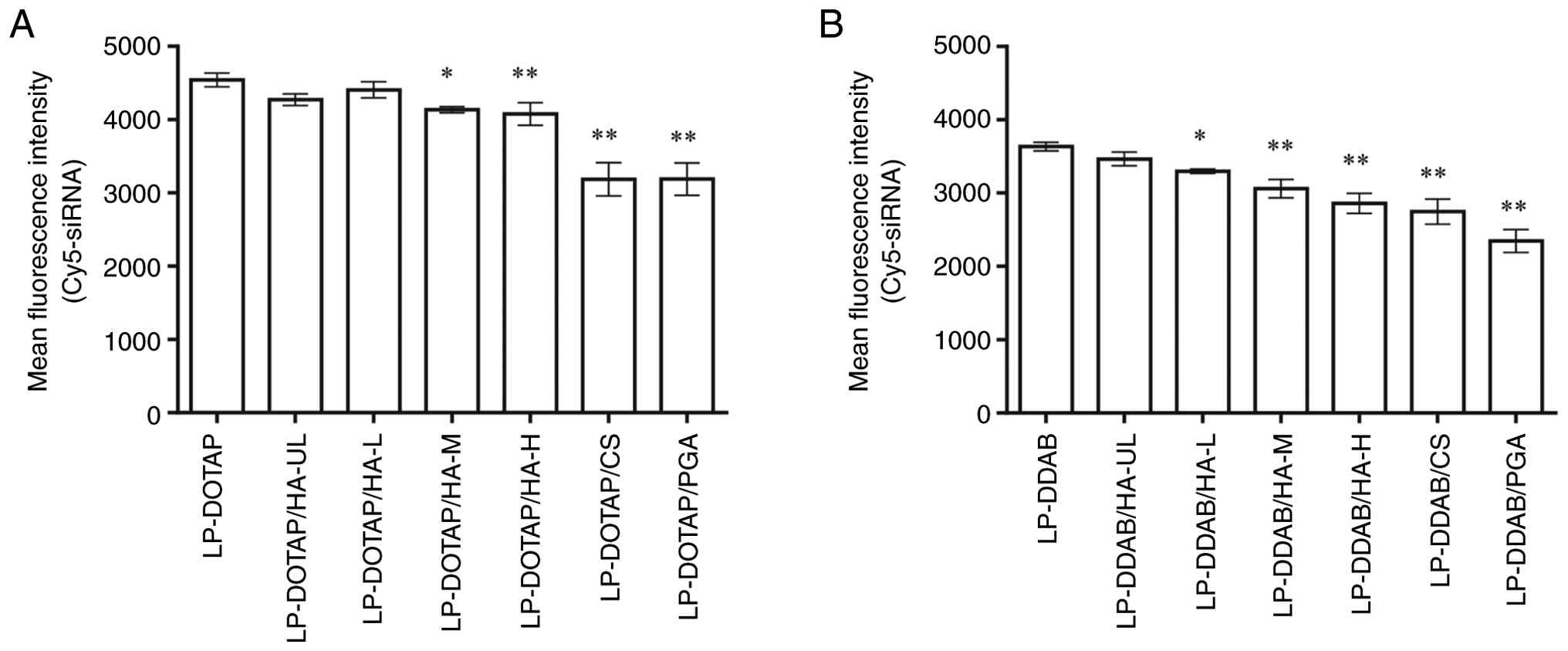
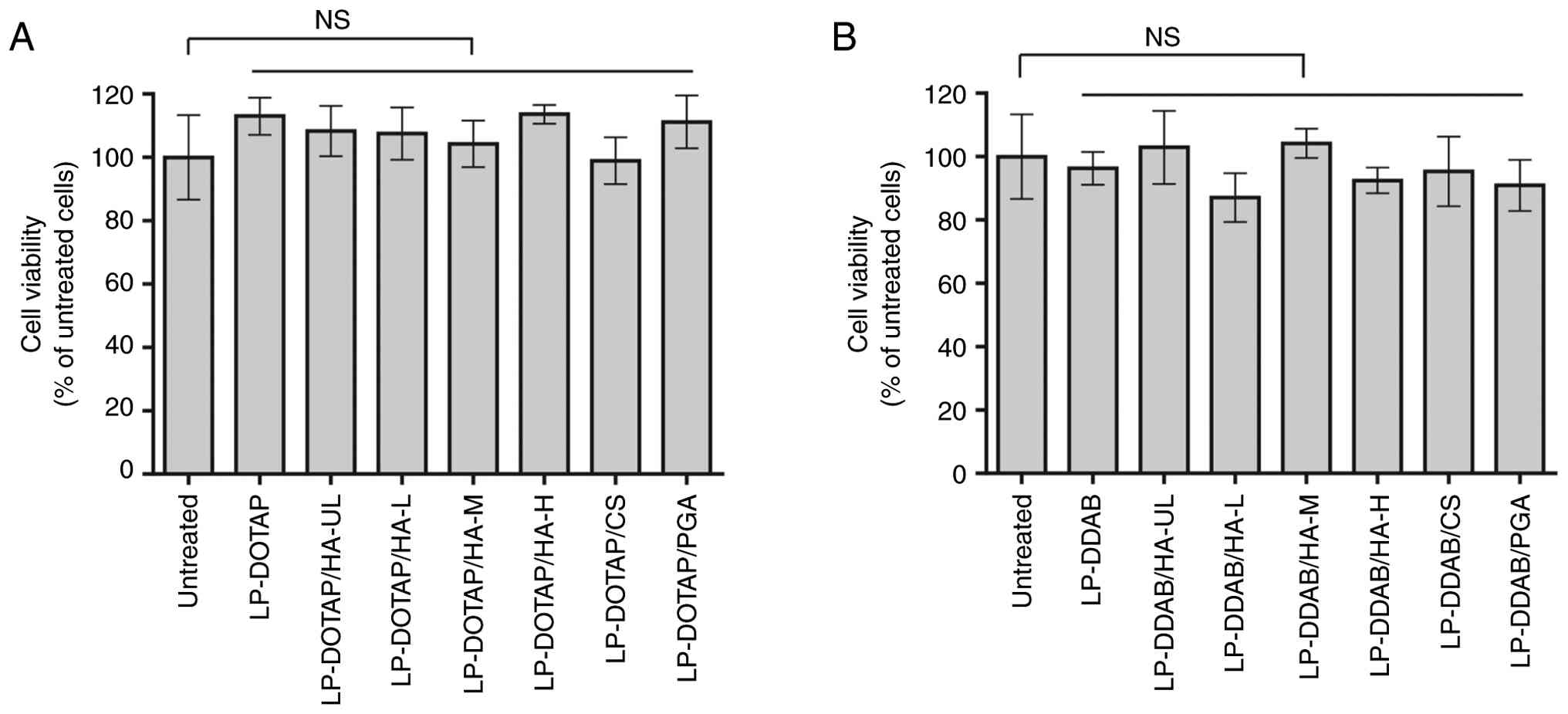
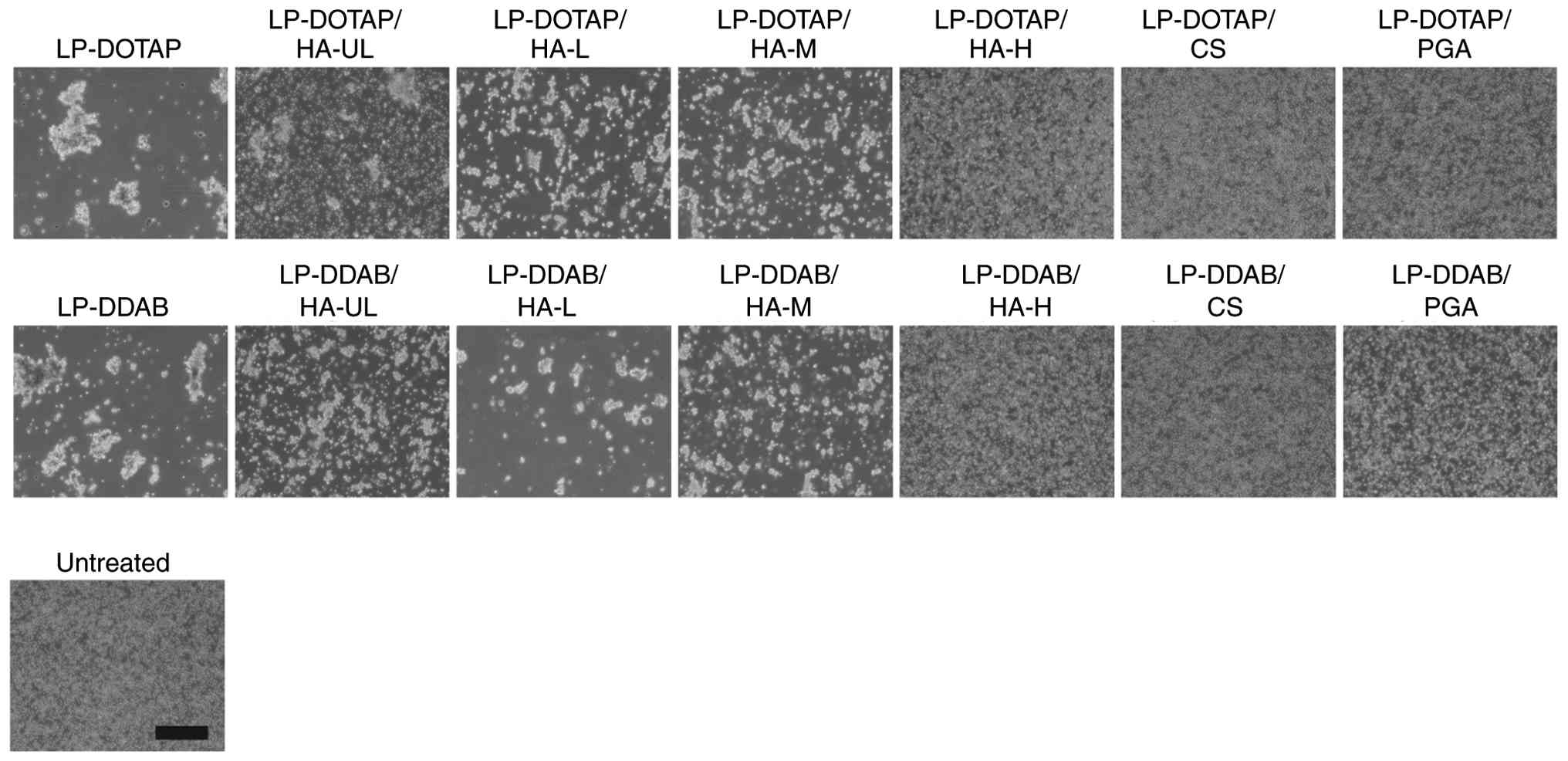
|
1
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Davis ME, Zuckerman JE, Choi CHJ, Seligson
D, Tolcher A, Alabi CA, Yen Y, Heidel JD and Ribas A: Evidence of
RNAi in humans from systemically administered siRNA via targeted
nanoparticles. Nature. 464:1067–1070. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zatsepin TS, Kotelevtsev YV and
Koteliansky V: Lipid nanoparticles for targeted siRNA
delivery-going from bench to bedside. Int J Nanomedicine.
11:3077–3086. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yan Y, Liu XY, Lu A, Wang XY, Jiang LX and
Wang JC: Non-viral vectors for RNA delivery. J Control Release.
342:241–279. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang S, Zhi D and Huang L: Lipid-based
vectors for siRNA delivery. J Drug Target. 20:724–735.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Simberg D, Weisman S, Talmon Y, Faerman A,
Shoshani T and Barenholz Y: The role of organ vascularization and
lipoplex-serum initial contact in intravenous murine lipofection. J
Biol Chem. 278:39858–39865. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Eliyahu H, Servel N, Domb AJ and Barenholz
Y: Lipoplex-induced hemagglutination: Potential involvement in
intravenous gene delivery. Gene Ther. 9:850–858. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Buyens K, De Smedt SC, Braeckmans K,
Demeester J, Peeters L, van Grunsven LA, de Mollerat du Jeu X,
Sawant R, Torchilin V, Farkasova K, et al: Liposome based systems
for systemic siRNA delivery: Stability in blood sets the
requirements for optimal carrier design. J Control Release.
158:362–370. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hattori Y, Tamaki K, Sakasai S, Ozaki KI
and Onishi H: Effects of PEG anchors in PEGylated siRNA lipoplexes
on in vitro gene-silencing effects and siRNA biodistribution
in mice. Mol Med Rep. 22:4183–4196. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hatakeyama H, Akita H and Harashima H: A
multifunctional envelope type nano device (MEND) for gene delivery
to tumours based on the EPR effect: A strategy for overcoming the
PEG dilemma. Adv Drug Deliv Rev. 63:152–160. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hattori Y, Yamasaku H and Maitani Y:
Anionic polymer-coated lipoplex for safe gene delivery into tumor
by systemic injection. J Drug Target. 21:639–647. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fukushige K, Tagami T and Ozeki T: The
offset effect of a hyaluronic acid coating to cationic carriers
containing siRNA: Alleviated cytotoxicity and retained gene
silencing in vitro. J Drug Deliv Sci Technol. 39:435–441. 2017.
|
|
13
|
Qian Y, Liang X, Yang J, Zhao C, Nie W,
Liu L, Yi T, Jiang Y, Geng J, Zhao X and Wei X: Hyaluronan reduces
cationic liposome-induced toxicity and enhances the antitumor
effect of targeted gene delivery in mice. ACS Appl Mater
Interfaces. 10:32006–32016. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hattori Y, Kurihara A and Shinkawa M:
Assessment of anionic siRNA lipoplexes prepared via modified
ethanol injection for tumor cell delivery. Biomed Rep.
24(12)2026.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hattori Y, Saito H, Nakamura K, Yamanaka
A, Tang M and Ozaki KI: In vitro and in vivo transfections using
siRNA lipoplexes prepared by mixing siRNAs with a lipid-ethanol
solution. J Drug Deliv Sci Technol. 75(103635)2022.
|
|
16
|
Hattori Y, Tang M, Suzuki H, Hattori A,
Endo S, Ishii A, Aoki A, Ezaki M and Sakai H: Optimization of
transfection into cultured cells with siRNA lipoplexes prepared
using a modified ethanol injection method. J Drug Deliv Sci
Technol. 99(106000)2024.
|
|
17
|
Hattori Y, Tang M, Aoki A, Ezaki M, Sakai
H and Ozaki KI: Effect of the combination of cationic lipid and
phospholipid on gene-knockdown using siRNA lipoplexes in breast
tumor cells and mouse lungs. Mol Med Rep. 28(180)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hattori Y and Shimizu R: Effective mRNA
transfection of tumor cells using cationic triacyl lipid-based mRNA
lipoplexes. Biomed Rep. 22(25)2025.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hattori Y, Tamaki K, Ozaki KI, Kawano K
and Onishi H: Optimized combination of cationic lipids and neutral
helper lipids in cationic liposomes for siRNA delivery into the
lung by intravenous injection of siRNA lipoplexes. J Drug Deliv Sci
Technol. 52:1042–1050. 2019.
|
|
20
|
Whitmore M, Li S and Huang L: LPD
lipopolyplex initiates a potent cytokine response and inhibits
tumor growth. Gene Ther. 6:1867–1875. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hattori Y, Arai S, Kikuchi T, Ozaki KI,
Kawano K and Yonemochi E: Therapeutic effect for liver-metastasized
tumor by sequential intravenous injection of anionic polymer and
cationic lipoplex of siRNA. J Drug Target. 24:309–317.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hattori Y, Tang M, Sato J, Tsuiji M and
Kawano K: Evaluation of mRNA lipoplexes prepared using modified
ethanol injection method as a tumour vaccine. J Drug Target.
32:1267–1277. 2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hattori Y, Nakamura A, Arai S, Nishigaki
M, Ohkura H, Kawano K, Maitani Y and Yonemochi E: In vivo siRNA
delivery system for targeting to the liver by poly-l-glutamic
acid-coated lipoplex. Results Pharm Sci. 4:1–7. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hattori Y, Nakamura A, Arai S, Kawano K,
Maitani Y and Yonemochi E: siRNA delivery to lung-metastasized
tumor by systemic injection with cationic liposomes. J Liposome
Res. 25:279–286. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nabar N, Dacoba TG, Covarrubias G,
Romero-Cruz D and Hammond PT: Electrostatic adsorption of
polyanions onto lipid nanoparticles controls uptake, trafficking,
and transfection of RNA and DNA therapies. Proc Natl Acad Sci USA.
121(e2307809121)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang T, Upponi JR and Torchilin VP: Design
of multifunctional non-viral gene vectors to overcome physiological
barriers: Dilemmas and strategies. Int J Nanomedicine. 427:3–20.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lesley J, Hascall VC, Tammi M and Hyman R:
Hyaluronan binding by cell surface CD44. J Biol Chem.
275:26967–26975. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sironen RK, Tammi M, Tammi R, Auvinen PK,
Anttila M and Kosma VM: Hyaluronan in human malignancies. Exp Cell
Res. 317:383–391. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lin WJ and Lee WC: Polysaccharide-modified
nanoparticles with intelligent CD44 receptor targeting ability for
gene delivery. Int J Nanomedicine. 13:3989–4002. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dosio F, Arpicco S, Stella B and Fattal E:
Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv
Drug Deliv Rev. 97:204–236. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Peer D and Margalit R: Tumor-targeted
hyaluronan nanoliposomes increase the antitumor activity of
liposomal Doxorubicin in syngeneic and human xenograft mouse tumor
models. Neoplasia. 6:343–353. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang Q, Deng C, Fu Y, Sun X, Gong T and
Zhang Z: Repeated administration of hyaluronic acid coated
liposomes with improved pharmacokinetics and reduced immune
response. Mol Pharm. 13:1800–1808. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mizrahy S, Raz SR, Hasgaard M, Liu H,
Soffer-Tsur N, Cohen K, Dvash R, Landsman-Milo D, Bremer MG,
Moghimi SM and Peer D: Hyaluronan-coated nanoparticles: The
influence of the molecular weight on CD44-hyaluronan interactions
and on the immune response. J Control Release. 156:231–238.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Qhattal HS and Liu X: Characterization of
CD44-mediated cancer cell uptake and intracellular distribution of
hyaluronan-grafted liposomes. Mol Pharm. 8:1233–1246.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Peng SF, Tseng MT, Ho YC, Wei MC, Liao ZX
and Sung HW: Mechanisms of cellular uptake and intracellular
trafficking with chitosan/DNA/poly(γ-glutamic acid) complexes as a
gene delivery vector. Biomaterials. 32:239–248. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kurosaki T, Ueda Y, Kato Y, Nakashima M,
Kitahara T, Sasaki H and Kodama Y: Effect of a novel siRNA delivery
system, siRNA ternary complex, on melanoma lung metastasis. J Drug
Target. 32:848–854. 2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hattori Y, Arai S, Okamoto R, Hamada M,
Kawano K and Yonemochi E: Sequential intravenous injection of
anionic polymer and cationic lipoplex of siRNA could effectively
deliver siRNA to the liver. Int J Pharm. 476:289–298.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dilliard SA, Cheng Q and Siegwart DJ: On
the mechanism of tissue-specific mRNA delivery by selective organ
targeting nanoparticles. Proc Natl Acad Sci USA.
118(e2109256118)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dilliard SA, Sun Y, Brown MO, Sung YC,
Chatterjee S, Farbiak L, Vaidya A, Lian X, Wang X, Lemoff A and
Siegwart DJ: The interplay of quaternary ammonium lipid structure
and protein corona on lung-specific mRNA delivery by selective
organ targeting (SORT) nanoparticles. J Control Release.
361:361–372. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hattori Y, Nakamura M, Takeuchi N, Tamaki
K, Ozaki KI and Onishi H: Effect of cationic lipid type in
PEGylated liposomes on siRNA delivery following the intravenous
injection of siRNA lipoplexes. World Acad Sci J. 1:74–85. 2019.
|
|
41
|
Hattori Y, Yoshiike Y, Kikuchi T, Yamamoto
N, Ozaki KI and Onishi H: Evaluation of the injection route of an
anionic polymer for small interfering RNA delivery into the liver
by sequential injection of anionic polymer and cationic lipoplex of
small interfering RNA. J Drug Deliv Sci Technol. 35:40–49.
2016.
|
|
42
|
Simonsen JB and Kromann EB: Pitfalls and
opportunities in quantitative fluorescence-based nanomedicine
studies-A commentary. J Control Release. 335:660–667.
2021.PubMed/NCBI View Article : Google Scholar
|