Introduction
Rhinosinusitis is an inflammatory condition that
affects both the nasal passages and paranasal sinuses. Chronic
rhinosinusitis (CRS) occurs frequently in both adult and paediatric
populations (1). Although the
clinical manifestations of CRS may exhibit similarities, there are
significant differences between adult and paediatric CRS across
various dimensions. The diagnostic criteria for adults include
hyposmia, facial pain/pressure, and nasal discharge or blockage
(2). In paediatric populations,
coughing has emerged as a substitute for reduced olfactory function
(3). This finding was corroborated
by a study that evaluated the clinical features of paediatric CRS,
which revealed that the most frequently reported symptoms in
children with CRS were a runny nose (96%) and cough (88%) (4). Currently, there is no systematic or
standardised treatment protocol for paediatric CRS. The most
commonly used interventions include nasal saline irrigation and
intranasal corticosteroid administration, which have demonstrated
therapeutic benefits in clinical practice (5). However, long-term outcomes remain
suboptimal. Therefore, it is crucial to develop consensus-based
guidelines for effective management of paediatric CRS to minimise
its impact during childhood.
During the 20th century, physical therapists
frequently employed thermotherapy utilising electromagnetic fields
such as those from ultrashort-wave diathermy to manage painful
musculoskeletal conditions (6).
This treatment approach was a widely adopted modality within the
field of physical therapy. Over the past 12 years, ultrashort waves
have been used to treat various inflammatory diseases. For example,
Yang et al (7) found that
ultrashort waves interact with heat shock protein 70 to ameliorate
pulmonary inflammation in mice with acute lung injury. In 2020,
during the peak of the COVID-19 pandemic, some studies reported
that ultrashort waves, as an alternative therapy, could inhibit
inflammation and enhance immune responses (8). Nasal ultrashort wave therapy involves
the placement of two electrodes on either side of the nose of the
child, followed by adjustment of a high-frequency electric field.
This process causes molecules within the nasal cavity and mucosa of
the child to oscillate repeatedly in the horizontal direction. The
friction generated between these molecules produces heat, which
enhances the permeability of the nasal blood vessels, improves
microcirculation, and promotes movement within the nasal mucosa.
This accelerates the clearance of nasal secretions. Furthermore,
ultrashort waves possess antibacterial properties that can
eliminate certain bacteria, while inhibiting their growth and
reproduction. This action helps suppress the development of
inflammation and contributes to achieving a therapeutic effect.
Currently numerous studies have confirmed the efficacy of
ultrashort-wave therapy on CRS (9,10).
However, the mechanism of action of ultrashort waves in paediatric
patients with CRS remains elusive.
In the present study, a mouse model of CRS was
developed, and an ultrashort-wave intervention was administered.
The aim of the pesent study was to preliminarily explore the role
and mechanism of ultrashort-wave therapy on CRS.
Materials and methods
Establishment of a CRS model in mice
and ultrashort wave therapy
All experimental procedures involving mice adhered
to the guidelines outlined in the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (11) and were approved (approval no.
20220601001) by the Ethics Committee of Lanxi People's Hospital
(Lanxi, China). For this experiment, SLAC Laboratory Animal Co.,
Ltd. supplied 24 adult male C57BL/6J mice, aged 8-10 weeks and
weighing between 24 and 27 g. All the mice were housed in specific
pathogen-free environments under standard laboratory conditions.
These conditions included a 12-h light/dark cycle, relative
humidity maintained between 40 and 55%, and temperatures ranging
from 22 to 25˚C. The animals had ad libitum access to food
and water. All mice exhibited a good mental state and showed no
signs of nasal congestion, rhinorrhoea, or sneezing. A week later,
the mice were randomly and evenly divided into four groups:
Control, sham, CRS, and CRS + ultrashort wave, with six mice in
each group. The CRS model was established as previously described
(12) with certain modifications.
Briefly, following the administration of an intraperitoneal
injection of pentobarbital sodium (50 mg/kg) for anaesthesia, a
surgical incision was made from the right side of the snout,
extending to the nasal bridge, to expose the right bony external
nares. A Merocel nasal pack (5 mm; Medtronic, Inc.) that had been
saturated with a solution of Streptococcus pneumoniae (20
µl; cat. no. BNCC360198; BeNa Culture Collection) was placed into
the right nasal cavity, after which the incision was closed with
sutures. For the mice in the sham group, a Merocel nasal pack was
impregnated with sterile saline and placed in the right nasal
cavity. Mice in the control group were not subjected to any
treatment. Successful modelling was assessed based on symptoms
observed in the mice, including nasal congestion, rhinorrhoea, and
nose scratching. Mice in the CRS + ultrashort wave group underwent
ultrashort wave intervention using an Ultrashort Wave Therapeutic
Instrument (Model DL-C; Shantou Medical Equipment Factory Co.,
Ltd.) for 15 min per day over a duration of 12 weeks, starting 24 h
post-modelling. The instrument needed to preheat for 5 min prior to
ultrashort wave therapy. Subsequently, two circular electrodes,
each with a diameter of 4 cm, were positioned parallel to one
another on either side of the mouse's nose, ensuring a separation
distance of ~2 cm. The ultrashort wave frequency was set at 40.68
MHz. The other groups did not receive any interventions. During
this experiment, concerted efforts were made to minimise animal
suffering. Subsequently, all the mice were euthanised by
intraperitoneal injection of pentobarbital sodium (200 mg/kg). The
nasal cavity was then rinsed to collect the nasal lavage fluid
(NLF), and sinus mucosal samples were obtained for further
analysis.
Haematoxylin and eosin (H&E)
staining
Sinus mucosal specimens were processed by fixation
(4% paraformaldehyde at 25˚C for 12 h), dehydration, and paraffin
embedding. Following sectioning into 4-µm thick slices, the samples
underwent dewaxing and rehydration before being stained with an
H&E staining kit (cat. no. ab245880; Abcam) at 25˚C for 60 min.
Images were acquired using a light microscope (Olympus
Corporation).
Immunofluorescence
Sinus mucosal sections (4 µm) underwent
deparaffinization in xylene for 10 min at room temperature and
rehydration with descending concentrations of ethanol (100, 95, 70%
for 3-5 min each), followed by antigen retrieval in heated citrate
buffer (10 mM; pH 6.0) at 80˚C for 25 min. Subsequently, the
samples were washed three times with PBS before being permeabilized
using 0.5% Triton X-100 in PBS. The sections were then blocked with
5% BSA (cat. no. ST023; Beyotime Institute of Biotechnology) at
room temperature for 1 h. Next, the sections were incubated with
primary antibodies against IFN-γ (cat. no. 15365-1-AP), IL-1α (cat.
no. 83644-1-RR), TNF-α (cat. no. 17590-1-AP), and IL-10 (cat. no.
60269-1-Ig) (all diluted 1:50; Proteintech Group, Inc.).
Subsequently, they were labelled with an Alexa Fluor 568-conjugated
secondary antibody (cat. no. A-11011; diluted 1:200; Invitrogen;
Thermo Fisher Scientific, Inc.). Following DAPI staining (at room
temperature for 15 min), fluorescence images of the sections were
acquired under a fluorescence microscope (Model BX53; Olympus
Corporation) and analysed using ImageJ software (version 1.8.0;
National Institutes of Health).
Terminal deoxynucleotidyl transferase
dUTP nick end labelling (TUNEL) assay
Sinus mucosa apoptosis was evaluated using the TUNEL
Cell Apoptosis kit (Beijing Solarbio Science & Technology Co.,
Ltd.) according to the manufacturer's instructions. Briefly, the
sinus mucosa tissues were fixed using 4% paraformaldehyde (48 h at
4˚C), embedded in paraffin and cut into 4-µm sections. The
deparaffinized tissue sections were incubated with 3% hydrogen
peroxide in methanol for 10 min at 25˚C in the dark, washed three
times with PBS and incubated with 0.1% Triton X-100 in freshly
prepared 0.01% sodium citrate for 8 min at 25˚C. Tissue sections
were then incubated with proteinase K working solution for 25 min
at 37˚C and washed three times with PBS (pH 7.4) for 5 min each. A
total of 50 µl TUNEL reagent was added to each sample and incubated
at 37˚C for 60 min. The sections were washed three times with PBS
(pH 7.4) and then cell nuclei were counterstained with 2 µg/ml DAPI
solution at room temperature for 10 min in the dark and mounted
with 50 µl anti-fade mounting medium. Fluorescence images were
obtained in five randomly-selected fields under an OLYMPUS
fluorescence microscope (Model BX53) and analysed using ImageJ
software (version 1.8.0; National Institutes of Health). The
percentages of TUNEL-positive cells were assessed and presented as
the apoptosis rate (%).
Enzyme-linked immunosorbent assay
(ELISA)
According to manufacturer's instructions, the
concentrations of IFN-γ (cat. no. KTE7003; Abbkine Scientific Co.,
Ltd.), IL-1α (cat. no. ED-20161), TNF-α (cat. no. ED-20852), and
IL-10 (cat. no. ED-20162; all from Xiamen LunChangShuo
Biotechnology Co., Ltd.) in NLF were determined through the
corresponding commercial kits.
Western blotting
Following incubation with RIPA lysis buffer
(Biosharp Life Sciences), total protein was extracted from the
sinus mucosal samples and quantified using a bicinchoninic acid
kit. Next, 25 µg of protein per well was loaded and separated via
10% polyacrylamide gel electrophoresis before being transferred
onto PVDF membranes. The membranes were blocked for 1 h at 25˚C
with a 5% skim milk solution (Biosharp Life Sciences). This was
succeeded by an overnight incubation at 4˚C with the respective
primary antibodies. The membranes were then incubated for an
additional hour with the corresponding secondary antibodies (cat.
no. A21020; diluted 1:10,000; Abbkine Scientific Co., Ltd.). For
detection purposes, the membranes were treated with
SuperKine™ West Pico PLUS Chemiluminescent Substrate
(Abbkine Scientific Co., Ltd.) and subsequently visualised
utilising the ChemiDoc imaging system (Bio-Rad Laboratories, Inc.).
The resulting images were analysed using the ImageJ software
(version 1.8.0). A detailed account of the specific primary
antibodies used in western blot analysis is presented in Table I.
 | Table IPrimary antibodies used in western
blotting. |
Table I
Primary antibodies used in western
blotting.
| Primary antibody | Cat. no. | Dilution ratio | Source |
|---|
| p38 | 66234-1-Ig | 1:4,000 | Proteintech Group,
Inc. |
| p-p38 | 28796-1-AP | 1:2,000 | Proteintech Group,
Inc. |
| JNK | 66210-1-Ig | 1:1,000 | Proteintech Group,
Inc. |
| p-JNK | 60666-1-Ig | 1:1,000 | Proteintech Group,
Inc. |
| ERK | 16443-1-AP | 1:2,000 | Proteintech Group,
Inc. |
| p-ERK | 28733-1-AP | 1:2,000 | Proteintech Group,
Inc. |
| GAPDH | 60004-1-Ig | 1:50,000 | Proteintech Group,
Inc. |
Statistical analysis
In vivo experiments were performed using six
mice per group. Each experiment was performed in triplicate. Data
analysis was performed using SPSS software version 22.0 (IBM
Corp.). To evaluate the differences among the data, one-way
analysis of variance (ANOVA) followed by Tukey's post hoc multiple
comparison test was applied. The results are presented as the mean
± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Ultrashort wave therapy improves
histopathology and inhibits apoptosis in sinus mucosal samples
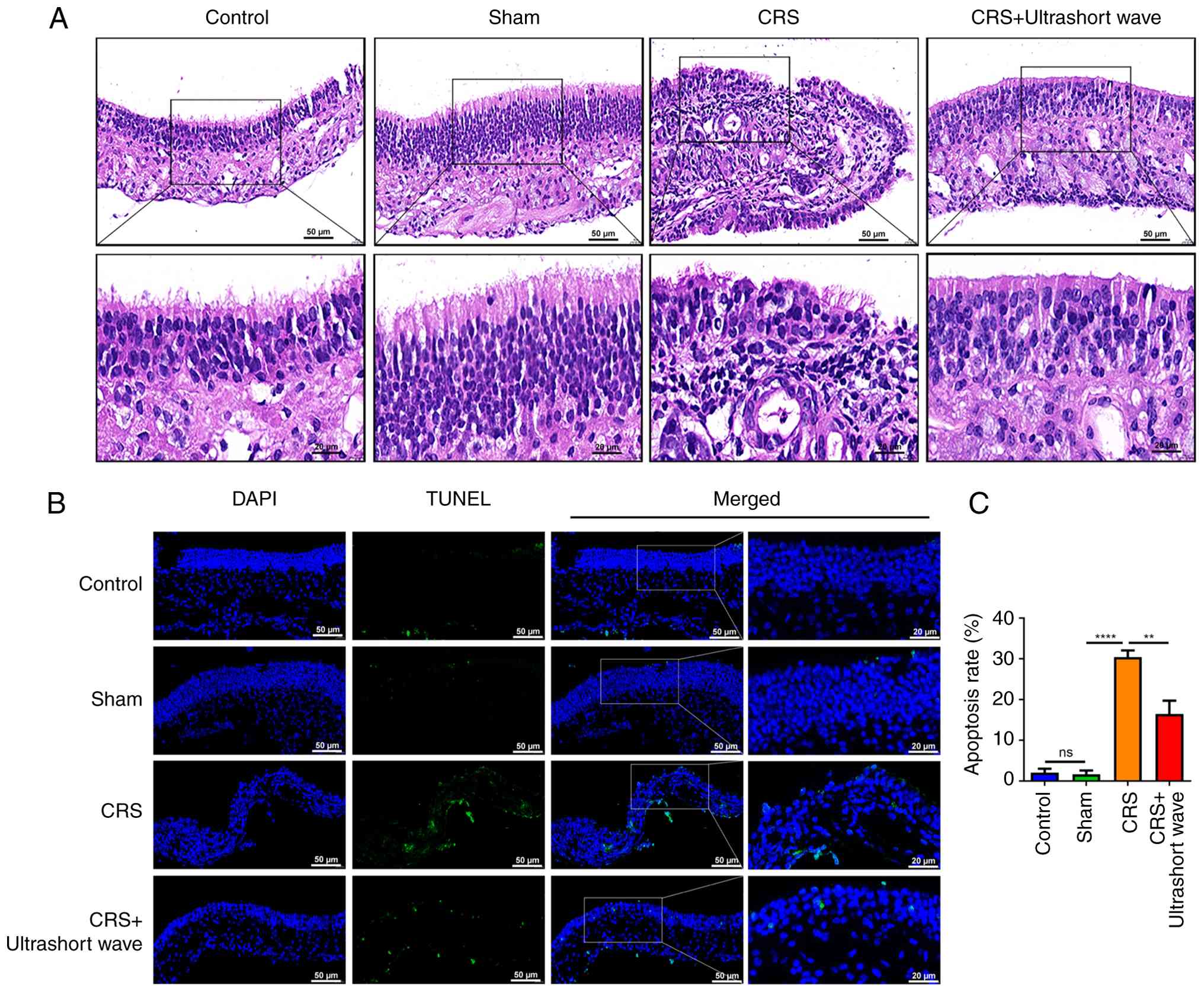
H&E staining was employed to observe the
histopathology of sinus mucosal samples. As illustrated in Fig. 1A, the epithelial cells in the sinus
mucosa of control mice were orderly arranged, with no evidence of
inflammatory cell infiltration observed in either the mucosa or
submucosa. The sinus mucosal epithelial cells and ciliary
structures in the sham group appeared intact and well-arranged,
with sporadic goblet cells present in the submucosal layer. By
contrast, the nasal sinus mucosa of mice with CRS exhibited signs
of chronic inflammation, characterized by a disordered arrangement
of mucosal epithelial cells, cilia shedding, partial cell necrosis,
and significant lymphocytic infiltration. Following ultrashort wave
therapy, there was an observable improvement in both the integrity
of mucosal and ciliary structures as well as a reduction in
lymphocyte numbers within the submucosal layer. The subsequent
TUNEL assay was conducted to evaluate the apoptosis of sinus
mucosal samples. The results indicated that there were no
significant differences in the apoptosis rate between the control
and sham groups. In comparison to the sham group, a notable
increase in the apoptosis rate was observed in the CRS group
(Fig. 1B and C; P<0.0001); however, this increase was
partially mitigated following intervention with ultrashort wave
therapy (P<0.01).
Ultrashort wave therapy inhibits
inflammatory responses in the sinus mucosal samples of mice with
CRS
The expression levels of the anti-inflammatory
cytokine IL-10, alongside pro-inflammatory cytokines such as IFN-γ,
IL-1α, and TNF-α, were subsequently assessed in the sinus mucosal
samples obtained from mice (Fig.
2A). No significant differences were found in the expression
levels of IFN-γ, IL-1α, TNF-α, and IL-10 between the control and
sham groups. The CRS group exhibited a marked increase in the
expression levels of IFN-γ, IL-1α and TNF-α (Fig. 2B-D; P<0.01), along with a
significant decrease in the expression of IL-10 (Fig. 2E; P<0.01), when compared with the
sham group. Following ultrashort wave therapy, these expression
changes were significantly reversed (Fig. 2B-E; P<0.05). Comparable patterns
were noted in the concentrations of IFN-γ, IL-1α, TNF-α, and IL-10
in the NLF samples (Fig. 2F-I;
P<0.05).
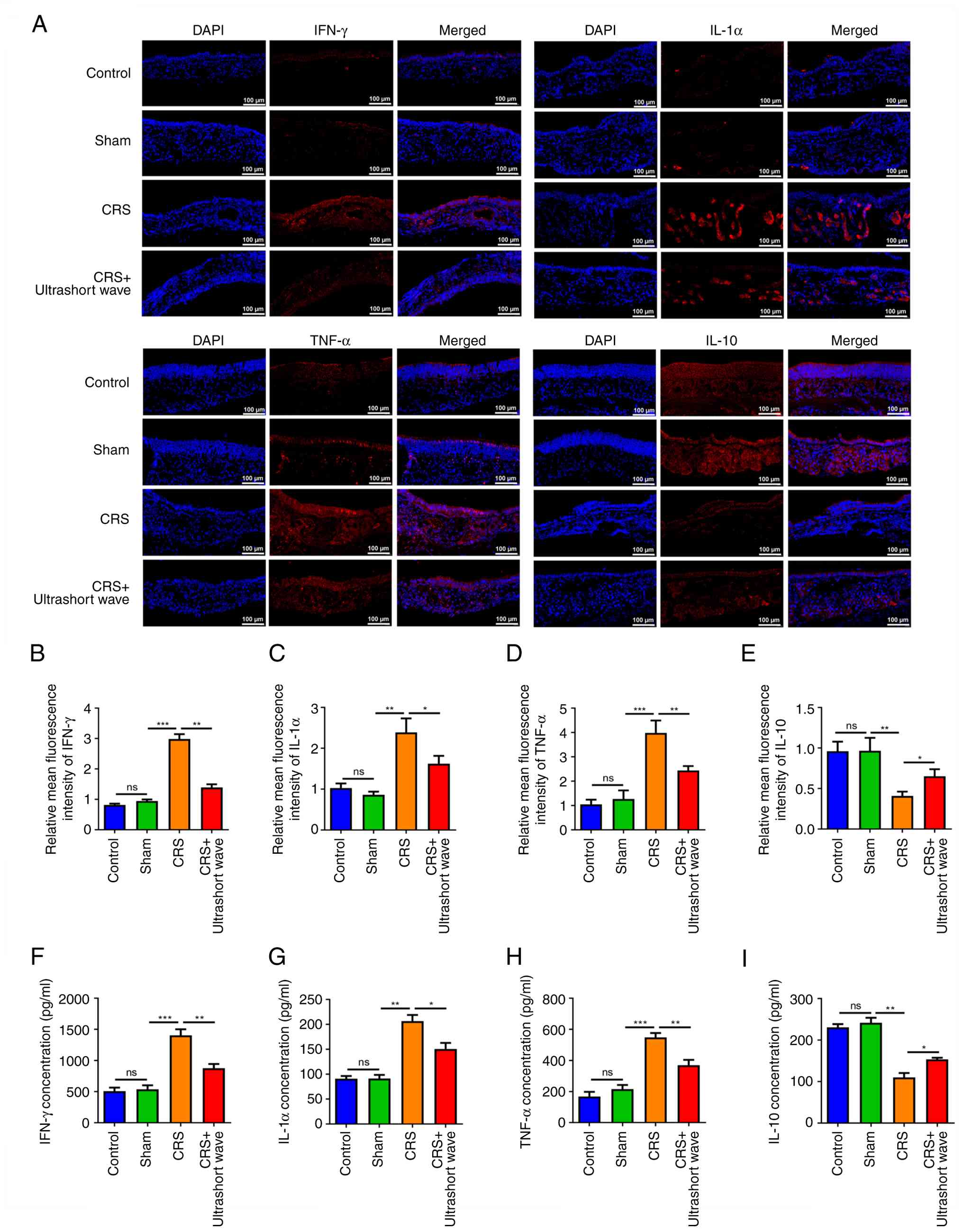 | Figure 2Ultrashort wave therapy inhibits
inflammatory responses in the sinus mucosal samples of mice with
CRS. (A-E) Immunofluorescence was performed to assess the
expression levels of IFN-γ, IL-1α, TNF-α, and IL-10 in sinus
mucosal samples of mice with CRS. (F-I) The concentrations of
IFN-γ, IL-1α, TNF-α, and IL-10 in nasal lavage fluid of mice with
CRS were measured using ELISA. Scale bar, 100 µm.
*P<0.05, **P<0.01 and
***P<0.001. N=6. CRS, chronic rhinosinusitis; ns, no
significance. |
Ultrashort wave therapy inhibits the
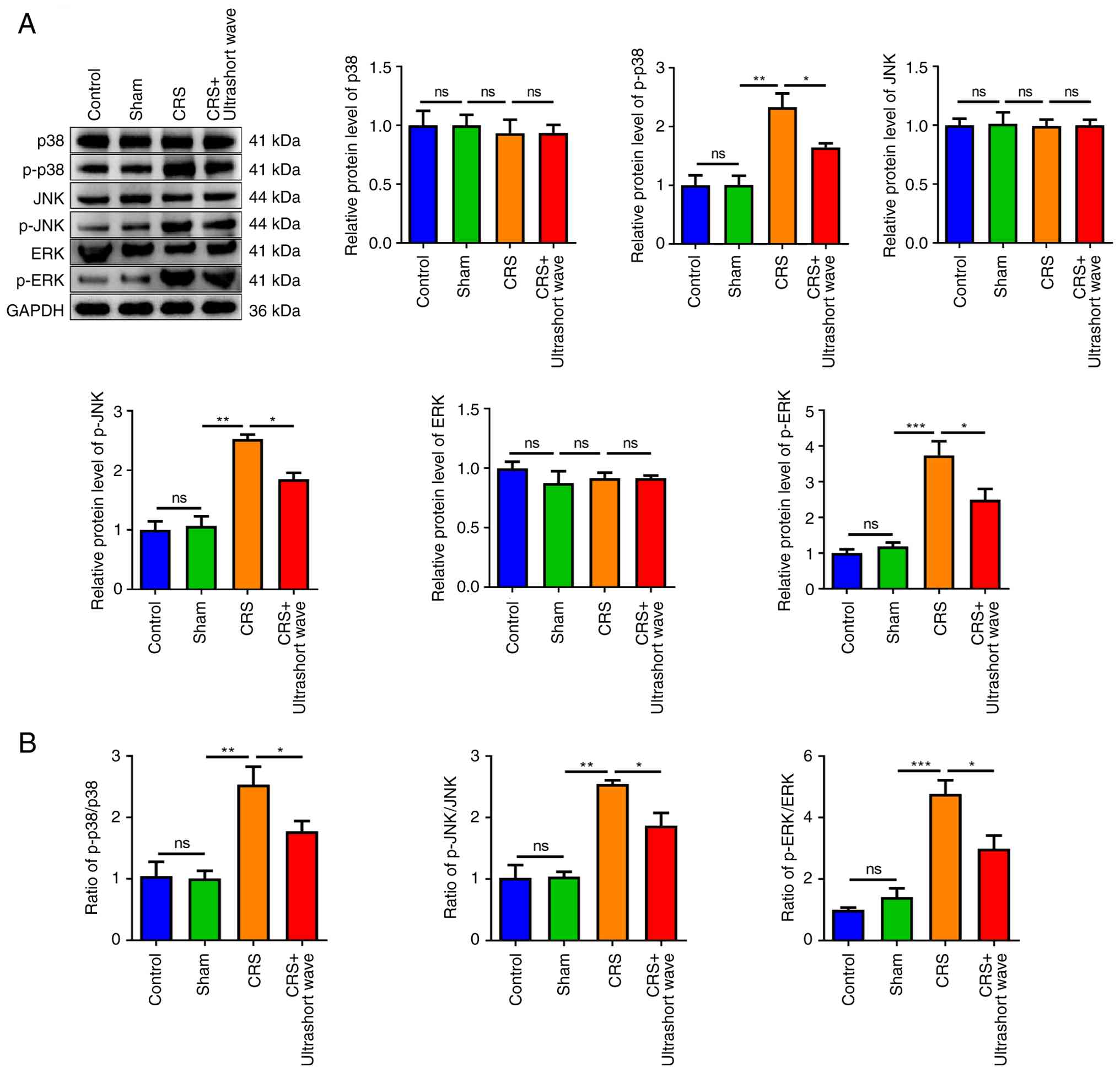
p38/JNK/ERK pathway in mice with CRS
The activation of MAPK pathway, which encompasses
p38, JNK and ERK, plays a crucial role in regulating apoptosis as
well as the release of pro-inflammatory cytokines (13). Subsequently, the effects of
ultrashort wave therapy on the p38/JNK/ERK signalling pathway were
investigated in mice with CRS. As illustrated in Fig. 3A, the protein levels of p38, JNK and
ERK across the various groups exhibited no significant changes.
However, the expression levels of phosphorylated proteins including
p-p38, p-JNK and p-ERK were significantly increased in the CRS
group compared with the sham group (P<0.01). By contrast, the
expression levels of p-p38, p-JNK and p-ERK were significantly
reduced when treated with ultrashort wave treatment (P<0.05). In
addition, a significant upregulation in the ratios of p-p38/p38,
p-JNK/JNK and p-ERK/ERK was observed in the CRS group (Fig. 3B; P<0.01). By contrast, these
ratios were significantly reduced in the CRS + ultrashort wave
group (P<0.05).
Discussion
CRS significantly impacts the quality of life and
hinders the social functioning of affected individuals (14). Of particular concern is paediatric
CRS, which imposes a considerable financial burden and strains
healthcare resources due to its widespread prevalence. A previous
study revealed that in the United States, there are approximately
3.7 to 7.5 million annual healthcare visits related to CRS among
children (15). Furthermore,
expenditures for the treatment of CRS in children aged 12 and under
soared to an astonishing $1.8 billion within a single year
(16). A significant portion of
these costs is associated with identifying the root cause of the
disorder and determining the appropriate treatment. Considering its
prevalence among the paediatric population, it is essential to
formulate strategies aimed at protecting children from CRS. In the
present study, the potential role and mechanisms by which
ultrashort wave therapy influences the progression of CRS in a
mouse model were elucidated. The findings demonstrated that
ultrashort waves effectively inhibited the p38/JNK/ERK signalling
pathway, leading to notable improvements in histopathological
outcomes while simultaneously reducing apoptosis and inflammation
within sinus mucosa.
During the progression of CRS, the sinus mucosal
epithelium undergoes characteristic apoptosis and desquamation
(17). This depletion of epithelial
cells likely compromises the barrier function of the epithelium,
rendering it more vulnerable to bacterial colonization, biofilm
formation, and sustained inflammatory responses (18). Furthermore, the sinus mucosa is
covered by a pseudostratified columnar ciliated epithelium. This
intricate epithelial layer comprises varying proportions of goblet
cells (20%), basal cells (5%), and ciliated cells (75%), all
situated on an acellular basement membrane (19). The cilia play a crucial role in
mucociliary clearance, a fundamental defense mechanism that ensures
the proper functioning of the paranasal sinuses. Effective
mucociliary clearance relies on coordinated ciliary movement and
adequate glandular secretions, both indispensable for maintaining
healthy sinus mucosa (20,21). In the present study, the damaged
ciliary structures within the sinus mucosa of mice with CRS
exhibited significant repair following ultrashort wave
intervention, characterized by a well-organized arrangement.
Furthermore, the apoptotic epithelial cells in the sinus mucosa of
mice with CRS demonstrated a notable reversal when subjected to
ultrashort wave therapy. These findings suggest that ultrashort
wave intervention may effectively improve ciliary structures and
reduce apoptosis in the sinus mucosa during the progression of
CRS.
Another notable characteristic of CRS is the
persistent inflammatory responses that occur within the sinuses.
Studies have demonstrated that the sinus mucosal epithelium plays a
pivotal role in the inflammatory cascades during CRS by secreting a
wide range of pro-inflammatory cytokines, including IFN-γ, IL-1α,
and TNF-α as well as anti-inflammatory cytokine IL-10(22). TNF-α, a multifunctional cytokine
known for its pro-inflammatory properties, can be synthesized by
epithelial cells and may further stimulate the production of IL-1α
and IFN-γ (23). Activated T cells
promote the expression of these pro-inflammatory cytokines in
epithelial cells. High levels of IFN-γ can induce apoptosis in
highly activated epithelial cells (24). The primary biological function of
IL-10 is to suppress antigen presentation by macrophages and
dendritic cells, as well as to inhibit TNF-α production by Th1
lymphocytes (25). Furthermore,
IL-10 can block the effects of IL-1α through the release of its
receptor antagonists (26). These
data suggest that during the development of CRS, the levels of
IFN-γ, IL-1α and TNF-α are elevated, whereas IL-10 levels are
reduced. This finding is consistent with our observations.
Moreover, the findings in the present study further indicated that
ultrashort wave intervention significantly decreased the levels of
IFN-γ, IL-1α, and TNF-α, while markedly increasing the level of
IL-10. These results revealed a notable anti-inflammatory effect
associated with ultrashort wave therapy throughout the progression
of CRS. Currently, the importance of the increased phosphorylation
of p38, JNK and ERK has been demonstrated in various human
inflammatory diseases (27-29).
Notably, numerous studies have respectively reported the roles of
the three signaling factors, p38, JNK and ERK, in CRS. For example,
Lee et al (30) reported
that increased levels of p-p38 exacerbate CRS by inducing
epithelial-mesenchymal transition. Victores et al (31) reported that olfactory loss in CRS is
associated with the activation of JNK. Wu et al (32) found that the occurrence of
inflammatory responses is accompanied by the activation of ERK.
Considering the anti-apoptotic and anti-inflammatory roles of
ultrashort wave therapy in the progression of CRS, it was therefore
hypothesized that the p38/JNK/ERK signalling pathway may be an
important intracellular target of ultrashort wave therapy in
inhibiting the development of CRS. As anticipated, the
phosphorylation levels of p38, JNK, and ERK were significantly
elevated in the sinus mucosa of mice with CRS. However, these
levels were markedly reduced following ultrashort wave therapy.
There are several limitations in the present study
that should be addressed in future research. Numerous studies have
reported the role of NF-κB and PI3K/Akt signalling in the
progression of CRS (33-35).
In addition, NF-κB and PI3K/Akt have been reported to be regulated
by ultrashort wave therapy in some inflammation-related diseases
such as spinal cord injury (36).
However, the interaction between the p38/JNK/ERK signalling pathway
and ultrashort wave therapy in the progression of CRS has rarely
been investigated. Therefore, exploring the role of the p38/JNK/ERK
signalling pathway in the present study may be considered a
somewhat subjective choice. In the future, apart from the
p38/JNK/ERK signalling pathway, exploring the effects of ultrashort
wave therapy on other pathways involved in CRS, such as NF-κB or
PI3K/Akt would strengthen the novelty of the findings.
Additionally, adult C57BL/6J mice aged 8-10 weeks were used for
modelling in the present study. By contrast, the immune system,
nasal cavity, and sinus structure of juvenile mice (particularly
those under 6-8 weeks of age) are still in the developmental
stages. For instance, the sinus mucosa is not fully mature, and the
functionality of immune cells has yet to be completely established.
Modeling may lead to additional lesions and even mortality in
juvenile mice. Furthermore, during the modeling process, these
young mice may undergo rapid growth and development, which could
disrupt the stability of disease progression. Therefore,
translating these findings into clinical practice, particularly in
paediatric patients, presents several potential challenges. First,
species and developmental differences must be carefully considered,
as results obtained from mouse models may not fully apply to human
paediatric patients due to anatomical and immunological
disparities. Thus, the specific mechanisms identified in animal
studies require validation in pediatric populations. Second,
compliance and sedation pose practical obstacles, since young
children may struggle to remain still during treatment,
necessitating sedation that carries anesthesia-related risks.
Finally, long-term safety remains a critical concern, and thus
extended follow-up in preclinical and clinical trials is
essential.
In summary, the present study provides preliminary
insights into the potential role and mechanisms of ultrashort wave
therapy in the progression of CRS in mice. This therapeutic
approach exhibited a notable protective effect during CRS
progression. It can mitigate inflammation and apoptosis as well as
protect the integrity of ciliary structures in sinus mucosa by
inhibiting the activation of the p38/JNK/ERK signalling pathway.
Consequently, ultrashort wave therapy may serve as a promising
avenue for developing effective treatments for CRS, either as an
independent intervention or in combination with other
pharmacological agents.
Acknowledgements
Not available.
Funding
Funding: The present study was supported by Key Projects of
Jinhua Science and Technology Bureau (grant no. 2022-3-003).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HX made substantial contributions to the conception
and design of the study. All authors (HX, JW, QB, YJ, CX, and LC)
made substantial contributions to the acquisition, analysis, and
interpretation of data in the present study. HX drafted the
manuscript. JW, QB, YJ, CX, and LC confirm the authenticity of all
the raw data. All authors revised the manuscript critically for
important intellectual content, read and approved the final version
of the manuscript, and agree to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Animal experiments were conducted in compliance with
the guidelines outlined in the NIH Guide for the Care and Use of
Laboratory Animals and approved (approval no. 20220601001) by the
Ethics Committee of Lanxi People's Hospital (Lanxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wallace DV: Treatment options for chronic
rhinosinusitis with nasal polyps. Allergy Asthma Proc. 42:450–460.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Snidvongs K, Sangubol M and Poachanukoon
O: Pediatric versus adult chronic rhinosinusitis. Curr Allergy
Asthma Rep. 20(29)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ramadan HH: Pediatric chronic
rhinosinusitis. Eur Arch Otorhinolaryngol. 281:1131–1137.
2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Poachanukoon O, Nanthapisal S and
Chaumrattanakul U: Pediatric acute and chronic rhinosinusitis:
Comparison of clinical characteristics and outcome of treatment.
Asian Pac J Allergy Immunol. 30:146–151. 2012.PubMed/NCBI
|
|
5
|
Siddiqui Z, Tahiri M, Gupta A, Kin Nam RH
and Rachmanidou A: The management of paediatric rhinosinusitis. Int
J Pediatr Otorhinolaryngol. 147(110786)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ventriglia G, Gervasoni F, Franco M, Magni
A, Panico G and Iolascon G: Musculoskeletal pain management and
thermotherapy: An exploratory analysis of italian physicians'
attitude, beliefs, and prescribing habits. J Pain Res.
16:1547–1557. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang X, Li K, Li M, Chen C, Yang X, Li J
and Zhang H: Ultrashort wave diathermy inhibits pulmonary
inflammation in mice with acute lung injury in a HSP70 independent
way: A pilot study. Mol Biol Rep. 51(750)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yu HPM, Jones AY, Dean E and Liisa Laakso
E: Ultra-shortwave diathermy-a new purported treatment for
management of patients with COVID-19. Physiother Theory Pract.
36:559–563. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kong L, Zhang JL and Zhu CM: Ultrashort
wave therapy for chronic rhinosinusitis with nasal polyps: protocol
for a randomized, double-blind, sham-controlled trial. Trials.
26(391)2025.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fouda KZ, Eladl HM, Ameer MA and Allam NM:
Effect of adding physiotherapy program to the conservative medical
therapy on quality of life and pain in chronic rhinosinusitis
patients. Ann Rehabil Med. 47:393–402. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals, 8th
edition. National Academies Press (US), Washington, DC, 2011.
|
|
12
|
Geng L, Wang S, Zhao Y and Hu H: Gene
expression profile in mouse bacterial chronic rhinosinusitis. Exp
Ther Med. 17:3451–3458. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yu H, Huang X, Xie C, Song J, Zhou Y, Shi
H, Chen M, Wu Y, Ruan Z, Deng L, et al: Transcriptomics reveals
apigenin alleviates airway inflammation and epithelial cell
apoptosis in allergic asthma via MAPK pathway. Phytother Res.
37:4002–4017. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Makary CA, Tumlin P, Asad F, Wasef K and
Ramadan HH: Quality of life measurement for adolescent patients
with sinonasal symptoms. Laryngoscope. 133:1052–1058.
2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Levy DA, Pecha PP, Nguyen SA and Schlosser
RJ: Trends in complications of pediatric rhinosinusitis in the
United States from 2006 to 2016. Int J Pediatr Otorhinolaryngol.
128(109695)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mirza AA, Shawli HY, Alandejani TA,
Aljuaid SM, Alreefi M, Basonbul RA, Alhomaiani SK, Althobaity BA,
Alhumaidi DA and Zawawi F: Efficacy and safety of paranasal sinus
balloon catheter dilation in pediatric chronic rhinosinusitis: A
systematic review. J Otolaryngol Head Neck Surg.
49(69)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma X, Guo S, Liu F, Li C, Shi X, Liu W, Qi
L, Yuan Y, Xie X, Wang P, et al: Unveiling the prevalence and
impact of silent rhinovirus infection in chronic rhinosinusitis
with nasal polyps. Ann Allergy Asthma Immunol. 134:420–430.e1.
2025.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Song J, Wang M, Wang C and Zhang L:
Olfactory dysfunction in chronic rhinosinusitis: Insights into the
underlying mechanisms and treatments. Expert Rev Clin Immunol.
19:993–1004. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ruysseveldt E, Martens K and Steelant B:
Airway basal cells, protectors of epithelial walls in health and
respiratory diseases. Front Allergy. 2(787128)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alekseenko S, Karpischenko S and
Barashkova S: Comparative analysis of mucociliary clearance and
mucosal morphology using high-speed videomicroscopy in children
with acute and chronic rhinosinusitis. Am J Rhinol Allergy.
35:656–663. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chegini Z, Noei M, Hemmati J, Arabestani
MR and Shariati A: The destruction of mucosal barriers, epithelial
remodeling, and impaired mucociliary clearance: Possible pathogenic
mechanisms of Pseudomonas aeruginosa and Staphylococcus aureus in
chronic rhinosinusitis. Cell Commun Signal. 21(306)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang C, Yan B and Zhang L: The
epithelium-derived inflammatory mediators of chronic rhinosinusitis
with nasal polyps. Expert Rev Clin Immunol. 16:293–310.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mitoma H, Horiuchi T, Tsukamoto H and Ueda
N: Molecular mechanisms of action of anti-TNF-α agents-comparison
among therapeutic TNF-α antagonists. Cytokine. 101:56–63.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chufistova AV, Shabaldina EV, Bedareva AV,
Vakhrameev IN, Abramova NA and Shabaldin AV: Features of
inflammatory endotypes and phenotypes in chronic rhinosinusitis.
Vestn Otorinolaringol. 89:60–67. 2024.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
25
|
Xuan L, Zhang N, Wang X, Zhang L and
Bachert C: IL-10 family cytokines in chronic rhinosinusitis with
nasal polyps: From experiments to the clinic. Front Immunol.
13(947983)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liang Y, Yin S, Chen X, Li C and Chen Q:
The causal relationship between autoimmune diseases and
rhinosinusitis, and the mediating role of inflammatory proteins: A
Mendelian randomization study. Exp Biol Med (Maywood).
249(10196)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yue Q, Liu T and Cheng Z: Protective
effect of colchicine on LPS-induced lung injury in rats via
inhibition of P-38, ERK1/2, and JNK activation. Pharmacology.
105:639–644. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jang EJ, Kim H, Baek SE, Jeon EY, Kim JW,
Kim JY and Kim CD: HMGB1 increases RAGE expression in vascular
smooth muscle cells via ERK and p-38 MAPK-dependent pathways.
Korean J Physiol Pharmacol. 26:389–396. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fawzy MA, Maher SA, Bakkar SM, El-Rehany
MA and Fathy M: Pantoprazole attenuates MAPK (ERK1/2, JNK,
p38)-NF-κB and apoptosis signaling pathways after renal
ischemia/reperfusion injury in rats. Int J Mol Sci.
22(10669)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee M, Kim DW, Khalmuratova R, Shin SH,
Kim YM, Han DH, Kim HJ, Kim DY, Rhee CS, Park JW and Shin HW: The
IFN-γ-p38, ERK kinase axis exacerbates neutrophilic chronic
rhinosinusitis by inducing the epithelial-to-mesenchymal
transition. Mucosal Immunol. 12:601–611. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Victores AJ, Chen MF, Smith A and Lane AP:
Olfactory loss in chronic rhinosinusitis is associated with
neuronal activation of c-Jun N-terminal kinase. Int Forum Allergy
Rhinol. 8:415–420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu YS, Sun KY, Tu YY, Li P, Hao D, Yu P,
Chen A, Wan Y and Shi L: miR-200a-3p regulates
epithelial-mesenchymal transition and inflammation in chronic
rhinosinusitis with nasal polyps by targeting ZEB1 via ERK/p38
pathway. Int Forum Allergy Rhinol. 14:41–56. 2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen W, Liu ZJ and Ye J: Relationship
among the expression of GSK3β, PI3K/Akt, and IL-6 in chronic
rhinosinusitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
48:128–134. 2013.PubMed/NCBI(In Chinese).
|
|
34
|
Yang HW, Kim HJ, Park JH, Shin JM and Lee
HM: Apigenin alleviates TGF-β1-induced nasal mucosa remodeling by
inhibiting MAPK/NF-kB signaling pathways in chronic rhinosinusitis.
PLoS One. 13(e0201595)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen XH, Chang LH, Li X, Huang J, Yang L,
Lai X, Huang Z, Wang Z, Wu X, Zhao J, et al: Tc17/IL-17A
Up-regulated the expression of MMP-9 via NF-κB pathway in nasal
epithelial cells of patients with chronic rhinosinusitis. Front
Immunol. 19(2121)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang S, Jia Y, Cao X, Feng S, Na L, Dong
H, Gao J and Zhang L: HUCMSCs transplantation combined with
ultrashort wave therapy attenuates neuroinflammation in spinal cord
injury through NUR77/NF-κB pathway. Life Sci.
267(118958)2021.PubMed/NCBI View Article : Google Scholar
|