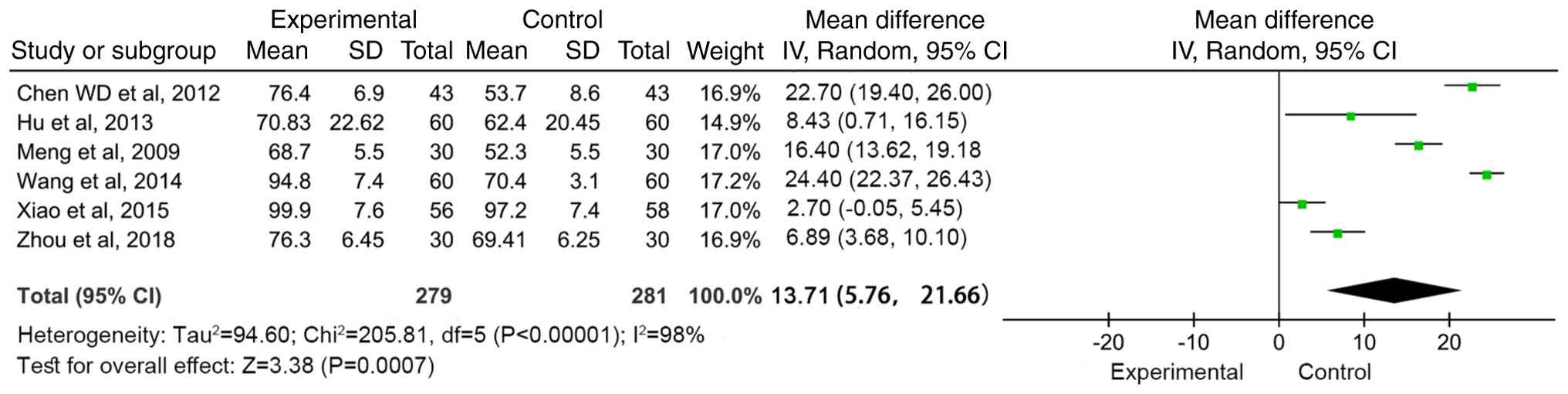
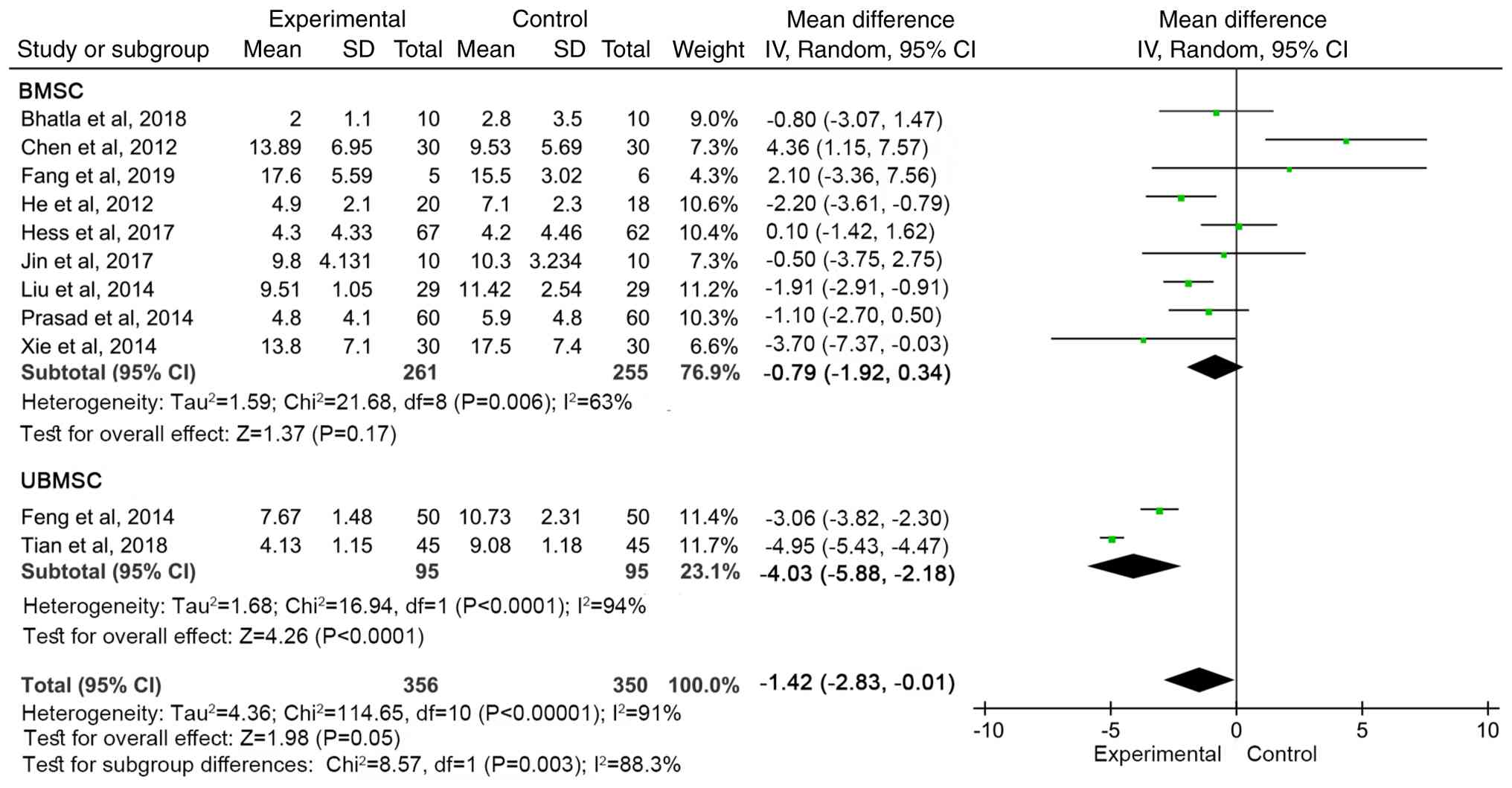
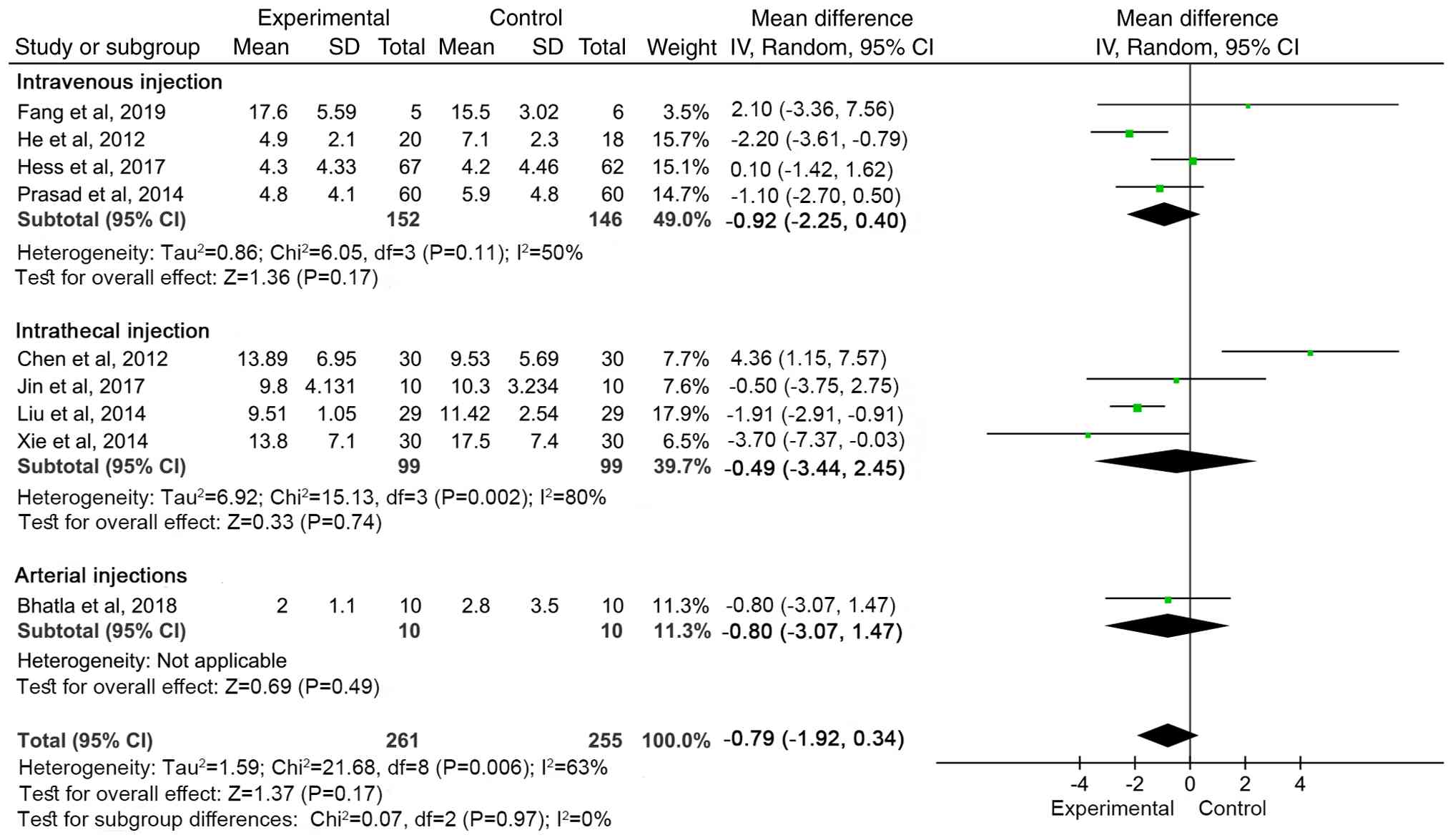
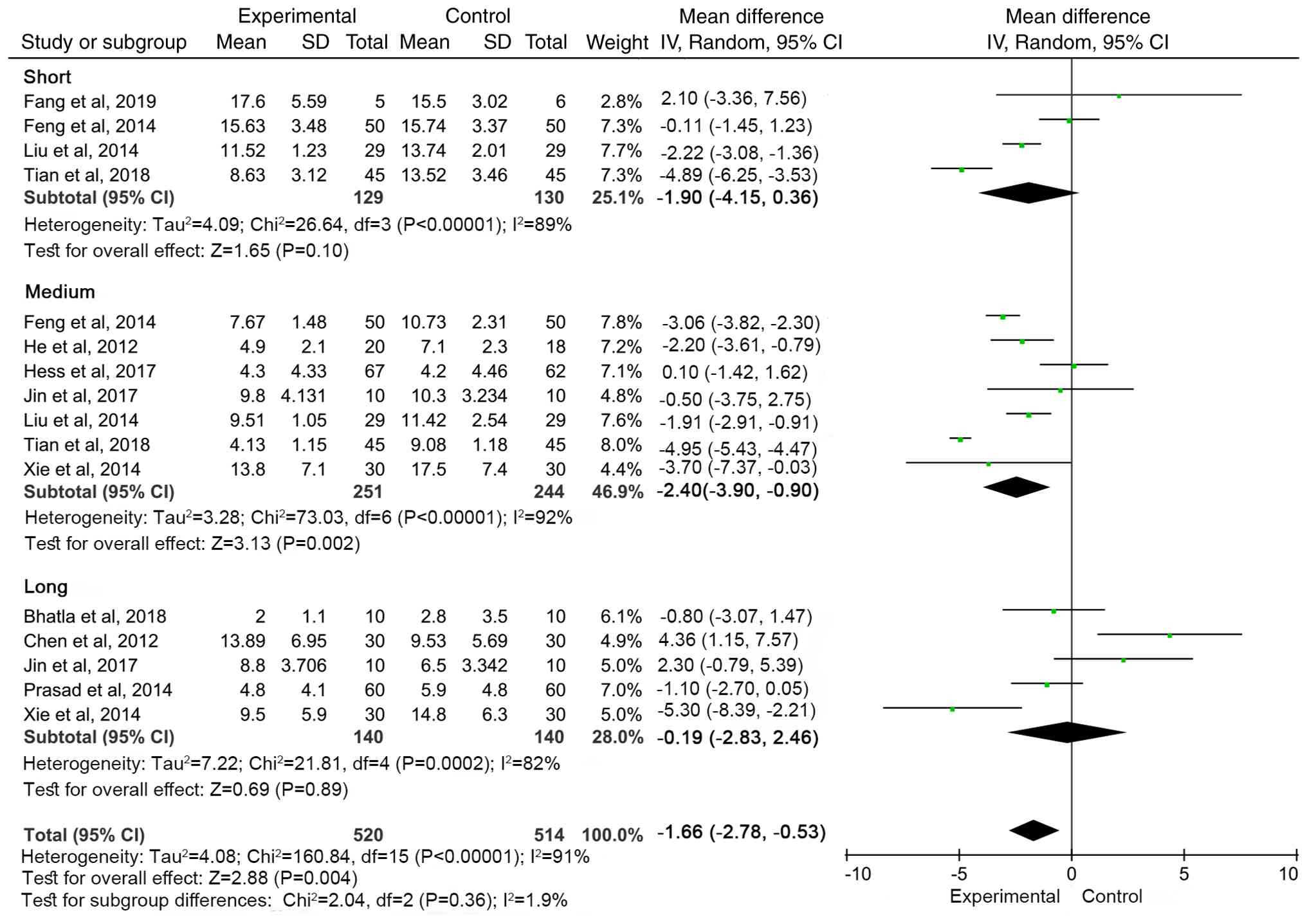
|
1
|
Jiang Y, Li XY, Hu N, Huang ZJ and Wu F:
Epidemiologic characteristics of cerebrovascular disease mortality
in China, 2004-2005. Zhonghua Yu Fang Yi Xue Za Zhi. 44:293–297.
2010.PubMed/NCBI(In Chinese).
|
|
2
|
Chen Y, Gu SL and Wang QQ: Status quo and
influencing factors of quality of life in elderly patients with
acute ischemic stroke hemiplegia after discharge. Chin J Mult Organ
Dis Elderly. 23:194–197. 2024.(In Chinese). https://qikan.cqvip.com/Qikan/Article/Detail?id=7111730250&from=Qikan_Search_Index.
|
|
3
|
Li Y, Hu G and Cheng Q: Implantation of
human umbilical cord mesenchymal stem cells for ischemic stroke:
Perspectives and challenges. Front Med. 9:20–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Banerjee S, Williamson DA, Habib N and
Chataway J: The potential benefit of stem cell therapy after
stroke: An update. Vasc Health Risk Manag. 8:569–580.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kuroda S: Current opinion of bone marrow
stromal cell transplantation for ischemic stroke. Neurol Med Chir
(Tokyo). 56:293–301. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goldstein LB and Samsa GP: Reliability of
the National Institutes of Health Stroke Scale. Extension to
non-neurologists in the context of a clinical trial. Stroke.
28:307–310. 1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fugl-Meyer AR, Jääskö L, Leyman I, Olsson
S and Steglind S: The post-stroke hemiplegic patient. 1. a method
for evaluation of physical performance. Scand J Rehabil Med.
7:13–31. 1975.PubMed/NCBI
|
|
8
|
Granger CV, Cotter AC, Hamilton BB and
Fiedler RC: Functional assessment scales: A study of persons after
stroke. Arch Phys Med Rehabil. 74:133–138. 1993.PubMed/NCBI
|
|
9
|
Hopman-Rock M, van Hirtum H, de Vreede P
and Freiberger E: Activities of daily living in older
community-dwelling persons: A systematic review of psychometric
properties of instruments. Aging Clin Exp Res. 31:917–925.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Saver JL, Chaisinanunkul N, Campbell BCV,
Grotta JC, Hill MD, Khatri P, Landen J, Lansberg MG,
Venkatasubramanian C and Albers GW: XIth Stroke Treatment Academic
Industry Roundtable. Standardized nomenclature for modified rankin
scale global disability outcomes: Consensus recommendations from
stroke therapy academic industry roundtable XI. Stroke.
52:3054–3062. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Higgins JPT, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chung JW, Chang WH, Bang OY, Moon GJ, Kim
SJ, Kim SK, Lee JS, Sohn SI and Kim YH: STARTING-2 Collaborators.
Efficacy and safety of intravenous mesenchymal stem cells for
ischemic stroke. Neurology. 96:e1012–e1023. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fang J, Guo Y, Tan S, Li Z, Xie H, Chen P,
Wang K, He Z, He P, Ke Y, et al: Autologous endothelial progenitor
cells transplantation for acute ischemic stroke: A 4-year follow-up
study. Stem Cells Transl Med. 8:14–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tian LP, Wang JJ and Zhao KL: Observation
of the clinical effect of human umbilical cord blood mesenchymal
stem cells in the treatment of cerebral infarction. Chin J Clin
Ration Drug Use. 11:109–110. 2018.(In Chinese). https://qikan.cqvip.com/Qikan/Article/Detail?id=676508155.
|
|
15
|
Zhou J: Clinical efficacy of cord blood
stem cell transplantation in improving functional independence in
stroke patients. Neural Inj Funct Reconstr. 13:42–43. 2018.(In
Chinese). https://qikan.cqvip.com/Qikan/Article/Detail?id=7000552239&from=Qikan_Search_Index.
|
|
16
|
Bhatia V, Gupta V, Khurana D, Sharma RR
and Khandelwal N: Randomized Assessment of the safety and efficacy
of intra-arterial infusion of autologous stem cells in subacute
ischemic stroke. AJNR Am J Neuroradiol. 39:899–904. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jin Y, Ying L, Yu G and Nan G: Analysis of
the long-term effect of bone marrow mononuclear cell
transplantation for the treatment of cerebral infarction. Int J
Clin Exp Med. 10:3059–3068. 2017.
|
|
18
|
Hess DC, Wechsler LR, Clark WM, Savitz SI,
Ford GA, Chiu D, Yavagal DR, Uchino K, Liebeskind DS, Auchus AP, et
al: Safety and efficacy of multipotent adult progenitor cells in
acute ischaemic stroke (MASTERS): A randomised, double-blind,
placebo-controlled, phase 2 trial. Lancet Neurol. 16:360–368.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xiao HX: Clinical efficacy of stem cell
transplantation in stroke. Medical Recapitulate. 21:1875–1876.
2015.(In Chinese). https://qikan.cqvip.com/Qikan/Article/Detail?id=665002239.
|
|
20
|
Xie XF, Liu SY, Jin GH, Qu XH, Zhang KN
and Wu XM: Clinical analysis of autologous bone marrow mesenchymal
stem cell transplantation for treatment of cerebral infarction. Lab
Med Clin. 11:2955–2957. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang X, Zhang Z, Jia FR and Yang H: A
clinical study on treatment of cerebral infarction using autologous
marrow mesenchymal stem cells intervening transplantation. Jilin
Med. 35:237–239. 2014.(In Chinese). https://qikan.cqvip.com/Qikan/Article/Detail?id=48441187&from=Qikan_Search_Index.
|
|
22
|
Prasad K, Sharma A, Garg A, Mohanty S,
Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP, et
al: Intravenous autologous bone marrow mononuclear stem cell
therapy for ischemic stroke: A multicentric, randomized trial.
Stroke. 45:3618–3624. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu DH, Han BJ, Hong SS, Wang QG, Du CY,
Gao H, Han LX, Wan MR and Ye Y: Transplanting autologous
mesenchymal nerve stem cells in the treatment of cerebral
infarction. Chin J Phys Med Rehabil. 36:425–428. 2014.(In Chinese).
https://qikan.cqvip.com/Qikan/Article/Detail?id=50201705&from=Qikan_Search_Index.
|
|
24
|
Feng TG, Li L and Zhou J: Clinical
efficacy study of human umbilical cord blood mesenchymal stem cells
in the treatment of cerebral infarction. PJCCPVD. 22:28–30.
2014.
|
|
25
|
Wang XH, Wang SP, Xu GX, Ma SB, Wang T and
Zou SF: Short-term efficacy of umbilical cord blood nucleated cells
in treatment of cerebral infarction sequelae. Transl Med J.
2:332–335. 2013.(In Chinese). https://qikan.cqvip.com/Qikan/Article/Detail?id=48118583&from=Qikan_Search_Index.
|
|
26
|
Hu Q, Cao MY, Li RF, Jiang HW and Ge LT:
Safety and efficacy on the treatment of cerebral infarction with
umbilical cord mesenchymal stem cells. Med J Wuhan Univ. 34:57–70.
2013.PubMed/NCBI View Article : Google Scholar : (In Chinese).
https://qikan.cqvip.com/Qikan/Article/Detail?id=44536233&from=Qikan_Search_Index.
|
|
27
|
He ZD: Study on the mechanism of bone
marrow mesenchymal stem cell transplantation to improve
neurological function in patients with cerebral infarctionf.
Asia-Pacific Tradit Med. 8:126–127. 2012.(In Chinese). https://qikan.cqvip.com/Qikan/Article/Detail?id=44178016&from=Qikan_Search_Index.
|
|
28
|
Chen WM, Zou QY, Lu JJ, Hu YX, Hu QL, Li
ZG and Zeng ZL: Reinfusion of autologous bone marrow mesenchymal
stem cells for treatment of stroke in 30 cases. Chin J Tissue Eng
Res. 16:6071–6075. 2012.(In Chinese). https://qikan.cqvip.com/Qikan/Article/Detail?id=43200003&from=Qikan_Search_Index.
|
|
29
|
Chen WD, Li JT, Zhang XB, Zhao H, Li W,
Yang SQ, Lu GZ, Li D and He L: Clinical analysis of bone marrow
mesenchymal stem cell transplantation for cerebral infarction.
Jilin Med. 33(4522)2012.(In Chinese). https://qikan.cqvip.com/Qikan/Article/Detail?id=42844101&from=Qikan_Search_Index.
|
|
30
|
Meng XG, Zhu SW, Gao H, Li YZ, Shi Q, Hou
HS and Li D: Treatment of cerebral infarction using autologous
marrow mesenchymal stem cells transplantation:A six-month
follow-up. J Clin Rehabil Tissue Eng Res. 13:6374–6378. 2009.(In
Chinese). https://qikan.cqvip.com/Qikan/Article/Detail?id=31368833&from=Qikan_Search_Index.
|
|
31
|
Wen YL, Yun JC, Phil HL, Huh K, Kang YM,
Kim HS, Ahn YH, Lee G and Bang OY: Mesenchymal stem cells for
ischemic stroke: Changes in effects after ex vivo culturing. Cell
Transplant. 17:1045–1059. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bonab MM, Alimoghaddam K, Talebian F,
Ghaffari SH, Ghavamzadeh A and Nikbin B: Aging of mesenchymal stem
cell in vitro. BMC Cell Biol. 7(14)2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lin YC, Ko TL, Shih YH, Lin MY, Fu TW,
Hsiao HS, Hsu JY and Fu YS: Human umbilical mesenchymal stem cells
promote recovery after ischemic stroke. Stroke. 42:2045–2053.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee
IH, Lin WS, Wu CH, Lin WY and Cheng SM: Mesenchymal stem cells from
human umbilical cord express preferentially secreted factors
related to neuroprotection, neurogenesis, and angiogenesis. PLoS
One. 8(e72604)2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Q, Huang C, Zeng F, Xue M and Zhang
X: Activation of the Hh pathway in periosteum-derived mesenchymal
stem cells induces bone formation in vivo: Implication for
postnatal bone repair. Am J Pathol. 177:3100–3111. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fernandez-Moure JS, Corradetti B, Chan P,
Van Eps JL, Janecek T, Rameshwar P, Weiner BK and Tasciotti E:
Enhanced osteogenic potential of mesenchymal stem cells from
cortical bone: A comparative analysis. Stem Cell Res Ther.
6(203)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bateman ME, Strong AL, Gimble JM and
Bunnell BA: Concise review: Using fat to fight disease: A
systematic review of nonhomologous adipose-derived stromal/stem
cell therapies. Stem Cells. 36:1311–1328. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang CY, Fei YP, Xu CS, Zhao Y and Pan Y:
Bone marrow mesenchymal stem cells ameliorate neurological deficits
and blood-brain barrier dysfunction after intracerebral hemorrhage
in spontaneously hypertensive rats. Int J Clin Exp Pathol.
8:4715–4724. 2015.PubMed/NCBI
|
|
39
|
Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH,
Joe E, Kim HO and Lee PH: Neuroprotective effects of human
mesenchymal stem cells on dopaminergic neurons through
anti-inflammatory action. Glia. 57:13–23. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Oh SH, Choi C, Chang DJ, Shin DA, Lee N,
Jeon I, Sung JH, Lee H, Hong KS, Ko JJ and Song J: Early
neuroprotective effect with lack of long-term cell replacement
effect on experimental stroke after intra-arterial transplantation
of adipose-derived mesenchymal stromal cells. Cytotherapy.
17:1090–1103. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Leu S, Lin YC, Yuen CM, Yen CH, Kao YH,
Sun CK and Yip HK: Adipose-derived mesenchymal stem cells markedly
attenuate brain infarct size and improve neurological function in
rats. J Transl Med. 8(63)2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ryu B, Sekine H, Homma J, Kobayashi T,
Kobayashi E, Kawamata T and Shimizu T: Allogeneic adipose-derived
mesenchymal stem cell sheet that produces neurological improvement
with angiogenesis and neurogenesis in a rat stroke model. J
Neurosurg. 132:442–455. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhou L, Wang J, Huang J, Song X, Wu Y,
Chen X, Tan Y and Yang Q: The role of mesenchymal stem cell
transplantation for ischemic stroke and recent research
developments. Front Neurol. 13(1000777)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Miao X, Wu X and Shi W: Umbilical cord
mesenchymal stem cells in neurological disorders: A clinical study.
Indian J Biochem Biophys. 52:140–146. 2015.PubMed/NCBI
|