Introduction
Non-small cell lung cancer (NSCLC) is the leading
cause of cancer-related death worldwide and is associated with poor
patient prognosis (1). One of the
most important prognostic factors is stage of disease. Since the
last version of the International Staging System (ISS) was updated
in 1997 (2,3), revision of this classification has
been proposed by different authors (4–6).
Particularly for stage III NSCLC, the 1997 ISS divides patients
into stage IIIA and IIIB; this group of patients is heterogeneous,
including those with surgically resectable tumors as well as those
with unresectable tumors treated by chemotherapy and/or
radiotherapy. Currently, increased attention is being focused on
new biological parameters for predicting prognosis. Many biological
markers have demonstrated independent prognostic significance in
NSCLC, though not in every single study, and none have yet proven
to be of clinical value (7).
Amino acid transporters are essential for growth and
proliferation in normal and transformed cells (8,9). The
amino acid transport system L is a Na+-independent large
neutral amino acid transport system (8,10).
L-type amino acid transporter 1 (LAT1) is one of the L-type amino
acid transporters and transports large neutral amino acids,
including leucine, isoleucine, valine, phenylalanine, tyrosine,
tryptophan, methinine and histidine (10–12).
LAT1 requires covalent association with the heavy chain of the 4F2
cell surface antigen (CD98) for its functional expression in the
plasma membrane (10). Previous
studies have shown LAT1 to be highly expressed in proliferating
tissues, numerous tumor cell lines (T24 bladder carcinoma cells,
RERF-LC-MA lung small-cell carcinoma cells and HeLa uterine
cervical carcinoma cells) and primary human tumors (12). LAT1 expression was found to be
closely related to the tumor cell growth of liver metastases in a
rat model (13). Recent studies
have demonstrated that positive expression of LAT1 is a significant
factor for predicting poor prognosis in NSCLC and correlates with
the grade of neuroendocrine tumors of the lung (14,15).
Nakanishi et al found that cooperative expression of LAT1
and CD98 was significantly correlated with both overall and
disease-free survival rates in transitional cell carcinoma of the
upper urinary tract (16).
Nawashiro et al described that the overall immunoreactivity
of LAT1 correlates well with the prognosis of patients with
astrocytic tumors, and that high CD98 immunoreactivity also
correlates with high LAT1 expression (17). However, it is unclear whether
cooperative expression of LAT1 and CD98 is associated with overall
survival in NSCLC. In our previous study, LAT1 expression was
analyzed in a limited number of patients with stage III NSCLC
(14). Thus, a large-scale study
is required in order to assess the prognostic significance of LAT1
and CD98 expression in patients with stage III NSCLC.
As stage III NSCLC is a heterogeneous group,
parameters other than disease stage must be examined in order to
improve the therapeutic strategy and prognostic assessment. The
purpose of the present study was to determine whether LAT1 and CD98
serve as significant prognostic factors, particularly in stage III
NSCLC. In addition, LAT1 expression was correlated with the
proliferative activity of the tumors as assessed by the Ki-67
labeling index (LI) and with tumor angiogenesis as assessed by
vascular endothelial growth factor (VEGF) expression, microvessel
density (MVD) and the vascular invasiveness of the tumors.
Materials and methods
Patients
We analyzed 207 consecutive patients with stage III
NSCLC who underwent resection either by lobectomy or pneumonectomy
with mediastinal lymph node dissection at Gunma University Hospital
and National Nishi-Gunma Hospital between June 1996 and December
2003. The involvement of mediastinal lymph nodes and malignant
effusions was not detected pre-operatively in any of the patients.
Nine patients who received induction chemotherapy or radiation
therapy and 1 patient who died from a surgery-related complication
were excluded. In 10 patients, no specimen was available for
immunohistochemical analysis. Thus, a total of 188 patients (121
men, 67 women) were evaluated, including 52 cases of stage III
disease involved in our previous study (14). The study protocol was approved by
the institutional review board.
At the time of surgery, the age of the patients
ranged from 36 to 80 years, with a mean age of 64 years.
Histological classification according to the criteria of the World
Health Organization revealed that 123 patients had adenocarcinoma
(AD), 53 had squamous cell carcinoma (SQC) and 12 had large-cell
carcinoma (LCC). Postoperative pathologic staging based on the
current tumor-node-metastasis classification (3) revealed the tumors to be of stage IIIA
(n=114) and IIIB (n=74). Postoperative adjuvant therapies in the
form of platinum-based chemotherapy and radiation were administered
to 6 and 8 patients, respectively. Intraoperative therapy was not
performed in any of the patients. The postoperative clinical course
was assessed by analyzing outpatient medical records and by
telephone inquiries. The date of surgery was considered the start
date for postoperative survival. The follow-up duration ranged from
6 to 125 months (mean 36).
Immunohistochemical staining
LAT1 and CD98
LAT1 expression was determined by
immunohistochemical staining with an affinity-purified rabbit
polyclonal anti-human LAT1 antibody (12). An oligopeptide corresponding to
amino acid residues 497–507 of human LAT1 (CQKLMQVVPQET) was
synthesized. The N-terminal cysteine residue was introduced for
conjugation with keyhole limpet hemocyanine. The antipeptide
antibody was produced as described elsewhere (18). For immunohistochemical analysis,
antiserum was affinity-purified as described previously (18).
Immunohistochemical staining was performed on
paraffin sections using a polymer peroxidase method (Envision+/HRP;
Dako Cytomation, Denmark). Briefly, deparaffinized rehydrated
sections were treated with 0.3% hydrogen peroxide in methanol for
30 min to block endogenous peroxidase activity. To expose antigens,
sections were autoclaved in 10 mmol/l sodium citrate buffer (pH
6.0) for 5 min and cooled for 30 min. After rinsing in 0.05 M
Tris-buffered saline containing 0.1% Tween-20, the sections were
incubated with affinity purified anti-LAT1 antibody (1.2 mg/ml;
1:3,200) overnight at 4°C. The LAT1 antibody at a concentration of
0.375 μg/ml was used to stain for LAT1. Thereafter, they were
incubated with Envision(+) rabbit peroxidase (Dako, Carpinteria,
CA, USA) for 30 min. The peroxidase reaction was performed using
0.02% 3,3′-diaminobenzidine tetrahydrochloride and 0.01% hydrogen
peroxide in 0.05 mol/l Tris-HCl buffer, pH 7.4. Finally, nuclear
counterstaining was performed with Mayer's hematoxylin. For the
negative control, the incubation step with the primary antibody was
omitted. The specificity of immunoreactions using the anti-LAT1
antibody was established in previous studies (17,19).
CD98 is an affinity purified goat polyclonal
antibody raised against a peptide mapping at the carboxy terminus
of CD98 of human origin. Immunohistochemical staining for CD98 was
performed by the avidin-biotin method. Briefly, formalin-fixed and
paraffin-embedded sections of resected specimens were dewaxed and
rehydrated. The sections were incubated with affinity purified goat
polyclonal antibody against CD98 (1:200; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) overnight at 4°C.
LAT1 and CD98 expression were considered positive
only when distinct membrane staining was present. Staining
intensity was scored as follows: 1, ≤10% of tumor area stained; 2,
11–25% stained; 3, 26–50% stained; 4, ≥51% stained. The tumors in
which stained tumor cells made up >10% of the tumor were graded
as positive. According to this scoring protocol, two investigators
from among the authors, without prior knowledge of the clinical
data, independently graded the staining intensity in all cases. To
test the intraobserver variability, each section was reassessed by
the same investigator after the first assessment was completed. The
time interval between the first and second assessment was at least
4 weeks. The interobserver variability was also determined by
comparing the values of the first measurements of the two
investigators.
Ki-67
The detailed protocol for Ki-67 immunostaining was
as published elsewhere (20).
Briefly, formalin-fixed and paraffin-embedded sections of resected
specimens were dewaxed, rehydrated, trypsinized and boiled in 0.01
mol/l citrate buffer for 20 min. For immunostaining, the murine
monoclonal antibody MIB-1 (Dako, Denmark), specific for human
nuclear antigen Ki-67, was used in a 1:40 dilution. The sections
were lightly counterstained with hematoxylin. Sections of normal
tonsil were used as a positive control for proliferating cells.
A highly cellular area of the immunostained sections
was evaluated. All epithelial cells with nuclear staining of any
intensity were defined as positive. Approximately 1,000 nuclei were
counted on each slide. Proliferative activity was assessed as the
percentage of MIB-1-stained nuclei (Ki-67 LI) in the sample.
VEGF, CD31 and CD34
Immunohistochemical staining for VEGF, CD31 and CD34
was performed by the avidinbiotin method. In brief, sections were
deparaffinized with xylene and rehydrated with ethanol. For VEGF,
the sections were trypsinized and incubated with blocking serum.
For CD31, antigen retrieval was carried out by placing the specimen
in 0.01 mol/l of citrate buffer at pH 6.0 and then exposing it to
microwave heating at 450 W for 20 min. For CD34, the sections were
treated by protease.
The antibodies used were: a monoclonal antibody
against VEGF (1:100; Immuno-Biological Laboratories Co., Ltd.,
Japan); a mouse monoclonal antibody against CD31 (1:50; Dako); and
a mouse monoclonal antibody against CD34 (1:200; Nichirei, Tokyo,
Japan).
The expression of VEGF was quantitatively assessed
according to the percentage of immunoreactive cells from a total of
1,000 neoplastic cells.
MVD was assessed using the criteria of Weidner et
al (21). The areas of highest
neovascularization were identified as regions of invasive carcinoma
with the highest numbers of discrete microvessels stained for CD31
and CD34. Any brown-stained endothelial cell or endothelial cell
cluster that was clearly separate from the adjacent microvessels,
tumor cells and other connective tissue elements was considered a
single countable microvessel. Microvessels in sclerotic areas
within the tumor where microvessels were sparse as well as
immediate adjacent areas of unaffected lung tissue were not
considered in vessel counts. The number of CD31- and CD34-positive
vessels was counted in four selected hot spots in a x400 field
(0.26-mm2 field area). MVD was defined as the mean count
of microvessels per 0.26-mm2 field area (21).
Statistical analysis
The Mann-Whitney U test, paired two group t-test and
χ2 test were used to examine the association of two
categorical variables. Statistical analysis of LAT1 and CD98 scores
was performed by the Mann-Whitney U test. In Ki-67 LI, VEGF and
microvessel counts for CD31 and CD34, the paired two group t-test
was performed. The Spearman rank-order correlation coefficient was
used to assess the relationship between LAT1, Ki-67 LI, VEGF, MVD
and other continuous variables.
The duration of survival was determined as the time
from tumor resection to death from any cause. For survivors, the
duration was determined according to the last date on which
patients were known to be alive. The Kaplan-Meier method was used
to estimate survival as a function of time, and differences in
survival were analyzed by the log-rank test. Multivariate analyses
were performed using the stepwise Cox proportional hazards model to
identify independent prognostic factors. A p-value <0.05 was
considered statistically significant. Statistical analysis was
performed using StatView J-4.5 for Windows.
Results
Immunohistochemical findings
LAT1, CD98, Ki-67, VEGF, CD31 and CD34
immunohistochemical staining was evaluated for the 188 surgically
resected primary lesions.
LAT1 and CD98
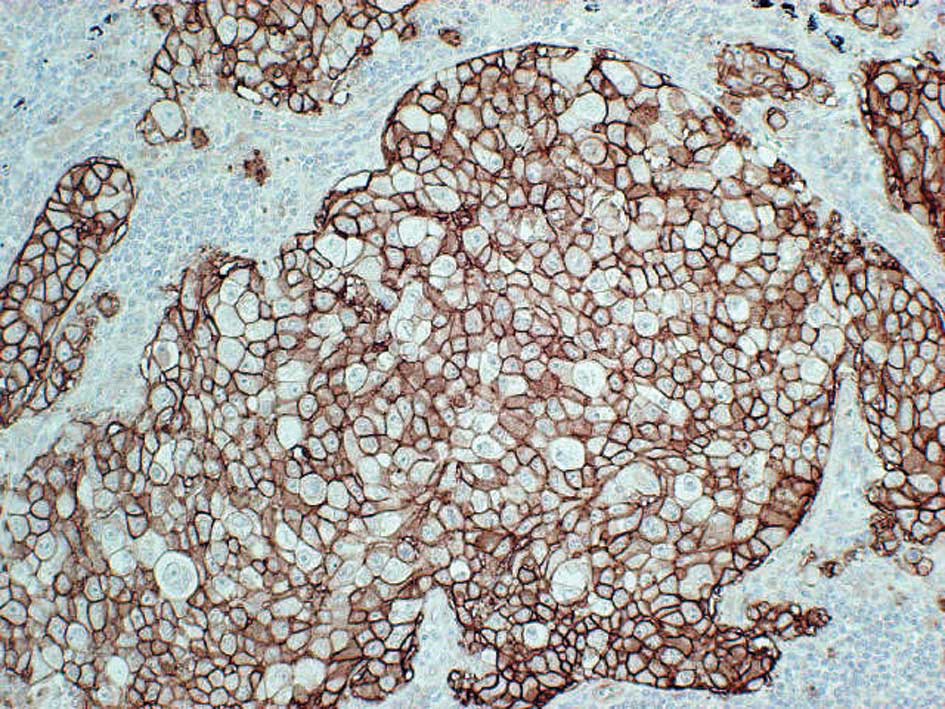
Expression of LAT1 and CD98 was localized
predominantly on the plasma membrane of carcinoma cells in the
tumor tissues (Fig. 1).
Cytoplasmic staining was rarely evident. In the present study, no
expression of LAT1 and CD98 was observed in any normal epithelial
cells of the lung, including bronchial epithelium and alveolar
cells. Positive LAT1 and CD98 expression was observed in 58%
(109/188) and 50% (94/188) of the samples, respectively (p=0.1473).
The average score of LAT1 and CD98 expression was 2.1±1.1 and
1.8±0.9, respectively (p=0.0113). A positive rate of LAT1
expression was significantly higher in SQC (90%, 48/53) and LCC
(100%, 12/12) than in AC (40%, 49/123). A positive rate of LAT1
with CD98 expression was also significantly higher in SQC (74%,
39/53) and LCC (75%, 9/12) than in AC (34%, 42/123).
The expression of LAT1 according to
clinicopathological parameters is shown in Table I. In NSCLC, LAT1 expression was
significantly associated with gender, disease stage, lymph node
metastases with N2–3 and vascular invasion. In AC, positive LAT1
expression was significantly associated with gender. In SQC, LAT1
expression was significantly associated with lymph node metastases
with N2–3. In Table II,
cooperative expression of LAT1 and CD98 (LAT1 with CD98) according
to the clinicopathological parameters is listed. The cooperative
expression of LAT1 with CD98 was also significantly associated with
gender, disease stage and vascular invasion. N3 lymph node
metastasis was observed in 2 (1.6%) of 125 patients with N2–3. N2
lymph node metastasis was observed in 109 (96%) of 114 patients
with stage IIIA and 16 (22%) of 74 patients with stage IIIB,
demonstrating a significant difference (p<0.001).
 | Table I.Association between LAT1 expression
and clinicopathological features. |
Table I.
Association between LAT1 expression
and clinicopathological features.
| Parameter | All casesa | Adenocarcinoma | Squamous cell
carcinoma |
|---|
|
|
|
|---|
| No. | LAT1-positive
| No. | LAT1-positive
| No. | LAT1-positive
|
|---|
| No. (%) | p-value | No. (%) | p-value | No. (%) | p-value |
|---|
| Total | 188 | 109 (58) | | 123 | 49 (40) | | 53 | 48 (90) | |
| Age | | | | | | | | | |
| ≤65 years | 87 | 49 (56) | 0.767 | 57 | 22 (39) | 0.854 | 25 | 22 (88) | 0.657 |
| >65 years | 101 | 60 (59) | | 66 | 27 (41) | | 28 | 26 (93) | |
| Gender | | | | | | | | | |
| Male | 121 | 80 (66) | 0.003b | 66 | 32 (39) | 0.042 | 44 | 40 (91) | 1.000 |
| Female | 67 | 29 (43) | | 57 | 17 (30) | | 9 | 8 (89) | |
| Disease stage
(p-stage) | | | | | | | | | |
| IIIA | 114 | 75 (66) | 0.009 | 67 | 30 (44) | 0.203 | 39 | 37 (95) | 0.108 |
| IIIB | 74 | 34 (46) | | 56 | 19 (34) | | 14 | 11(79) | |
| Lymph node
status | | | | | | | | | |
| N0–1 | 63 | 33 (40) | <0.001 | 41 | 15 (37) | 0.697 | 16 | 12 (75) | 0.025 |
| N2–3 | 125 | 75 (60) | | 82 | 34 (41) | | 37 | 36 (97) | |
| Lymphatic
permeation | | | | | | | | | |
| Positive | 154 | 89 (58) | 1.000 | 102 | 40 (39) | 0.809 | 44 | 41 (93) | 0.195 |
| Negative | 34 | 20 (58) | | 21 | 9 (42) | | 9 | 7 (79) | |
| Vascular
invasion | | | | | | | | | |
| Positive | 110 | 72 (65) | 0.016 | 68 | 32 (47) | 0.095 | 33 | 31 (94) | 0.353 |
| Negative | 78 | 37 (47) | | 55 | 17 (31) | | 20 | 17 (85) | |
| Pleural
involvement | | | | | | | | | |
| Positive | 110 | 66 (60) | 1.000 | 74 | 31 (42) | 0.579 | 28 | 27 (96) | 0.176 |
| Negative | 78 | 43 (55) | | 49 | 18 (37) | | 25 | 21 (84) | |
 | Table II.Association between cooperative
expression of LAT1 with CD98 and clinicopathological features. |
Table II.
Association between cooperative
expression of LAT1 with CD98 and clinicopathological features.
| Parameter | All casesa (LAT1 with CD98) | Adenocarcinoma
(LAT1 with CD98) | Squamous cell
carcinoma (LAT1 with CD98) |
|---|
|
|
|
|---|
| No. | Positive
| No. | Positive
| No. | Positive
|
|---|
| No. (%) | p-value | No. (%) | p-value | No. (%) | p-value |
|---|
| Total | 188 | 88 (47) | | 123 | 41 (33) | | 53 | 39 (74) | |
| Age | | | | | | | | | |
| ≤65 years | 87 | 41 (47) | 0.771 | 57 | 18 (32) | 0.848 | 25 | 19 (76) | 1.000 |
| >65 years | 101 | 50 (49) | | 66 | 23 (35) | | 28 | 21 (75) | |
| Gender | | | | | | | | | |
| Male | 121 | 63 (52) | 0.034b | 66 | 26 (39) | 0.179 | 44 | 32 (73) | 1.000 |
| Female | 67 | 24 (36) | | 57 | 15 (26) | | 9 | 7 (78) | |
| Disease stage
(p-stage) | | | | | | | | | |
| IIIA | 114 | 62 (54) | 0.011 | 67 | 24 (36) | 0.568 | 39 | 31 (79) | 0.103 |
| IIIB | 74 | 26 (35) | | 56 | 17 (30) | | 14 | 8 (57) | |
| Lymph node
status | | | | | | | | | |
| N0–1 | 63 | 24 (38) | 0.163 | 41 | 14 (34) | 0.839 | 16 | 8 (50) | 0.017 |
| N2–3 | 125 | 62 (50) | | 82 | 26 (32) | | 37 | 31 (84) | |
| Lymphatic
permeation | | | | | | | | | |
| Positive | 154 | 72 (47) | 1.000 | 102 | 33 (32) | 0.618 | 44 | 34 (77) | 0.661 |
| Negative | 34 | 16 (47) | | 21 | 8 (38) | | 9 | 5 (71) | |
| Vascular
invasion | | | | | | | | | |
| Positive | 110 | 60 (55) | 0.025 | 68 | 29 (43) | 0.021 | 33 | 25 (76) | 0.751 |
| Negative | 78 | 29 (37) | | 55 | 12 (22) | | 20 | 14 (70) | |
| Pleural
involvement | | | | | | | | | |
| Positive | 110 | 51 (46) | 1.000 | 74 | 26 (35) | 0.697 | 28 | 21 (75) | 1.000 |
| Negative | 78 | 36 (46) | | 49 | 15 (31) | | 25 | 18 (72) | |
Ki-67
The median value of Ki-67 LI was 41% (range 2–85),
and the value of 40% was established as the cutoff point. High
expression (LI >40%) was noted in 95 (51%) of 188 patients. The
mean value of Ki-67 LI differed significantly between SQC (53±14)
and AC (31±18) (p<0.001).
VEGF, CD31, and CD34
The staining pattern of VEGF was uniformly localized
in the cytoplasm and/or membrane of neoplastic cells as shown in
Fig. 1. The median rate of VEGF
positivity was 35% (range 1–90), and the value of 35% was
established as the cutoff point. High expression (>35) was noted
in 97 (52%) of 188 patients. The median rate of MVD as assessed by
CD31 was 26 (range 1–69), and the value of 25% was chosen as the
cutoff point. High expression (>25) was noted in 103 (55%) of
188 patients. The median rate of MVD as assessed by CD34 was 36
(range 2–121), and the value of 35% was chosen as the cutoff point.
High expression (>35) was noted in 99 (53%) of 188 patients. The
positive expression of VEGF, CD31 and CD34 did not differ
significantly between SCC and AC. Analysis of the relationship
between VEGF and the number of microvessels in the areas of the
highest vascularization showed a significant association between
the mean microvessel count and VEGF expression in the pulmonary
lesions. VEGF expression was significantly associated with the
number of microvessel counts [CD31-MVD (γ=0.6218, p<0.0001) and
CD34-MVD (γ=0.6264, p<0.0001), respectively].
Correlation between LAT1, CD98, cell
proliferation and angiogenesis
LAT1 expression was significantly correlated with
Ki-67 LI, VEGF, CD31 and CD34 expression (Table III), and was closely correlated
with CD98 expression (γ=0.7320, p<0.0001) and Ki-67 LI
(γ=0.7342, p<0.0001). An association was also noted between LAT1
expression and histological type. In patients with AC, LAT1
expression was significantly correlated with Ki-67 LI, VEGF, CD31
and CD34, while in patients with SQC, it was significantly
correlated with Ki-67 LI and CD98, but not with VEGF, CD31 and
CD34.
 | Table III.Correlation between the expression of
LAT1 and other immunohistochemical markers. |
Table III.
Correlation between the expression of
LAT1 and other immunohistochemical markers.
| Markers | Spearman γ | 95% confidence
interval | p-value |
|---|
| CD98 | | | |
| All casesa (n=188) | 0.7320 | 0.6553–0.7937 | <0.0001 |
| AC (n=123) | 0.7382 | 0.6424–0.8113 | <0.0001 |
| SQC (n=53) | 0.5347 | 0.3016–0.7075 | <0.0001 |
| Ki-67 | | | |
| Alla (n=188) | 0.7342 | 0.6580–0.7955 | <0.0001 |
| AC (n=123) | 0.6616 | 0.5451–0.7530 | <0.0001 |
| SQC (n=53) | 0.3632 | 0.0948–0.5824 | 0.0075 |
| VEGF | | | |
| Alla (n=188) | 0.1442 | 0.0032–0.2854 | 0.0484 |
| AC (n=123) | 0.2611 | 0.0828–0.4232 | 0.0035 |
| SQC (n=53) | 0.2103 | −0.0718–0.4612 | 0.1307 |
| CD31 | | | |
| Alla (n=188) | 0.3073 | 0.1675–0.4349 | <0.0001 |
| AC (n=123) | 0.3953 | 0.2285–0.5379 | <0.0001 |
| SQC (n=53) | 0.1769 | −0.1062–0.4335 | 0.2050 |
| CD34 | | | |
| Alla (n=188) | 0.3590 | 0.2235–0.4808 | <0.0001 |
| AC (n=123) | 0.4237 | 0.2617–0.5625 | <0.0001 |
| SQC (n=53) | 0.2288 | −0.0525–0.4764 | 0.0994 |
Postoperative survival according to
immunohistochemical markers
Postoperative survival was compared to the
expression of LAT1 as shown in Table
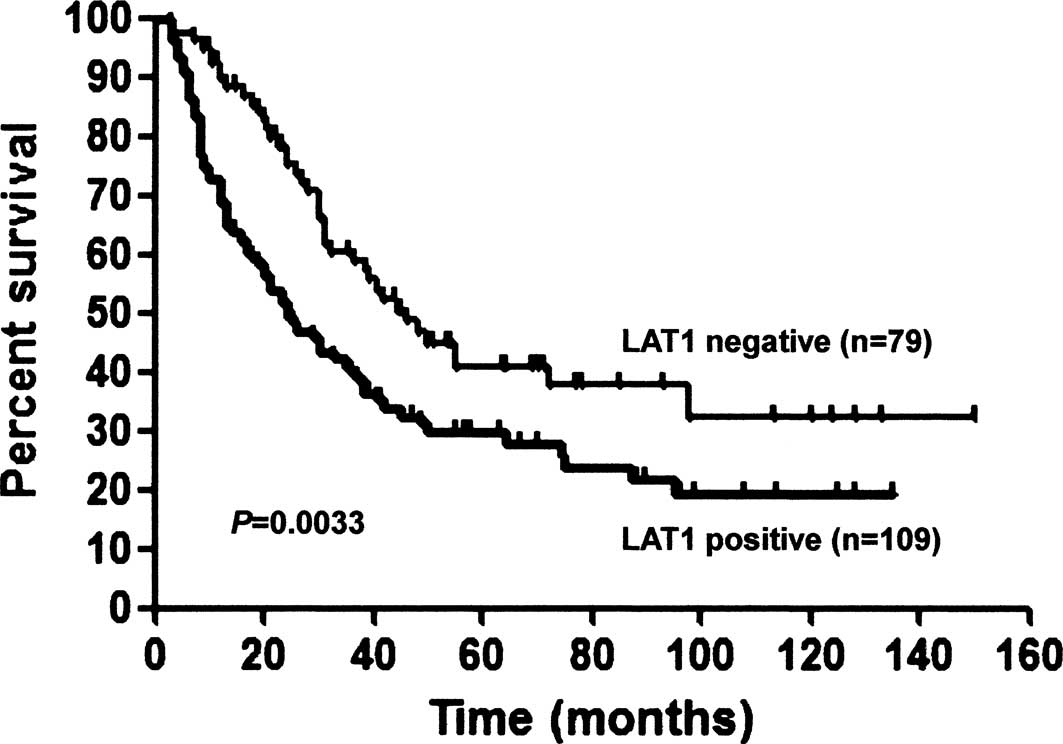
IV. For all patients, the 5-year survival rates of the
LAT1-positive and -negative patients were 27.9 and 40.6%,
respectively (p=0.0033, Fig. 2).
Postoperative survival was also analyzed according to age, gender
and pathologic stage. A significant difference in the prognosis
between the LAT1-positive and -negative patients was demonstrated
for the parameters: age >65 years, female and AC. Next, we
analyzed the postoperative survival of LAT1 with CD98-positive
patients and the other patients. For all patients, the 5-year
survival rates of patients with cooperative LAT1 and CD98
positivity and of the other patients were 24.1 and 43.6%,
respectively (p=0.0004, Fig. 2). A
significant difference in prognosis between LAT1 with CD98-positive
patients and the other patients was demonstrated in terms of age,
gender, pathologic stage and histology (Table V).
 | Table IV.Five-year survival according to LAT1
expression. |
Table IV.
Five-year survival according to LAT1
expression.
| Variable | Five-year survival
rate (%)a
|
|---|
| LAT1-positive | LAT1-negative | p-valueb |
|---|
| All patients | 27.9 | 40.6 | 0.0033 |
| Age | | | |
| ≤65 years | 33.9 | 38.5 | 0.2583 |
| >65 years | 27.0 | 42.5 | 0.0033 |
| Gender | | | |
| Male | 31.7 | 35.4 | 0.1987 |
| Female | 23.6 | 46.6 | 0.0061 |
| Pathologic
stage | | | |
| IIIA | 29.5 | 38.7 | 0.0412 |
| IIIB | 25.1 | 43.1 | 0.0317 |
| Histology | | | |
|
Adenocarcinoma | 23.3 | 38.7 | 0.0032 |
| Squamous cell
carcinoma | 33.0 | 60.0 | 0.1449 |
 | Table V.Five-year survival according to the
cooperative expression of LAT1 and CD98. |
Table V.
Five-year survival according to the
cooperative expression of LAT1 and CD98.
| Variable | Five-year survival
rate (%)a
|
|---|
| LAT1 with
CD98-positivity | Other | p-valuea |
|---|
| All patients | 24.1 | 43.6 | 0.0004 |
| Age | | | |
| ≤65 years | 26.0 | 45.8 | 0.0269 |
| >65 years | 22.8 | 46.2 | 0.0002 |
| Gender | | | |
| Male | 27.4 | 41.4 | 0.0299 |
| Female | 15.4 | 50.1 | 0.0007 |
| Pathologic
stage | | | |
| IIIA | 25.1 | 41.2 | 0.0043 |
| IIIB | 19.6 | 42.0 | 0.0167 |
| Histology | | | |
|
Adenocarcinoma | 19.4 | 39.7 | 0.0002 |
| Squamous cell
carcinoma | 26.4 | 61.5 | 0.0090 |
Postoperative survival was also analyzed according
to the expression of Ki-67, VEGF, CD31 and CD34. For all patients,
the 5-year survival rates of the Ki-67 LI-positive and -negative
patients were 25.7 and 43.4%, respectively (p=0.0006). No
statistically significant difference in postoperative survival was
observed in patients according to VEGF, CD31 and CD34 expression.
Univariate analysis confirmed that positive expression of LAT1,
LAT1 with CD98 and Ki-67 was a significant factor predicting poor
prognosis. There was no significant difference in the 5-year
survival rates according to age, gender, disease stage, histology,
N factor, VEGF, CD31 and CD34.
Multivariate analysis of prognostic
factors
Multivariate analysis confirmed that positive
expression of LAT1 and CD98 was an independent factor for
predicting poor prognosis (Table
VI). Next, a multivariate analysis was performed according to
histological type. In AC patients, multivariate analysis confirmed
that positive expression of LAT1 and CD98 was an independent factor
for predicting poor prognosis. In SQC patients, multivariate
analysis confirmed that positive expression of CD98 was an
independent significant factor for predicting poor prognosis;
however, the positive expression of LAT1 was not an independent
prognostic factor.
 | Table VI.Multivariate analysis of the
prognostic factors in stage III NSCLC. |
Table VI.
Multivariate analysis of the
prognostic factors in stage III NSCLC.
| Prognostic
factor | Hazard ratio | 95% Confidence
interval | p-value |
|---|
| Age (≤65
years/>65 years) | 0.809 | 0.381–1.716 | 0.5798 |
| Gender
(male/female) | 0.921 | 0.603–1.408 | 0.7045 |
| Disease stage
(IIIA/IIIB) | 0.914 | 0.607–1.378 | 0.6692 |
| Histology
(AC/non-AC) | 1.350 | 0.846–2.156 | 0.2082 |
| LAT1
(positive/negative) | 1.529 | 1.053–2.219 | 0.0255 |
| CD98
(positive/negative) | 2.118 | 1.140–3.934 | 0.0175 |
| Ki-67 labeling
index (low/high) | 1.531 | 0.839–2.794 | 0.1652 |
| N factor
(N0–1/N2–3) | 0.664 | 0.410–1.136 | 0.1231 |
Discussion
The present study evaluated the clinical
significance of LAT1 and CD98 expression in surgically resected
stage III NSCLC. The results of the study clearly demonstrate that
the expression of LAT1 and CD98 is a significant independent factor
for predicting poor prognosis in patients with surgically resected
stage III NSCLC. LAT1 expression was higher in SQC than in AC. In
patients with AC, the expression of LAT1 and CD98 was a significant
independent prognostic factor. However, in patients with SQC, the
expression of CD98 was a significant independent prognostic
factor.
We recently reported that LAT1 expression was a
significant prognostic factor in stage I–III NSCLC (14). In a previous study, the positive
rate of LAT1 expression was 23% (38/164) in stage I AC and 88%
(61/69) in stage I SQC. Accordingly, the expression of LAT1 was
higher in stage III as compared to stage I AC. However, the
expression of LAT1 was equivalent between patients with stage I and
III SQC. It is uncertain why the incidence of LAT1 expression was
low in AC as compared to SQC. LAT1 expression was closely
associated with cell proliferation and CD98 expression. The present
study demonstrated that Ki-67 LI and CD98 were significantly higher
in SQC than in AC. These factors may be associated with difference
in the LAT1 expression profile of AC and SQC. On the other hand,
one in vitro study demonstrated a link between amino acid
transporter expression and mammalian target-of-rapamycin (mTOR)
function (23). Although the
mechanism of LAT1 or CD98 expression may be associated with the
mTOR signaling pathway, this association was not examined in the
present study. Further investigation of this association is
warranted.
A previous study found LAT1 expression to be closely
related to the tumor cell growth of liver metastases in a rat model
(13). Tumor size in the LAT1 with
CD98-positive group was significantly larger than tumor size in the
other groups. Moreover, we previously reported that the expression
of LAT1 and CD98 was significantly higher in the metastatic sites
than the primary tumor sites of various human neoplasms (24). These results suggest that the
overexpression of LAT1 and CD98 plays an important role in the
metastatic process of human neoplasms, including NSCLC. In the
present study, the expression of LAT1 was significantly higher in
patients with mediastinal lymph node metastases than in patients
without mediastinal lymph node metastases. Since the incidence of
mediastinal lymph node metastases was significantly higher in stage
IIIA than in stage IIIB cases, LAT1 expression may be higher in
stage IIIA than that in stage IIIB NSCLC (Tables I and II). Another previous study also
demonstrated that LAT1 expression is significantly higher in NSCLC
patients with lymph node metastases than in NSCLC patients without
(14). The overexpression of LAT1
may play an important role in the metastatic process of lymph
nodes, and may be correlated with patient prognosis.
CD98 is a disulphide-linked 125-kDa heterodimeric
membrane glycoprotein that is found on the cell surface of most
normal cells. However, CD98 is also involved in cellular
proliferation, transformation, fusion and adhesion, as well as in
the LAT system, and its expression is up-regulated in a variety of
tumors (16,17,25,26).
Ohkame et al reported the overexpression of CD98 in
metastatic liver tumors in a rat model (13). In the present study, no expression
of CD98 protein was observed in any normal epithelial cells of the
lung, including the bronchial epithelium and alveolar cells.
Overexpression of LAT1 with CD98 was more closely associated with
poor prognosis than LAT1 expression alone. Notably, in SQC the
coexpression of LAT1 and CD98 was an independent prognostic factor,
while the expression of LAT1 was not noted. These results suggest
that CD98 may play a significant role in tumor progression.
Therefore, the up-regulation of both LAT1 and CD98 may be required
for poor prognosis rather than that of LAT1 alone.
Amino acid transport systems play an important role
in the regulation of cellular proliferation, whereas the details
concerning its function as a promoter of tumor cell proliferation
have not been clarified (11).
Previous studies in addition to the present study indicate that
LAT1 expression is significantly associated with Ki-67 LI,
suggesting the role of LAT1 in cellular proliferation (14,27).
Ki-67 LI was significantly higher in SQC and LCC than in AC. A
meta-analysis revealed that the expression of Ki-67 is a factor of
poor survival prognosis in NSCLC (28). The present study also revealed that
high Ki-67 LI was associated with an unfavorable prognosis in NSCLC
patients. On the other hand, LAT1 expression was significantly
associated with angiogenesis as determined by the expression of
VEGF, CD31 and CD34 in AC, although these markers were not factors
of poor prognosis in NSCLC. In contrast to a previous study, whicch
showed that the expression of VEGF is correlated with poor
prognosis in stage I NSCLC (29),
the present study revealed that neither VEGF nor MVD were
independent prognostic markers in stage III NSCLC.
Several clinical investigations have shown an
increased uptake of radio-labelled amino acids in human neoplasms
(30–32). L-[318-F]-methyltyrosine
(18F-FMT) was developed as a tracer for amino acid
transporter using positron emission tomography (33). 18F-FMT is transported
via LAT1, and its uptake correlates with LAT1 expression in NSCLC
(27,30,34).
A recent study demonstrated that the uptake of 18F-FMT
was significantly associated with CD98 expression, cell
proliferation and angiogenesis (35). Future studies are warranted to
clarify the association of 18F-FMT uptake with prognosis
and also with the cooperative expression of LAT1 and CD98 in NSCLC
patients.
2-Aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) is an
amino acid-related compound that has been used as a selective
inhibitor of the system L amino acid transporter, including LAT1
(8,34). Kim et al described that
inhibition of the amino acid transporter LAT1 by BCH led to
apoptotic cell death in cancer cells by inducing intracellular
depletion of essential neutral amino acids, such as L-leucine
(36). Their experimental study
suggested that LAT1 may be a new target for the suppression of the
growth of cancer cells. Nawashiro et al also found that BCH
inhibited the growth of C6 glioma cells in vitro and in
vivo in a dose-dependent manner, and that treatment with BCH
significantly improved the survival of rats inoculated with C6
glioma (17). Thus, LAT1 may be a
molecular target for the therapy of human neoplasms.
Stage III NSCLC comprises a heterogeneous group of
patients. The 1997 ISS is not sufficiently accurate to allow for
the prediction of individual prognosis. Revision of the ISS has
been proposed, having potential clinical implications. However,
none of these revisions include novel biological markers. In the
present study, LAT1 and CD98 expression appears to supplement
prognostic information in the group of patients with stage III
NSCLC. Our study analyzed surgically resected tumors, while other
studies have employed both biopsy and surgical samples (37,38).
Surgical samples are preferable for the immunohistochemical
assessment of potential biological markers.
Patients with stage III NSCLC are a heterogeneous
group that require multimodal therapy, including chemotherapy
and/or radiotherapy. In this study, only patients with surgical
resection were eligible. It is unknown whether LAT1 and CD98
expression is an independent prognostic factor in patients treated
with chemotherapy and/or radiotherapy, and meta-analyses are needed
to answer this question. Our results indicated that there was
significant difference in LAT1 and CD98 expression between AC and
SQC. Moreover, LAT1 expression alone was an independent prognostic
factor in AC, but not in SQC. Therefore, prognostic factors may
differ according to the histological types of NSCLC.
This study had several limitations. The optimal
treatment of stage III NSCLC has not been clearly defined, and many
aspects of its therapy remain controversial. While there are many
potential treatment options, none yield a high probability of cure.
Additionally, the present study may not reflect the general
population of stage III NSCLC, as the currently studied population
had nonbulky stage III disease, and all patients were treated with
surgical resection. However, the survival rate of our study was
better than that reported in previous studies on stage III NSCLC
(36,37). Berghmans et al described
that the undertaking of surgery is a significant independent
prognostic factor for predicting a favorable prognosis in stage III
NSCLC (36).
In conclusion, the expression of LAT1 and CD98 is a
significant biological marker for predicting poor prognosis in
patients with surgically resected stage III NSCLC. LAT1 expression
was significantly correlated with CD98 expression, tumor cell
proliferation and angiogenesis. The overexpression of LAT1 and CD98
plays an important role in the cellular proliferation and
progression of NSCLC. The inhibition of LAT1 and CD98 function may
in future serve as an effective therapeutic target for the
treatment of stage III NSCLC.
Acknowledgements
The authors thank T. Hikino for
technical assistance in the immunohistochemical staining of LAT1,
Ki-67, CD98, VEGF, CD31 and CD34.
References
|
1.
|
Shottenfeld D: Epidemiology of Lung
Cancer. Lippincott-Raven; Philadelphia, PA: 1996
|
|
2.
|
Graziano SL: Non-small cell lung cancer:
clinical value of new biological predictors. Lung Cancer. 17(Suppl
1): 37–58. 1997. View Article : Google Scholar
|
|
3.
|
Mountain CF: Revision in the International
System for Staging Lung Cancer. Chest. 11:1710–1717. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Andre F, Grunenwald D, Pignon JP, et al:
Survival of patients with resected N2 non-small cell lung cancer:
evidence for a subclassification and implications. J Clin Oncol.
18:2981–2989. 2000.PubMed/NCBI
|
|
5.
|
Leong SS, Rocha Lima CM, Sherman CA and
Green MR: The 1997 International Staging System for non-small cell
lung cancer: have all the issues been addressed? Chest.
115:242–248. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jassem J, Skokowski J, Dziadziuszko R, et
al: Results of surgical treatment of non-small cell lung cancer:
validation of the new postoperative pathologic TNM classification.
J Thorac Cardiovasc Surg. 119:1141–1146. 2000. View Article : Google Scholar
|
|
7.
|
Clinical practice guidelines for the
treatment of unresectable non-small cell lung cancer: adopted on
May 16, 1997 by the American Society of Clinical Oncology. J Clin
Oncol. 15:2996–3018. 1997.PubMed/NCBI
|
|
8.
|
Christensen HN: Role of amino acid
transport and countertransport in nutrition and metabolism. Physiol
Rev. 70:43–77. 1990.PubMed/NCBI
|
|
9.
|
McGivan JD and Pastor-Anglada M:
Regulatory and molecular aspects of mammalian amino acid transport.
Biochem J. 299:321–334. 1994.PubMed/NCBI
|
|
10.
|
Oxender DL and Christensen HN: Evidence
for two types of mediation of neutral amino acid transport in
Ehrlich cells. Nature. 197:765–767. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kanai Y, Segawa H, Miyamoto K, Uchino H,
Takeda E and Endou H: Expression cloning and characterization of a
transporter for large neutral amino acids activated by the heavy
chain of 4F2 antigen (CD98). J Biol Chem. 273:23629–23632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yanagida O, Kanai Y, Chairoungdua A, et
al: Human L-type amino acid transporter 1 (LAT1): characterization
of function and expression in tumor cell lines. Biochim Biophys
Acta. 1514:291–302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ohkame H, Masuda H, Ishii Y and Kanai Y:
Expression of L-type amino acid transporter 1 (LAT1) and 4F2 heavy
chain (4F2hc) in liver tumor lesions of rat models. J Surg Oncol.
78:265–272. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kaira K, Oriuchi N, Imai H, et al:
Prognostic significance of L-type amino acid transporter 1
expression in resectable stage I–III nonsmall cell lung cancer. Br
J Cancer. 98:742–748. 2008.
|
|
15.
|
Kaira K, Oriuchi N, Imai H, et al:
Expression of L-type amino acid transporter 1 (LAT1) in
neuroendocrine tumors of the lung. Pathol Res Pract. 204:553–561.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nakanishi K, Ogata S, Matsuo H, et al:
Expression of LAT1 predicts risk of progression of transitional
cell carcinoma of the upper urinary tract. Virchows Arch.
451:681–690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Nawashiro H, Otani N, Shinomiya N, et al:
L-type amino acid transporter 1 as a potential molecular target in
human astrocytic tumors. Int J Cancer. 119:484–492. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Chairoungdua A, Segawa H, Kim JY, et al:
Identification of an amino acid transporter associated with the
cystinuria-related type II membrane glycoprotein. J Biol Chem.
274:28845–28848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Matsuo H, Tsukada S, Nakata T, et al:
Expression of a system L neutral amino acid transporter at the
blood-brain barrier. Neuroreport. 11:3507–3511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Buck AC, Schirrmeister HH, Guhlmann CA, et
al: Ki-67 immunostaining in pancreatic cancer and chronic active
pancreatitis: does in vivo FDG uptake correlate with proliferative
activity? J Nucl Med. 42:721–725. 2001.PubMed/NCBI
|
|
21.
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mineo TC, Ambrogi V, Baldi A, et al:
Prognostic impact of VEGF, CD31, CD34 and CD105 expression and
tumor vessel invasion after radical surgery for IB–IIA non-small
cell lung cancer. J Clin Pathol. 57:591–597. 2004.PubMed/NCBI
|
|
23.
|
Fucks BC, Finger RE, Onan MC and Bode BP:
ASCT2 silencing regulates mammalian target-of-rapamycin growth and
survival signaling in human hepatoma cells. Am J Physiol Cell
Physiol. 293:55–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kaira K, Oriuchi N, Imai H, et al: L-type
amino acid transporter 1 (LAT1) and CD98 expression in the primary
site and the metastatic site of human neoplasms. Cancer Sci.
99:2380–2386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Rintoul RC, Buttery RC, Mackinnon AC, et
al: Cross-linking CD98 promotes integrin-like signaling and
anchorage-independent growth. Mol Biol Cell. 13:2841–2852. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Esteban F, Ruiz-Cabello F, Concha A, Perez
Ayala M, Delgado M and Garrido F: Relationship of 4F2 antigen with
local growth and metastatic potential of squamous cell carcinoma of
the larynx. Cancer. 66:1493–1498. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kaira K, Oriuchi N, Otani Y, et al:
Fluorine-18-α-methyltyrosine positron emission tomography for
diagnosis and staging of lung cancer: a clinicopathological study.
Clin Cancer Res. 13:6369–6378. 2007.
|
|
28.
|
Martin B, Paesmans M, Mascaux C, et al:
Ki-67 expression and patient survival in lung cancer: systematic
review of the literature with meta-analysis. Br J Cancer.
91:2018–2025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Han H, Silverman JF, Santucci TS, et al:
Vascular endothelial growth factor expression in stage I non-small
cell lung cancer correlates with neoangiogenesis and a poor
prognosis. Ann Surg Oncol. 8:72–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Oriuchi N, Higuchi T, Ishikita T, et al:
Present role and future prospect of positron emission tomography in
clinical oncology. Cancer Sci. 97:1291–1297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kaira K, Oriuchi N, Otani Y, et al:
Diagnostic usefulness of fluorine-18-α-methyltyrosine positron
emission tomography in combination with
18F-fluorodeoxyglucose in sarcoidosis patients. Chest.
131:1019–1027. 2007.
|
|
32.
|
Inoue T, Koyama K, Oriuchi N, et al:
Detection of malignant tumors: whole-body PET with
fluorine-18-α-methyl tyrosine versus FDG-preliminary study.
Radiology. 220:54–62. 2001.
|
|
33.
|
Tomiyoshi K, Amed K, Muhammad S, et al:
Synthesis of new fluorine-18 labeled amino acid
radiopharmaceutical: L-F-alpha-methyl tyrosine using separation and
purification system. Nucl Med Commun. 18:169–175. 1997. View Article : Google Scholar
|
|
34.
|
Kim DK, Kanai Y, Choi HW, et al:
Characterization of the system L amino acid transporter in T24
human bladder carcinoma cells. Biochim Biophys Acta. 1565:112–122.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Kaira K, Oriuchi N, Shimizu K, et al:
Evaluation of thoracic tumors with 18F-FMT and
18F-FDG PET-CT: a clinicopathological study. Int J
Cancer. 124:1152–1160. 2009.PubMed/NCBI
|
|
36.
|
Kim CS, Cho SH, Chun HS, et al: BCH, an
inhibitor of system L amino acid transporters, induces apoptosis in
cancer cells. Biol Pharm Bull. 31:1096–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Berghmans T, Meert AP, Martin B, Ninane V
and Sculier JP: Prognostic role of epidermal growth factor receptor
in stage III non-small cell lung cancer. Eur Respir J. 25:329–335.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Berghmans T, Mascaux C, Martin B, Ninane V
and Sculier JP: Prognostic role of thyroid transcription factor-1
in stage III non-small cell lung cancer. Lung Cancer. 52:219–224.
2006. View Article : Google Scholar : PubMed/NCBI
|