Introduction
Bronchiolitis in children is a serious self-limiting
disease (mortality rate <1%) of respiratory tract infections.
However, in high-risk groups such as children with bronchopulmonary
dysplasia, congenital heart disease or cystic fibrosis, mortality
increases to 5–10%. The leading cause of bronchiolitis are viral
infections, with the most common agent being respiratory syncytial
virus infection (RSV) (60–80% of cases) (1–4).
During RSV infection, the cytokine cascade is activated, leading to
the activation of Th1 and Th2 lymphocytes. Thus, an increase in the
concentrations of cytokines such as interleukin (IL)-2, -6, -10,
-12, -13 and a decrease in interferon (IFN)-γ and IL-4
concentrations are observed (5–12).
During non-RSV viral infections, the Th1 type response of the
immunologic system with an increase in the IFN-γ concentration is
commonly observed (5,8,13,14).
IL-15 and IL-18 are relatively newly discovered
cytokines that are produced principally by macrophages during
immune response. IL-15 has multiple biological properties,
including the induction of the production of other cytokines and
the inhibition of T-cell apoptosis (15,16).
IL-18 is a pro-inflammatory cytokine with pleiotropic properties
and plays a crucial role in the maintenance of Th1-cell response.
This cytokine activates NK cells, leads to eosinophilia and
increases histamine concentrations (17–20).
There are various reports indicating that, during viral infection,
IL-15, IL-18 and IFN-γ concentrations are elevated (21). A secondary increase in NK cell
activity has also been reported (22). However, the role of IL-15 and IL-18
in viral bronchiolitis in children remains unknown.
The aim of this study was to analyze IL-15, IL-18
and IFN-γ concentrations and the activity of NK cells as well as
CD4+ and CD8+ lymphocytes in children with
acute viral bronchiolitis.
Patients and methods
Twenty-three children with clinical presentation of
viral bronchiolitis aged 3–30 months (median 9) were included in
the study as the patient group. The concentrations of cytokines and
T lymphocytes were analyzed in blood samples drawn within the first
2 h of hospital admission.
The control group consisted of 15 age-matched
children for IL-18, 14 for IL-15 and 30 for IFN-γ. As a cut-off for
the normal range of analyzed cytokines, values up to the 95th
percentile were allowed.
The concentrations of IL-15, IL-18 and IFN-γ were
determined using ELISA: OPT EIA Human IL-15 (Pharmingen), Human
ELISA IL-18 (R&D Systems), and the OPT EIA Human IFN Gamma kit
(Pharmingen).
CD4+, CD8+ and NK cell
activity was analyzed using the Coulter Epics XL 40256 flow
cytometer. Monoclonal antibodies against CD3, CD4, CD8, CD16 and
CD56 (Dako) were applied. The cells with a
CD3−(CD16+CD56)+ phenotype were
defined as NK cells. Results were expressed as age- and
gender-matched z-scores ± 1 SDS when compared to Polish population
reference values (23). The
Z-score was calculated using the equation: z-score = X −
Xmean/SD, where X = the result and Xmean and
SD are the mean value and standard deviation. The normal values
were between the 5th and 95th percentile of the calculated z-scores
(mean ± 1.645 SD of z-scores).
The etiology of infection was identified with the
serological Becton-Dickinson Directigen™ RSV test kit
and with the Lencomm Euroimmun Pneumo FIDE M (RTP1) kit, which
detect viruses such as RSV, adenovirus, influeza and parainfluenza.
In cases where viral agents could not be diagnosed using these
methods, the viral etiology of infection was defined according to
the following criteria: WBC <12,000/μl with lymphocytosis,
C-reactive protein (CRP) <5 mg/dl and procalcitonin (PCT)
<0.5 ng/dl. In cases where bacterial infection was suspected
based on a physical examination conducted in the pediatric
emergency department, a chest X-ray (CXR) examination was
additionally performed. Only cases in which the CXR was without
inflammatory changes and peripheral oedema or atelectasis were
included (17,24,25).
Children with confirmed RSV infection were excluded from the
study.
The clinical scoring of the severity of the
bronchiolitis was determined according to the criteria proposed by
Papadopoulos et al (26),
with modifications (Table I).
 | Table I.Bronchiolitis severity scale. |
Table I.
Bronchiolitis severity scale.
| Parameter | Score
|
|---|
| 1 | 2 | 3 |
|---|
| Heart rate
(beats/min) | <120 | 120–160 | >160 |
| Breath rate
(breaths/min) | <40 | 40–60 | >60 |
| Wheezing | Expiratory | Inspiratory | Heard without
auscultation |
| Cyanosis | Normal skin | Mild cyanosis | Moderate and
serious cyanosis |
|
Feedinga | Readily | Reluctantly | Oral feeding
impossible |
| SaO2
(%) | >98 | 94–98 | <94 |
Statistical analysis was carried out with Statistica
PL 6.0 using a Student's t-test and χ2 test with Yates'
correction when appropriate. The level of significance was set at
p<0.05.
The study was approved by the local ethics committee
of the Collegium Medicum in Bydgoszcz, Nicolaus Copernicus
University.
Results
In the children with bronchiolitis, the mean
concentrations of IL-15, IL-18 and IFN-γ were 9.39±11.55,
884.03±645.44 and 17.92±27.14 pg/ml, respectively, and were
significantly higher than in the control group [2.34±0.61 pg/ml
(p=0.0295), 248.69±98.73 pg/ml (p=0.0009) and 2.75±1.72 pg/ml
(p=0.0035), respectively] (Table
II).
 | Table II.Concentration of cytokines in
children with bronchiolitis. |
Table II.
Concentration of cytokines in
children with bronchiolitis.
| IL-15 (pg/ml) | IL-18 (pg/ml) | IFN-γ (pg/ml) |
|---|
|
|
|
|---|
| Control | ZO | Control | ZO | Control | ZO |
|---|
| No. | 14 | 23 | 15 | 23 | 30 | 23 |
| Mean | 2.34 | 9.39 | 248.69 | 884.03 | 2.75 | 17.92 |
| SD | 0.61 | 11.55 | 98.73 | 645.44 | 1.72 | 27.14 |
| Median | 2.45 | 6.50 | 274.80 | 699.60 | 2.13 | 9.46 |
| Min | 1.12 | 2.72 | 58.00 | 143.32 | 0.30 | 2.27 |
| Max | 3.18 | 59.09 | 358.50 | 2,621.20 | 6.12 | 103.83 |
| P-value | 0.0295 | 0.0009 | 0.0035 |
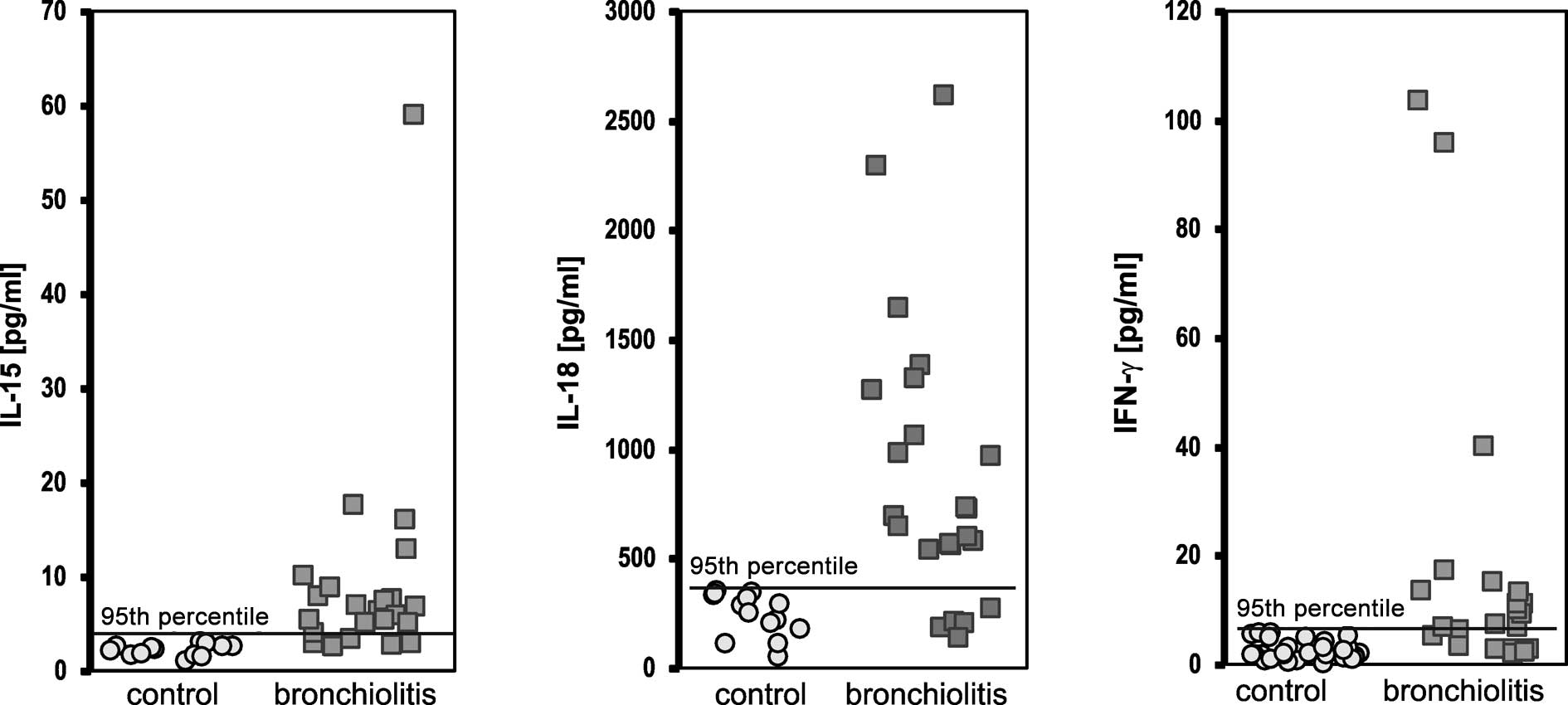
At the time of presentation, the concentrations of
cytokines in the children with viral bronchiolitis above the 95th
percentile of the control values were elevated for IL-15 in 19/23
(82.6%) cases, for IL-18 in 18/23 (78.3%) cases and for IFN-γ in
17/23 (73.9%) cases, and were significantly higher than in the
control group [1/35 (2.8%, p<0.0001) cases, 2/35 (5.7%,
p<0.0001) cases and 2/33(6.1%, p<0.0001) cases, respectively]
(Fig. 1).
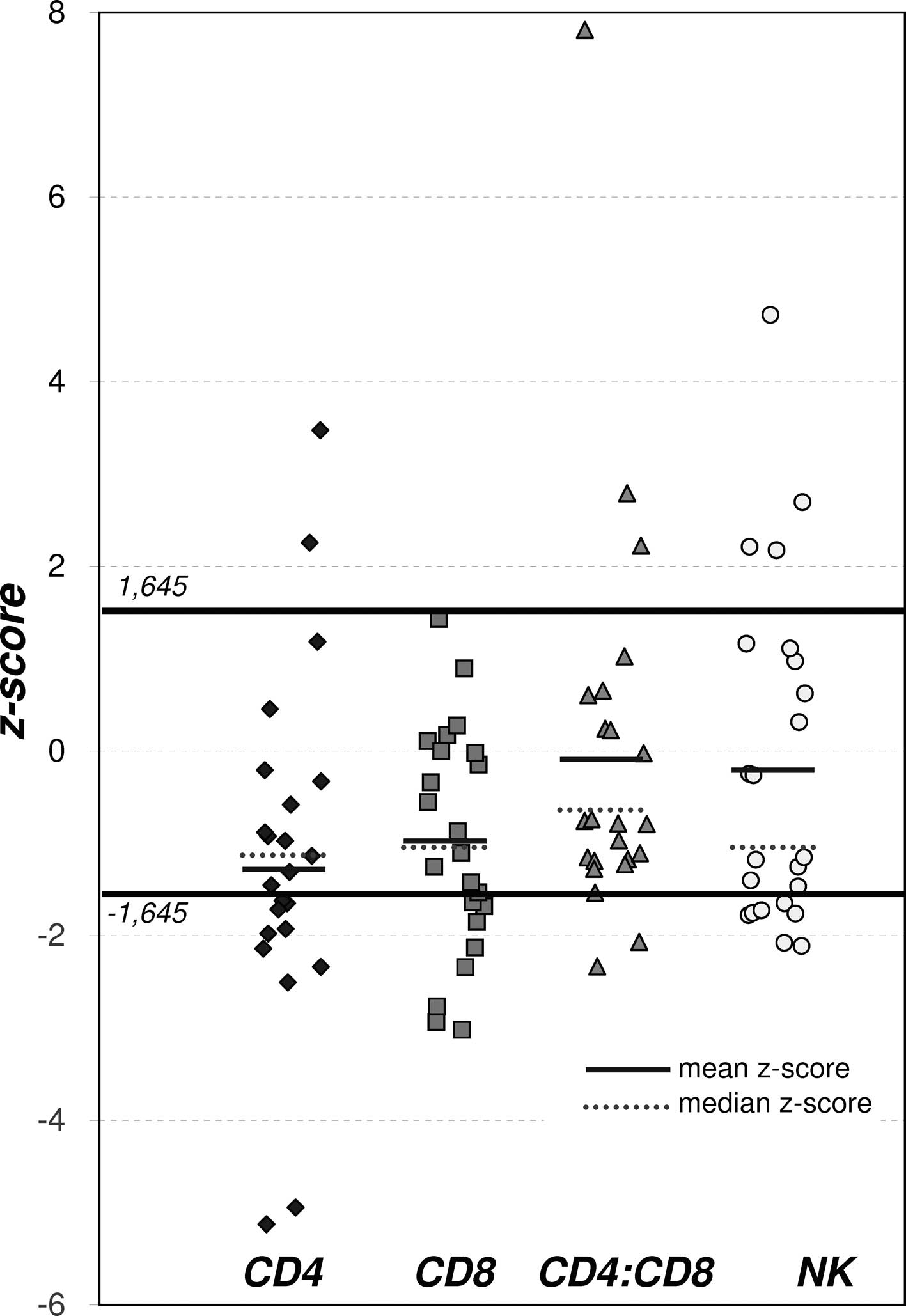
The mean value of z-scores for CD4+ and
CD8+ cells in the bronchiolitis group was significantly
lower than that of the general Polish population (CD4+,
p<0.001 and CD8+, p<0.01) (Table III, Fig. 2). However, neither the mean z-score
of the CD4+/CD8+ ratio nor the NK cell count
differed significantly between the patients and the controls. At
presentation, 12/23 (52.17%) and 7/23 (30.4%) of patients had
CD4+, CD8+ SDS values below −1.645 SDS
(<5th percentile) (Table III,
Fig. 2).
 | Table III.z-scores for NK cell activity and
CD4+, CD8+ and
CD4+/CD8+ ratio. |
Table III.
z-scores for NK cell activity and
CD4+, CD8+ and
CD4+/CD8+ ratio.
| No. | z-score
| z-score
distribution
|
|---|
| Mean ± SD | Median
(min-max) | <−1.645 | −1.645 to
1.645 | >1.645 | P-valuea |
|---|
| CD4 | 23 | −1.15±1.90 | −1.31 (−5.13 to
3.47) | 12 (52.17%) | 10 (43.48%) | 1 (4.35%) | 0.001 |
| CD8 | 23 | −0.90±1.23 | −1.10 (−3.02 to
1.43) | 7 (30.43%) | 15 (65.22%) | 1 (4.35%) | 0.01 |
| CD4:CD8 | 23 | −0.06±2.13 | −0.78 (−2.33 to
7.81) | 2 (8.70%) | 18 (78.26%) | 3 (13.04%) | NS |
| NK cells | 23 | −0.17±1.85 | −1.15 (−2.11 to
4.72) | 6 (26.09%) | 13 (56.52%) | 4 (17.39%) | NS |
No relationships were found between cytokine
concentrations and parameters such as age at the time of
hospitalization, duration of hospitalization, respiratory rate,
saturation, bronchiolitis clinical scoring, CRP and PCT. A positive
correlation was found between IL-15 and time elapsed between the
first symptoms and hospitalization (r=0.4893, p=0.024). IL-15 was
also significantly related to IFN-γ (r=0.7776, p=0.0001). There
were no significant correlations between CD4+,
CD8+, CD4+/CD8+ and age, time
elapsed until hospitalization, duration of hospitalization,
saturation, clinical scoring or CRP, but a significant correlation
was found between the CD4+ count and PCT concentrations
(r=0.9234, p=0.0001). No correlations were observed between NK cell
count and IL-15, IL-18 and IFN-γ concentrations. A significant
negative correlation was found between breath rate and NK cell
activity (r=−0.4880, p=0.025).
Discussion
To our knowledge, this is the first study to
demonstrate that the cytokines IL-15 and IL-18 along with IFN-γ are
involved in the pathogenesis of viral bronchiolitis in children.
The mean concentrations of these cytokines, determined within the
first 24 h following hospital admission, were significantly higher
in children with bronchiolitis than in the control group. The
incidence of increased concentrations of cytokines, over the 95th
percentile of the normal values, was also observed more frequently
in the bronchiolitis group. The activity of CD4+ and
CD8+ cells was lower than in the controls, whereas the
activity of NK cells did not differ significantly compared to the
controls.
Our results are corroborated by the indirect
findings of Okamura et al (21) and Mueller et al (22), who found a significant increase in
IL-15, IL-18 and NK cell activity during viral infection. Others
have reported a predominant increase in IFN-γ concentrations in
children with bronchiolitis (5).
This increase in IFN-γ concentrations appears to be secondary to
the increase in IL-18 concentrations, as this interleukin is one of
the most powerful agents stimulating the production and release of
IFN-γ (20). The relationships
between infection and IL-15 or NK cell activity are similar. IL-15
is a well-known activator of NK cells and T lymphocytes (27). Therefore, in most viral infections,
a primary elevation in IL-15 and IL-18 and a secondary increase in
NK cell activity and IFN-γ concentration are observed (28,29).
This increase is noted in the presence of activation, predominantly
of the TH1 type, of the cytokine cascade (30,31).
Contrary to these findings, no elevation of CD4+,
CD8+ or NK cell activity was noted in the present study,
while an unexpected and significant decrease in the subpopulation
of T lymphocytes was observed. The activity of NK cells did not
differ significantly from that of the controls. However, in the
first phase of viral infection, a decrease was observed in NK cell,
CD4+ and CD8+ activity (14,32).
It is possible that our data reflect these phenomena. Diminished
values of NK cell, CD4+ and CD8+ activity may
be risk factors for more serious infection, indicating the
necessity of hospitalization. An increase in IL-15 concentrations
in relation to the time elapsed before hospitalization was observed
in our study, suggesting that IL-15 may also be a sensitive marker
of the severity of the disease (33–35).
Therefore, in future studies we will investigate the concentrations
of IL-15, IL-18, IFN-γ, T lymphocytes and NK cells throughout the
period of hospitalization for bronchiolitis.
Interpretations of our results have some
limitations. We used serological methods to define the etiology of
bronchiolitis infection and excluded the RSV pathogen. These
methods are of limited value, but at the time of the study
molecular methods were not available. In that respect, we could not
entirely exclude the RSV etiology, and it is likely that in some of
the children more than one virus was identified (36). Moreover, our data were gathered
using blood samples; in future, concentrations of the investigated
cytokines should be determined from respiratory secretions as well.
All of the aforementioned limitations may have impacted the
obtained results.
In summary, our results suggest that IL-15, IL-18
and IFN-γ participate in the generation of inflammatory response
during bronchiolitis in children. During the initial phase of
disease, a significant increase in IL-15, IL-18 and IFN-γ was
noted, with a decrease in the activity of CD4+ and
CD8+ and NK cells.
References
|
1.
|
Kotagal UR, Robbins JM, Kini HD and
Kirschbaum MS: Impact of bronchiolitis guideline – a multisided
demonstration project. Chest. 121:1789–1797. 2002.
|
|
2.
|
Everard ML: Bronchiolitis. Origins and
optimal management. Drugs. 49:885–896. 1995.PubMed/NCBI
|
|
3.
|
Chehab MS, Bafagih HA and Al-Dabbagh MM:
Overview of bronchiolitis. Saudi Med J. 26:177–190. 2005.PubMed/NCBI
|
|
4.
|
Smyth RI and Openshaw PJM: Bronchiolitis.
Lancet. 368:312–322. 2006. View Article : Google Scholar
|
|
5.
|
Van Schaik SM, Tristram DA, Nagpal IS,
Hintz KM, Welliver RC II and Welliver RC: Increased production of
IFN-gamma and cysteinyl leukotrienes in virus-induced wheezing. J
Allergy Clin Immunol. 103:630–636. 1999.PubMed/NCBI
|
|
6.
|
Bont L, Heijnen CJ, Kavelaars A, et al:
Peripheral blood cytokine responses and disease severity in
respiratory syncytial virus bronchiolitis. Eur Respir J.
14:144–149. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Aoyagi M, Shimojo N, Sekine K, Nishimuta T
and Kohno Y: Respiratory syncytial virus infection suppresses
IFN-gamma production of gamma delta T cells. Clin Exp Immunol.
131:312–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Joshi P, Shaw A, Kakakios A and Isaacs D:
Interferon-gamma levels in nasopharyngeal secretions of infants
with respiratory syncytial virus and other respiratory viral
infections. Clin Exp Immunol. 131:143–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Semple MG, Cowell A, Dove W, Greensill J,
McNamara PS, Halfhide C, Shears P, Smyth RL and Hart CA: Dual
infection of infants by human metapneumovirus and human respiratory
syncytial virus is strongly associated with severe bronchiolitis. J
Infect Dis. 191:382–386. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wang SZ and Harrod KS: The immunobiology
of respiratory syncytial virus infection. Clin Applied Immunol Rev.
6:37–52. 2006. View Article : Google Scholar
|
|
11.
|
Castro M, Schweiger T, Yin-Declue H, et
al: Cytokine response after severe respiratory syncytial virus
bronchiolitis in early life. J Allergy Clin Immunol. 122:726–733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lee YM, Miyahara N, Takeda K, Prpich J, Oh
A, Balhorn A, Joetham A, Gelfand EW and Dakhama A: IFN-gamma
production during initial infection determines the outcome of
reinfection with respiratory syncytial virus. Am J Respir Crit Care
Med. 177:208–218. 2008. View Article : Google Scholar
|
|
13.
|
Konno S, Grindle KA, Lee WM, et al:
Interferon-gamma enhances rhinovirus-induced RANTES secretion by
airway epithelial cells. Am J Respir Cell Mol Biol. 26:594–601.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Brooks DG, Teyton L, Oldstone MBA and
McGawern DB: Intrinsic functional dysregulation of CD4 T cells
occurs rapidly following persistent viral infection. J Virol.
79:10514–10527. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Park YB, Kim DS, Lee WK, Suh CH and Lee
SK: Elevated serum interleukin-15 levels in systemic lupus
erythematosus. Yonsei Med J. 40:343–348. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Cassatella MA and McDonald PP:
Interleukin-15 and its impact on neutrophil function. Curr Opin
Hematol. 7:174–177. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Gendrel D, Josette R, Coste J, et al:
Comparison of procalcitonin with C-reactive protein, interleukin 6
and interferon-alpha for differentiation of bacterial vs. viral
infections. Pediatr Infect Disease J. 18:875–881. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang SZ, Bao YX, Rosenberger CL, Tesfaigzi
Y, Stark JM and Harrod KS: IL-12p40 and IL-18 modulate inflammatory
and immune responses to respiratory syncytial virus infection. J
Immunol. 173:4040–4049. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Vankayalapati R, Wisel B, Weis SE, Samten
B, Girard WM and Barnes PF: Production of interleukin-18 in human
tuberculosis. J Infect Dis. 182:234–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ngoumou G, Schaefer D, Mattes J and Kopp
MV: Interleukin-18 enhances the production of interferon-gamma
(IFN-γ) by allergen-specific and unspecific stimulated cord blood
mononuclear cells. Cytokine. 25:172–178. 2004.
|
|
21.
|
Okamura H, Tsutsi H, Komatsu T, Yutsudo M,
Hakura A and Tanimoto T: Cloning of a new cytokine that induces
IFN-gamma production by T cells. Nature. 378:88–91. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mueller YM, Petrovas C, Bojczuk PM, et al:
Interleukin-15 increases effector memory CD8+ T cells
and NK cells in simian immunodeficiency virus-infected macaques. J
Virol. 79:4877–4885. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zeman K, Fornalczyk-Wachowska E, Pokoca L,
et al: Skład odsetkowy podstawowych subpopulacji limfocytów oraz
komórek NK we krwi obwodowej populacji polskiej. Central Europ J
Immunol. 21:107–113. 1996.
|
|
24.
|
Putto A, Ruuskanen O, Meurman O, et al: C
reactive protein in the evaluation of febrile illness. Arch Dis
Child. 61:24–29. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Prat C, Dominiquez J, Rodrigo C, et al:
Procalcitonin, C-reactive protein and leucocyte count in children
with lower respiratory tract infection. Pediatr Infect Dis J.
22:963–968. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Papadopoulos NG, Moustaki M, Tsolia M, et
al: Association of rhinovirus infection with increased disease
severity in acute bronchiolitis. Am J Resp Crit Care Med.
165:1285–1289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Fehniger TA and Caligiuri MA: Interleukin
15: biology and relevance to human disease. Blood. 97:14–32. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Van Benten IJ, van Drunen CM, Koopman LP,
et al: RSV-induced bronchiolitis but not upper respiratory tract
infection is accompanied by an increased nasal IL-18 response. J
Med Virol. 71:290–297. 2003.PubMed/NCBI
|
|
29.
|
Domachowske JB and Rosenberg HF: Advances
in the treatment and prevention of severe viral bronchiolitis.
Pediatr Ann. 34:35–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Stark JM and Busse WW: Respiratory virus
infections and airway hyper-reactivity in children. Pediatr Allergy
Immunol. 2:95–110. 1991. View Article : Google Scholar
|
|
31.
|
Semple MG, Dankert HM, Ebrahimi B, et al:
Severe respiratory syncytial virus bronchiolitis in infants is
associated with reduced airway interferon gamma and substance P.
PLoS ONE. 2:e10382007. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Biet F, Locht C and Kremer L:
Immunoregulatory functions of interleukin 18 and its role in
defense against bacterial pathogens. J Mol Med. 80:147–162. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Pitrez PM, Machado DC, Jones MH, Andrade
F, Camozzato C and Stein RT: Th-1 and Th-2 cytokine production in
infants with virus-associated wheezing. Braz J Med Biol Res.
38:51–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Brooks GD, Buchta KA, Swenson CA, Gern JE
and Busse WW: Rhinovirus-induced interferon-gamma and airway
responsiveness in asthma. Am J Respir Crit Care Med. 168:1091–1094.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Chung JY, Han TH, Kim JS, Kim SW, Park CG
and Hwang ES: Th1 and Th2 cytokine levels in nasopharyngeal
aspirates from children with human bocavirus bronchiolitis. J Clin
Virol. 43:223–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Henrickson KJ, Hoover S, Kehl KS and Hua
W: National disease burden of respiratory viruses detected in
children by polymerase chain reaction. Pediatr Infect Dis J.
23(Suppl 1): 11–18. 2004. View Article : Google Scholar
|