Introduction
A peptide-based cancer vaccine is one of the new
treatment modalities for cancer. We recently reported that it has a
clinical benefit for advanced prostate cancer patients in a
randomized clinical trial (1).
However, peptide-based immunotherapy for cancer patients is highly
restricted by HLA-A alleles, which in turn is hampering the
development of peptide-based cancer vaccines at the commercial
level. Therefore, the identification of candidate peptides widely
applicable for patients with different HLA-A alleles is required.
We previously found and reported (2–5) such
epitope peptides, which bind to more than one HLA-class IA allele.
Therefore, in the present study we examined whether or not the 13
different peptides that have been reported to induce HLA-A3
supertype-restricted cytotoxic T lymphocyte (CTL) activity
(2,3,6–10)
also induce CTL activity restricted to the HLA-A2, HLA-A24 and
HLA-A26 alleles in the peripheral blood mononuclear cells (PBMCs)
of prostate cancer patients.
Materials and methods
Peptide-HLA stabilization assay
To assess the binding and stabilizing activity of
peptides to HLA-A*0201, -A*0206,
-A*0207, -A*2402 and -A*2601
molecules, a previously reported method was employed, with several
modifications (2,3,6–10).
Briefly, RMA-S-A*0201, -A*0206,
-A*0207, -A*2402 and -A*2601
(5×105 cells/well in a 24-well plate) were incubated in
500 μl RPMI-1640 (Invitrogen) supplemented with 20% fetal bovine
serum (FBS) (MP Biomedicals Inc., Eschwege, Germany) for 20 h at
26°C in 5% CO2. Then, the cells were incubated in 500 μl
Opti-MEM (Invitrogen, Carlsbad, CA, USA) containing 0.1–100 μM
peptides and human β2 microglobulin (2 μg/ml) at 26°C for 2 h, and
then for 3 h at 37°C in 5% CO2. Cells were washed and
incubated for 30 min on ice with an appropriate dilution of
anti-HLA-A24 or BB7.2 supernatant (anti-HLA-A2). After being washed
with phosphate-buffered saline (PBS), the cells were stained by
Alexa Fluor 488 goat anti-mouse IgG (Invitrogen) for 30 min on ice.
The mean fluorescence intensity (MFI) was measured by flow
cytometry, and peptides which exhibited a >25% increase in the
MFI were defined as positive binding peptides.
Cell lines
The cell lines used as target cells for cytotoxicity
were the PC3 (HLA-A*2402) and LNCaP
(HLA-A*0201) prostate cancer cell lines, and LNCap
transfected with the HLA-A*2402 gene (LNCaP-A24) as
previously reported (11). PC3 and
LNCaP-A24 tumor cells were used as relevant tumor cells for the
measurement of HLA-A24-restricted CTL activity, whereas LNCaP cells
were used as irrelevant target cells. The cell cultures were
maintained in RPMI-1640 medium supplemented with 10% FBS. For the
pulsing of peptides to the assessed induction of peptide-specific
CTLs from PBMCs as reported previously (1,8,11),
we used C1R-A*2402 cells (an HLA-A*2402
gene-stable transfectant of the B lymphoblastoid cell line, kindly
provided by Dr M. Takiguchi, Kumamoto University, Japan), as
reported previously (11,12). RMA-S cells were derived from a
mouse mutant cell line deficient in antigen processing, which
showed decreased cell surface expression of MHC class I molecules.
The HLA-A*0201, -A*0206, -A*0207,
-A*2402 and -A*2601 genes were also
individually transfected into RMA-S cells using the FuGENE
transfection reagent (Roche, Mannheim, Germany). Clones of stably
HLA gene-transfected cells were established from a separate well in
the presence of geneticin (0.5 mg/ml). The detailed methods for
establishing these transfectants have been reported previously
(7).
Peptides
Peptides with >90% purity were purchased from
Hokkaido System Science (Sapporo, Japan) or Genenet (Fukuoka,
Japan) and dissolved in dimethyl sulfoxide (DMSO) at a
concentration of 10 μg/ml. Fourteen peptides that were previously
shown to be capable of inducing HLA-A3 supertype (-A3, -A11, -A31
and -A33)-restricted CTLs (2,3,6–10)
were used in this study. In addition, Epstein-Barr virus
(EBV)-derived and human immunodeficient virus (HIV)-derived
peptides were used as controls binding to the HLA-A2 and -A24
alleles, as reported previously (13–16).
A peptide derived from positions 155 to 163 of prostate acid
phosphatase (PAP) was used as a positive control for binding to the
HLA-A*0201, -A*0206 and -A*2402
alleles, but not the other alleles, as reported previously
(2). NS31582–1590 was
also used as a positive-control peptide for HLA-A26 (2).
Patients
The Institutional Ethical Review Board of Kurume
University approved the study protocol, which conformed to the
ethical guidelines of the 1975 Declaration of Helsinki. Informed
written consent was obtained from all participants who donated
PBMCs for this study. PBMCs were obtained from 9 prostate cancer
patients and from 3 healthy donors (HDs) who were homozygous for
the HLA-A24 allele. None of the participants were infected with
HIV. The patient characteristics are presented in brief in Table I. PBMCs were isolated from blood
samples by density centrifugation using Ficoll-Conray (density
1.077), and were cryopreserved until use. The expression of HLA-A24
molecules on PBMCs was discriminated by staining with anti-HLA-A24
mAb and analyzed by flow cytometry (2).
 | Table I.Characteristics of the prostate
cancer patients. |
Table I.
Characteristics of the prostate
cancer patients.
| Patient no. | Age | Gender | TMN category | GS | PSA (before
surgery) | PSA (after
surgery) |
|---|
| 1 | 74 | Male | cT2aN0M0 | 3+4=7 | 9.99 | 0.348 |
| 2 | 65 | Male | T2bN0M0 | 3+4=7 | 4.11 | 0.529 |
| 3 | 76 | Male | | | | |
| 4 | 58 | Male | cT2aN0M0 | 4+4=8 | 5.05 | 0.028 |
| 5 | 65 | Male | cT1cN2M0 | 4+3=7 | 9.09 | <0.005 |
| 6 | 66 | Male | cT2aN0M0 | 3+4=7 | 14.70 | 0.006 |
| 7 | 75 | Male | cT3bN0M0 | 4+5=9 | 46.92 | <0.005 |
| 8 | 81 | Male | cT1cN0M0 | 3+3=6 | 4.70 | 0.144 |
| 9 | 70 | Male | cT1cN0M0 | 3+4=7 | 7.45 | 0.049 |
Induction of peptide-specific CTLs from
PBMCs
The induction of peptide-specific CTLs and the
detection of interferon (INF)-γ produced by CTLs were carried out
according to a previously reported method with several
modifications (6). Briefly, PBMCs
(1×105 cells per well in a 96-well U-bottom-type plate)
were incubated with 10 μg/ml of each peptide in culture medium. The
culture medium consisted of 45% RPMI-1640, 45% AIM-V medium
(Invitrogen, Gaithersburg, MD, USA), 10% FBS, 100 U/ml
interleukin-2 and 0.1 mM MEM Non-Essential Amino Acids Solution
(Life Technologies) at 37°C in 5% CO2. On Day 15 of
culture, the cells were divided into four wells. Two of these wells
were mixed with the corresponding peptide-pulsed
C1R-A*2402 cells, while the other two were mixed with
the irrelevant (HIV) peptide and incubated for 18 h at 37°C in 5%
CO2. The IFN-γ production of CTLs was determined by an
enzyme-linked immunosorbent assay. Discrimination of the induction
of peptide-specific CTLs was considered to be successful when the
P-value was <0.05 and when the difference in IFN-γ production
compared to the control HIV peptide exceeded 50 pg/ml.
Cytotoxicity assay
Peptide-stimulated PBMCs were tested for their
cytotoxicity against PC3, LNCaP and LNCap-A24 prostate cancer cells
by a standard 6-h 51Cr-release assay (2). Phytohemagglutinin (PHA)-activated T
cells from HLA-A24-positive patients were used as a negative
control. The PBMCs were also tested for their cytotoxicity against
CIR-A*2401 cells that were pre-pulsed with either a
corresponding peptide or the HIV peptide.
51Cr-labeled target cells (2,000
cells/well) were mixed with effector cells at the indicated
effector-to-target (E/T) ratios in 96 round-well plates.
Immediately before the cytotoxicity assay, CD8+ T cells
were positively isolated using a CD8 Positive Isolation kit (Dynal,
Oslo, Norway) according to the manufacturer's manual. After
incubation for 20 h, the plates were centrifuged and the
supernatant was collected to measure radioactive quantitation by a
gamma counter. The specific 51Cr release was according
to the formula (test cpm - spontaneous cpm). Spontaneous
51Cr release was calculated by measuring the radioactive
quantitation of the 51Cr-labeled target cell supernatant
alone, and the total 51Cr release was then calculated by
measuring the radioactive quantitation of 51Cr-labeled
target cell lysis by 1% Triton X-100 (Wako Pure Chemical
Industries, Osaka, Japan). For the blocking assay, 10 μg/ml of
either anti-HLA class I (W6/32: mouse IgG2a), anti-HLA-DR (L243:
mouse IgG2a) or anti-HLA-B,C (B1-23, IgG2a; kindly donated by Dr
Pierre G. Coulie, Catholique de Louvain University, Brussels,
Belgium) was added to the medium at the initiation of the mixed
culture.
The peptide-stimulated CTLs were confirmed by
specific peptide recognition using a cold inhibition assay. In
brief, 51Cr-labeled target cells (2×104 cells
per well) were mixed with the effector cells (2×104
cells per well) in 96 round-well plates with 2×104 cold
target cells and peptide-pulsed C1R-A*2402 cells.
Statistical analysis
The Student's t-test was used to test statistical
significance, and P-values of <0.05 were considered
significant.
Results
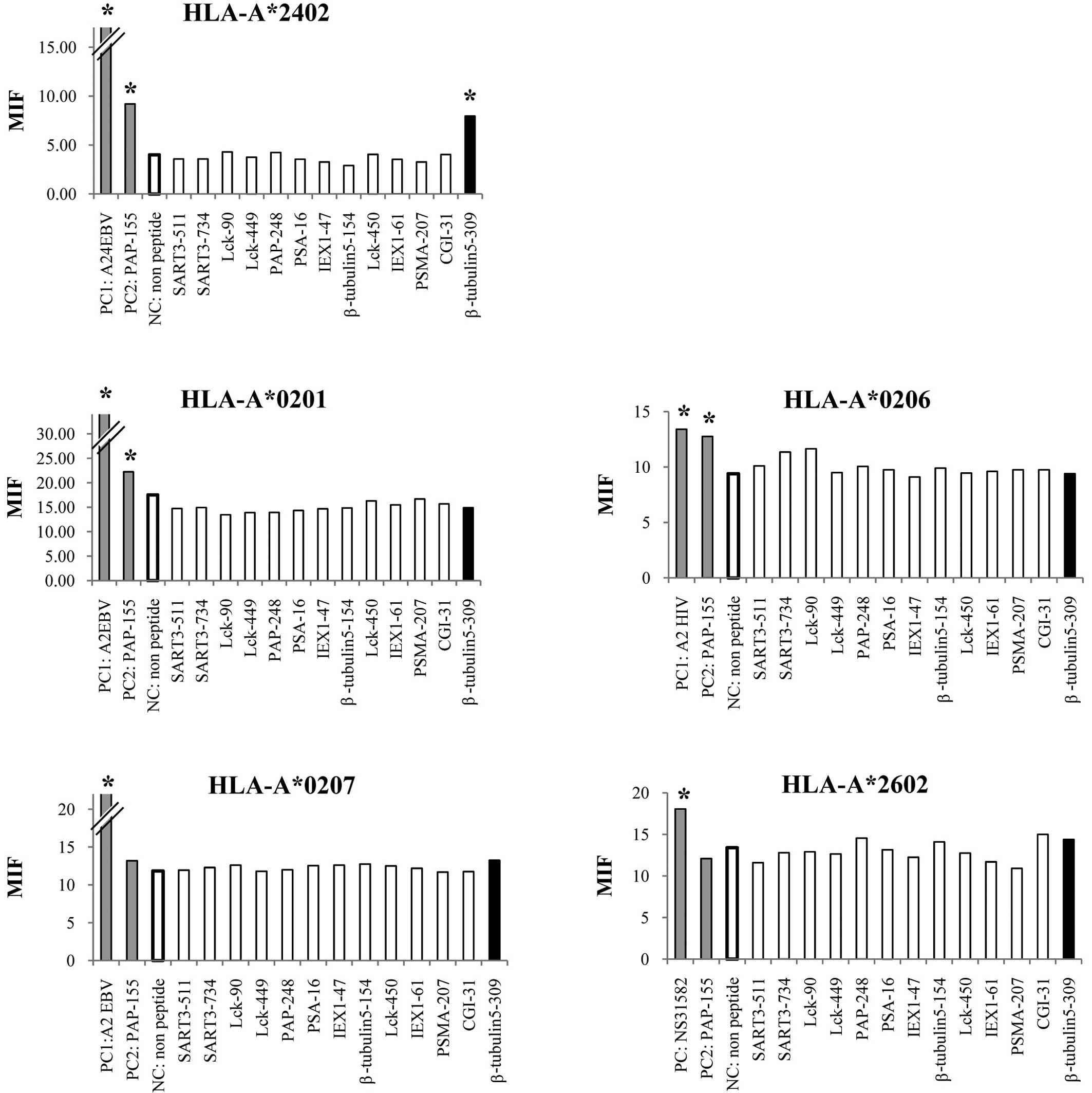
HLA stabilization assay
We first screened the binding activity of each of
the 13 different HLA-A3 supertype peptides (100 μM) to the
HLA-A*0201, -A*0206, -A*0207,
-A*2402 and -A*2601 alleles by means of an
HLA stabilization assay using RMA-S cells expressing each HLA
molecule. A PAP-derived peptide consisting of the amino acid
sequence from positions 155 to 163 was used as a positive control
for binding to the HLA-A*0201, -A*0206 and
-A*2402 alleles, but not the other alleles, as reported
previously (4). As a result, one
peptide from positions 309 to 318 of β-tublin 5 (β-tubulin
5309–318) showed binding activity to
HLA-A*2402 molecules, but not to any of the other
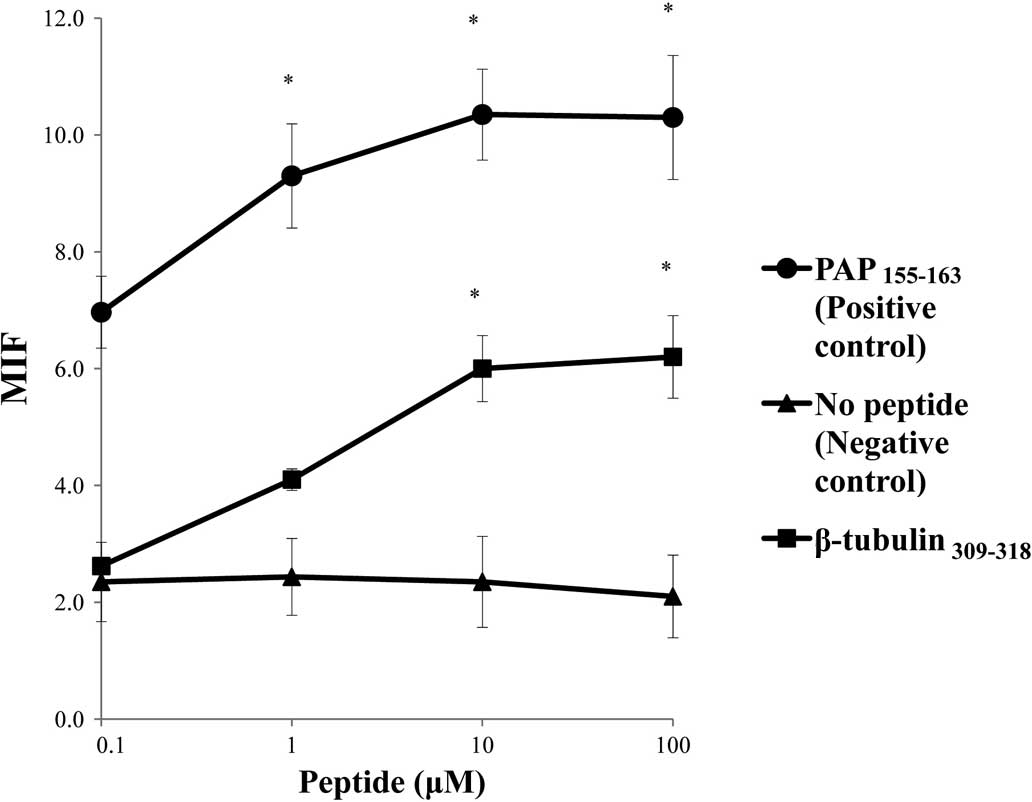
molecules tested (Fig. 1). The
surface expression of the HLA-A*2402 molecules on
RMA-S-A*2402 cells was stabilized in a dose-dependent
manner when cells were cultured with either a positive control or
the β-tubulin 5309–318 peptide (Fig. 2).
 | Figure 1.Stabilization assay of the β-tubulin
5309–318 peptide for various HLA alleles. The binding
activities of the β-tubulin 5309–318 peptide to various
HLA-A alleles were examined using the stable transfectant cell
lines RMA-S-A*0201 (A), -A*0206 (B),
-A*2402 (C), -A*0207 (D) and
-A*2601 (E) with a positive-control peptide and negative
control (DMSO). The positive-control peptides used for each HLA
were HIV-A2 (A and B), HIV-A24 (C), EBV-A2 (D) and
NS31582–1590 (E). A peptide derived from positions 155
to 163 of prostate acid phosphatase (PAP) was used as a positive
control for binding to the HLA-A*0201,
-A*0206 and -A*2402 alleles, but not the
other alleles, as reported previously (4). The mean fluorescence intensity (MFI)
was indicated at 100 μM of the peptide against
HLA-A*2402 (A), HLA-A*0201 (B),
HLA-*0206 (C), HLA-A*0207 (D) and
HLA-A*2602 (E). Representative results from at least
three separate experiments are shown. *Statistically
significant at P<0.05. |
Induction of peptide-specific CTL
activity
We attempted to determine by means of an IFN-γ
production assay whether or not the β-tubulin 5309–318
peptide has the potential to generate peptide-specific CTLs from
prostate cancer patients and HDs. PBMCs from HLA-A24/A24
homozygotes were stimulated in vitro with the β-tubulin
5309–318 peptide, a positive (EBV) control peptide or a
negative (HIV) control peptide, followed by measurement of IFN-γ
production in response to the appropriate peptide-pulsed cells. The
results showed that this peptide induced peptide-specific CTL
activity in the PBMCs from 5 of the 9 patients tested (Table II), but not in any of the 3 HDs
tested (data not shown). Of the 9 patients, 6 showed CTL activity
reactive to the EBV-derived peptide (a positive control), while
none showed CTL activity reactive the HIV-derived peptide (a
negative control) (Table II).
 | Table II.Interferon-γ production in
peptide-stimulated prostate cancer patient peripheral blood
mononuclear cells. |
Table II.
Interferon-γ production in
peptide-stimulated prostate cancer patient peripheral blood
mononuclear cells.
| Patient no. | β-tubulin
5-309 | Positive (EBV) | Negative (HIV) |
|---|
| 1 | 86 | 51 | NS |
| 2 | ns | 242 | NS |
| 3 | ns | 539 | NS |
| 4 | 50 | ns | NS |
| 5 | 50 | 1,501 | NS |
| 6 | 272 | ns | NS |
| 7 | 178 | ns | NS |
| 8 | ns | 457 | NS |
| 9 | ns | 50 | NS |
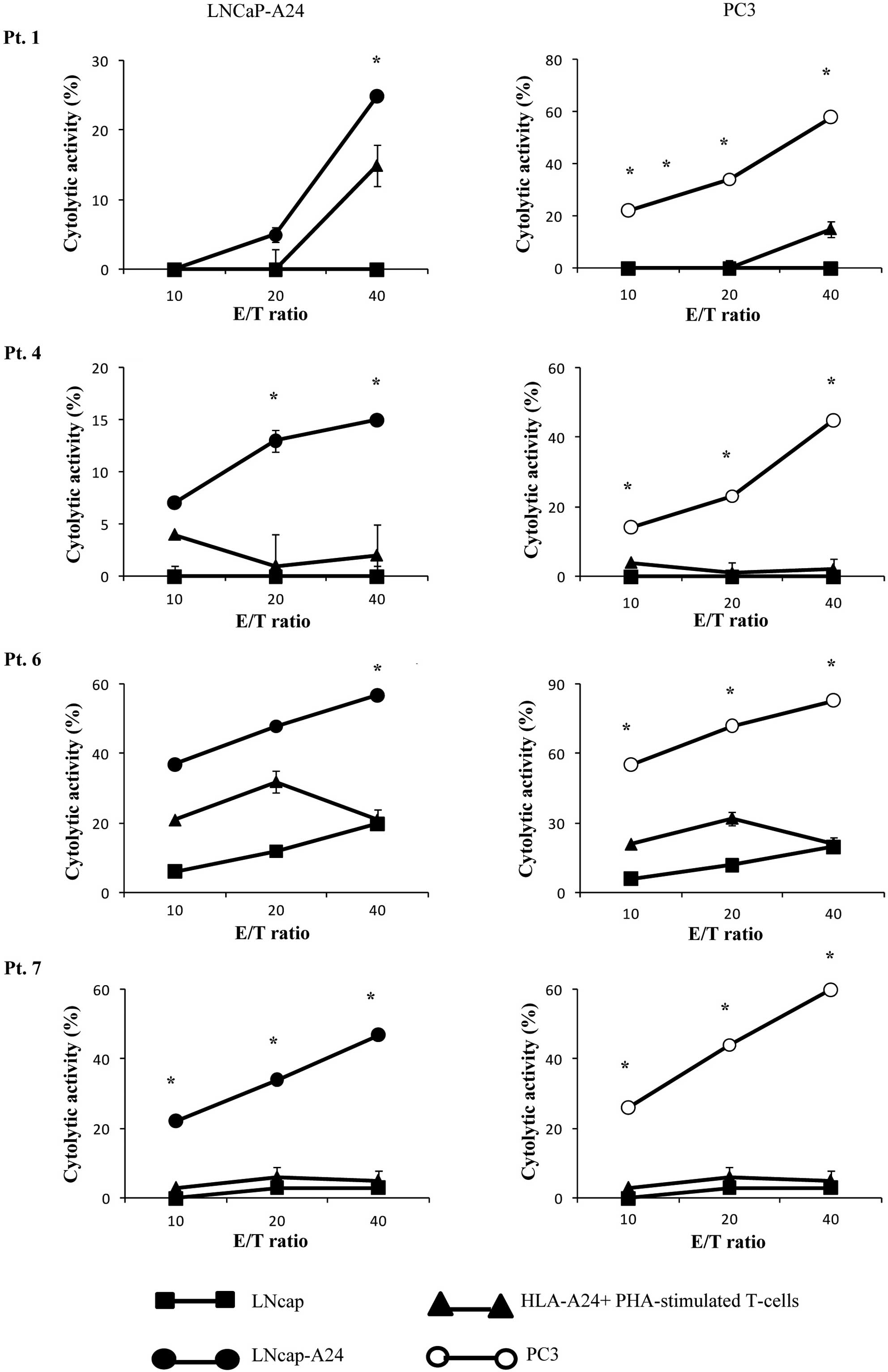
Cytotoxicity assay
We then determined whether or not the CTLs induced
by in vitro stimulation with the β-tubulin
5309–318 peptide showed cytotoxicity against prostate
cancer cells in PBMCs from 4 of the 5 patients (pt. 1, 4, 6, 7) who
exhibited a positive CTL response as indicated by the IFN-release
assay (Table II). The
peptide-stimulated PBMCs from all 4 of the patients exhibited
significant levels of cytotoxicity against both PC3 and LNCaP-A24
cells, but not against LNCaP cells or HLA-A24+
PHA-stimulated T-cell blasts as indicated by the 51Cr
release assay (Fig. 3). By
contrast, as shown in Table II,
PBMCs from none of the 3 patients (pt. 3, 8 and 9) whose samples
responded negatively in the IFN-γ assay showed detectable levels of
CTL activity with this assay (data not shown). The PBMCs from the
remaining 3 patients were not eligible for the assay.
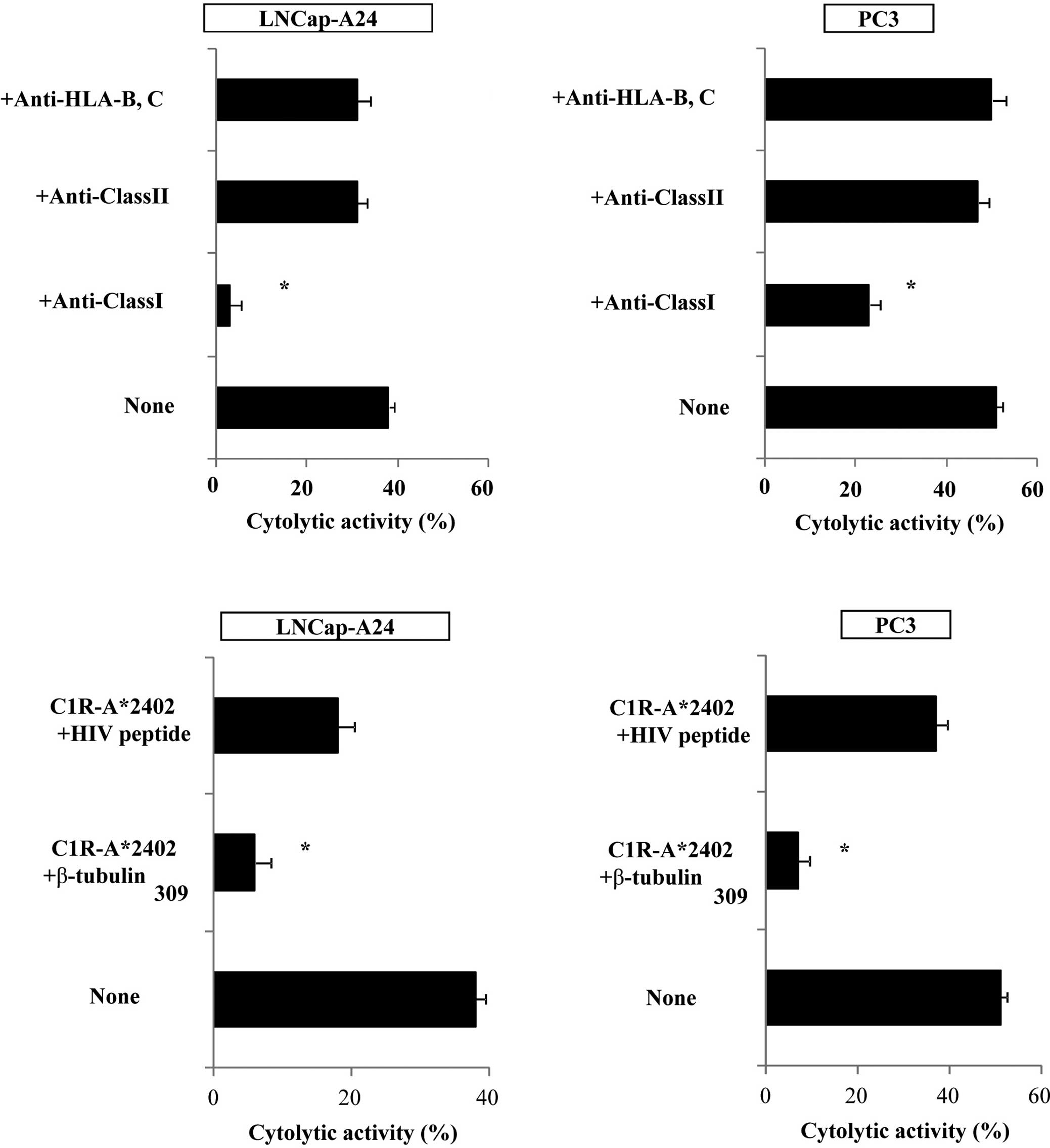
We then attempted to identify the cells responsible
for the cytotoxicity of β-tubulin 5309–318
peptide-stimulated PBMCs. Purified CD8+ T cells were
used in the following experiments. The levels of cytotoxicity by
CD8+ T cells purified from the peptide-stimulated PBMCs
against PC3, as well as LNCaP tumor cells, were significantly
decreased by the addition of anti-HLA class I mAb (W6/32), but not
by the addition of either anti-HLA class II (HLA-DR) or
anti-HLA-B,C (B1–23, IgG2a) mAbs. Representative cases are shown in
Fig. 4A. In addition, cytotoxicity
was significantly inhibited by the addition of a corresponding
peptide-pulsed unlabeled C1R-A*2402, but not by the
addition of an HIV peptide-pulsed unlabeled C1R-A*2402.
Representative cases are shown in Fig.
4B. These results indicate that CTL activity was determined to
be specific to this peptide, and was mediated by CD8+ T
cells in an HLA-class I-restricted manner.
Discussion
The binding score of the β-tubulin
5309–318 peptide (RYLTVAAVFR) to HLA-A*2402
was lower than it was to HLA-A*3101 and
-A*3302, but higher than it was to HLA-A3 or
-A*1101, based on information from the BioInformatics
and Molecular Analysis Section (BIMAS) website (2). In this study, we showed that the
β-tubulin 5309–318 peptide, a previously reported
peptide capable of inducing HLA-A3 supertype-restricted CTLs
(7), did bind to
HLA-A*2402, one of the dominant HLA-A types in Asian
populations, including the Japanese. HLA-A24 binding peptides are
characterized by the presence of Y or F residues at amino acid
position 2; and of L, F, I or W residues at position 9 (12,17).
These findings suggest that the peptide binds to
HLA-A*2402 molecules. On the other hand, no binding of
this peptide to HLA-A0201-transfected cells was expected, since its
binding score to HLA-A*0201 on BIMAS is zero. Indeed,
β-tubulin 5309–318 showed no binding activity to the
HLA-A*0201, -A*0206 or -A*0207
molecules.
We previously reported that the use of the CTL assay
with a 14-day incubation period and with stimulation administered
five times did not detect CTL precursors by de novo
sensitization to an epitope peptide (18). The sensitivity of this employed CTL
assay was 1 out of 3,000 to 1 out of 5,000 CTL precursors. Thus, it
is likely that the immune response against the β-tubulin
5309–318 peptide is relatively restricted in cancer
patients whose tumors overexpress β-tubulin 5 antigen (19–21).
This is primarily because naïve T cells from HDs do not induce CTL
activity as readily as those from prostate cancer patients. Indeed,
PBMCs from any of the three HDs homozygous for the HLA-A24 allele
showed CTL activity (data not shown). By contrast, it was
relatively easy to induce β-tubulin 5309–318-specific
CTLs in prostate cancer patients, and such CTLs were detectable in
5 of the 9 patients tested. CTL precursors were not detectable in
the remaining 4 patients, which may have been due, in part, to the
immune suppression associated with prostate cancer. Alternatively,
the employed CTL assays may not have been sufficiently sensitive,
based on the finding that the CTL precursors to the EBV-derived
peptide, which were used as a positive control, were also
detectable in some of the patients tested.
Significantly higher fractions of β-tubulin class II
and V mRNA were reported as compared to the other isotypes in lung
tumor samples (22). In regard to
biological function, β-tubulin 5, which is located in the cytoplasm
and one of the structural subunit of microtubules, is important for
cell proliferation (19). Tubulin
is one of the major target molecules of anticancer drugs such as
docetaxel, based on the fact that the expression of tubulin is
reported more often in cancer cells than in normal cells (19–21).
Together with the results presented herein, these
findings suggest that the β-tubulin 5 peptide has potential utility
as a cancer vaccine, both in prostate and other types of
cancer.
Acknowledgements
This study was supported in part by
Grants-in-Aid from the Ministry of Education, Science, Sports and
Culture of Japan (no. 12213134 to K.I., no. 21591652 to S.S. and
no. 18591449 to M.N.), and by the Toshi-Area Program (to K.I., S.S.
and M.N.).
Abbreviations:
|
CTL,
|
cytotoxic T lymphocyte;
|
|
DMSO,
|
dimethyl sulfoxide;
|
|
EBV,
|
Epstein-Barr virus;
|
|
FBS,
|
fetal bovine serum;
|
|
HDs,
|
healthy donors;
|
|
HIV,
|
immunodeficient virus;
|
|
IFN-γ,
|
interferon-γ;
|
|
PAP,
|
prostate acid phosphatase;
|
|
PBMCs,
|
peripheral blood mononuclear
cells;
|
|
PHA,
|
phytohemagglutinin
|
References
|
1.
|
Noguchi N, Kakuma T, Uemura H, et al: A
randomized phase II trial of personalized peptide vaccine plus low
dose estramustine phosphate (EMP) versus standard dose EMP in
patients with castration resistant prostate cancer. Cancer Immunol
Immunother. 59:1001–1009. 2010. View Article : Google Scholar
|
|
2.
|
Terasaki Y, Shichijo S, Nui Y, et al: An
HLA-A3-binding prostate acid phosphatase-derived peptide can induce
CTLs restricted to HLA-A2 and -A24 alleles. Cancer Immunol
Immunother. 58:1877–1885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Mohamed RE, Naito M, Terasaki Y, et al:
Capability of SART3109–118 peptide to induce cytotoxic
T-lymphocytes from prostate cancer patients with HLA class I-A11,
-A31 and -A33 alleles. Int J Oncol. 34:529–536. 2009.
|
|
4.
|
Niu Y, Komatsu N, Komohara K, et al: A
peptide derived from hepatitis C virus (HCV) core protein inducing
cellular responses in patients with HCV with various HLA class IA
alleles. J Med Virol. 81:1232–1240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Niu Y, Terasaki Y, Komatsu N, Noguchi M,
Shichijo S and Itoh K: Identification of peptides applicable as
vaccines for HLA-A26-positive cancer patients. Cancer Sci.
100:2167–2174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Matsueda S, Takedatsu H, Yao A, et al:
Identification of peptide vaccine candidates for prostate cancer
patients with HLA-A3 supertype alleles. Clin Cancer Res.
11:6933–6943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Takedatsu H, Shichijo S, Katagiri K,
Sawamizu H, Sata M and Itoh K: Identification of peptide vaccine
candidates sharing among HLA-A3+, -A11+,
-A31+, and -A33+ cancer patients. Clin Cancer
Res. 10:1112–1120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Minami T, Matsueda S, Takedatsu H, et al:
Identification of SART3-derived peptides having the potential to
induce cancer-reactive cytotoxic T lymphocytes from prostate cancer
patients with HLA-A3 supertype alleles. Cancer Immunol Immunother.
56:689–698. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Matsueda S, Takedatsu H, Sasada T, et al:
New peptide vaccine candidates for epithelial cancer patients with
HLA-A3 supertype alleles. J Immunother. 30:274–281. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Naito M, Komohara Y, Ishihara Y, et al:
Identification of Lck-derived peptides applicable to anti-cancer
vaccine for patients with human leukocyte antigen-A3 supertype
alleles. Br J Cancer. 97:1648–1654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yao A, Harada M, Matsueda S, et al:
Identification of parathyroid hormone-related protein-derived
peptides immunogenic in human histocompatibility leukocyte
antigen-A24+ prostate cancer patients. Br J Cancer.
91:287–296. 2004.
|
|
12.
|
Torikai H, Akatsuka Y, Miyauchi H, et al:
The HLA-A*0201-restricted minor histocompatibility
antigen HA-1H peptide can also be presented by another HLA-A2
subtype, A*0206. Bone Marrow Transplant. 40:165–174.
2007.
|
|
13.
|
Steven NM, Annels NE, Kumar A, Leese AM,
Kurilla MG and Rickinson AB: Immediate early and early lytic cycle
proteins are frequent targets of the Epstein-Barr virus-induced
cytotoxic T cell response. J Exp Med. 185:1605–1607. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lee SP, Tierney RJ, Thomas WA, Brooks JM
and Rickinson AB: Conserved CTL epitopes within EBV latent membrane
protein 2. A potential target for CTL-based tumor therapy. J
Immunol. 158:3325–3334. 1997.PubMed/NCBI
|
|
15.
|
Parker KC, Bednarek MA, Hull LK, et al:
Sequence motifs important for peptide binding to the human MHC
class I molecule, HLA-A2. J Immunol. 149:3580–3587. 1992.PubMed/NCBI
|
|
16.
|
Ikeda-Moore Y, Tomiyama H, Miwa K, et al:
Identification and characterization of multiple HLA-A24-restricted
HIV-1 CTL epitopes: strong epitopes are derived from V regions of
HIV-1. J Immunol. 159:6242–6252. 1997.PubMed/NCBI
|
|
17.
|
Rammensee HG, Flak K and Rotzschke O:
Peptides naturally presented by MHC class I molecules. Annu Rev
Immunol. 11:213–244. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hida N, Maeda Y, Katagiri K, Takasu H,
Harada M and Itoh K: A simple culture protocol to detect
peptide-specific cytotoxic T lymphocyte precursors in the
circulation. Cancer Immunol Immunother. 51:219–228. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Jordan MA and Wilson L: Microtubules and
actin filaments: dynamic targets for cancer chemotherapy. Curr Opin
Cell Biol. 10:123–130. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hashimoto Y, Tajima O, Hashiba H, Nose K
and Kuroki T: Elevated expression of secondary, but not early,
responding genes to phorbol ester tumor promoters in papillomas and
carcinomas of mouse skin. Mol Carcinog. 3:302–308. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kato K, Ito H, Inaguma Y, Okamoto K and
Saga S: Synthesis and accumulation of αB crystallin in C6 glioma
cells is induced by agents that promote the disassembly of
microtubules. J Biol Chem. 271:26989–26994. 1996.
|
|
22.
|
Cucchiarelli V, Hiser L, Smith H, et al:
Beta-tubulin isotype classes II and V expression patterns in
non-small cell lung carcinomas. Cell Motil Cytoskeleton.
65:675–685. 2008. View Article : Google Scholar : PubMed/NCBI
|