Introduction
Through invasion and metastasis, head and neck
squamous cell carcinoma (HNSCC) becomes fatal. If an involvement of
the inflammatory process in the mechanisms of metastasis is
confirmed, then potential novel targets for cancer treatment may be
identified (1). However, the
mechanisms involved in the invasive growth of tumors and the
induction of metastasis are not yet completely known. Since most
cancer-related deaths are caused by metastasis, this field has been
the focus of study in the past several years. According to
previously reported theories, the processes involved in the
invasive growth and metastasis of tumors are complex and vary
depending not only on the intrinsic characteristics of the tumor
cells themselves, but also on the microenvironments where tumors
originate. In particular, inflammation occurring in the vicinity of
tumors contributes to tumor cells acquiring invasive and metastatic
potential by cytokines, chemokines and growth factors released by
the infiltrated inflammatory cells (2,3).
Interleukin-1β (IL-1β) is involved in tumor progression, treatment
resistance (4,5) and increased expression of
cyclooxygenase-2 (COX-2) in HNSCC (6–8).
COX-2 is an inducible enzyme involved in the initiation of
inflammation and mitogenic response. In addition to its action on
the regulation of inflammation and cell growth, COX-2 is associated
with carcinogenesis and tumorigenesis (9–11).
Several prostaglandins (PGs), particularly PGE2, play
the role of accelerator in the process of tumorigenesis by
stimulating angiogenesis and suppressing immune surveilance
(9). COX-2 is regulated by IL-1β,
lipopolysaccharide, tumor necrosis factor-α and reactive oxygen
species (ROS) (12).
Epithelial-mesenchymal transition (EMT), the process
of cells losing the characteristics of epithelial cells and
acquiring the characteristics of mesenchymal cells, has been
implicated in the process of tumor progression in carcinoma cases.
EMT has been reported to be closely associated with the invasion
and metastasis of tumors and is associated with a poor patient
prognosis (13,14). As EMT-inducing factors, Slug,
Twist, SIP1, Zeb1 and E47 induce EMT by suppressing the expression
of E-cadherin and subsequently inducing the invasion and metastasis
of tumors (15). Slug is a member
of the Snail family. It plays an important role in the regulation
of EMT by suppressing various epithelial markers. E-cadherin is a
cell adhesion molecule located in the cell adhesion site of
epithelial cells; it plays an important role in the suppression of
tumor invasion. When E-cadherin is decreased or inactivated, the
malignant potential of tumors is increased and metastasis is
induced (16).
In the present study, we investigated whether
inflammatory mediators are involved in EMT by comparing and
examining the significance of the expression of the proinflammatory
mediators IL-1β and COX-2, and Slug and E-cadherin, determined by
immunohistochemical techniques applied to HNSCC tissues.
Furthermore, the relationship between the expression pattern of
Slug and E-cadherin and the expression pattern of the
proinflammatory mediators IL-1β and COX-2 was examined. The aim of
the study was to identify novel approaches to cancer treatment.
Materials and methods
Patients
The study consisted of 146 consecutive patients with
HNSCC who underwent surgical treatment for primary tumors at the
Department of Otorhinolaryngology, Chosun University, from 1994 to
2002. The clinical, epidemiologic and histopathologic
characteristics of the patients are listed in Table I. None of the patients had
previously received pre-operative chemotherapy or radiotherapy.
Fifty-eight of the 146 patients (39.7%) had histologically
confirmed cervical lymph node metastasis, whereas the remaining 88
patients (60.3%) had no clinical or histopathologic evidence of
neck disease. Tumors were staged according to the AJCC TNM
classification (17) and graded as
follows: well, moderately and poorly differentiated.
 | Table I.Clinical, epidemiologic and histologic
characteristics of 146 patients with head and neck squamous cell
carcinoma. |
Table I.
Clinical, epidemiologic and histologic
characteristics of 146 patients with head and neck squamous cell
carcinoma.
| Characteristic | No. (%) |
|---|
| No. of patients | 146 (100) |
| Age (years) | |
| Mean | 61.6 |
| Range | 26–87 |
| Gender | |
| Male | 130 (89.0) |
| Female | 16 (11.0) |
| Tumor stage | |
| Early (I,
II) | 91 (62.3) |
| I | 37 |
| II | 54 |
| Advanced (III,
IV) | 55 (37.7) |
| III | 16 |
| IV | 39 |
| Tumor site | |
| Oral cavity | 37 (25.3) |
| Pharynx | 48 (32.9) |
| Larynx | 61 (41.8) |
| Degree of tumor
differentiation | |
| Well
differentiated | 83 (56.9) |
| Moderately
differentiated | 53 (36.3) |
| Poorly
differentiated | 10 (6.9) |
| Lymph node
metastasis | |
| Yes | 58 (39.7) |
| No | 88 (60.3) |
Immunohistochemistry
Upon approval of the Institutional Review Board,
immunohistochemistry was carried out on formalin-fixed,
paraffin-embedded HNSCC tissues from the Pathology Department
archives. All tumors investigated in the study were tested for
rabbit polyclonal IL-1β (dilution 1:200) (Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA), mouse monoclonal COX-2 (dilution 1:300)
(Cayman Chemical, Ann Arbor, MI, USA), goat polyclonal Slug
(dilution 1:100) (Santa Cruz Biotechnology Inc.) and mouse
monoclonal E-cadherin (dilution 1:100) (Santa Cruz Biotechnology
Inc.). Immunolocalization for IL-1β was performed using a Polink-2
HRP Plus rabbit DAB detection system (Golden Bridge International,
Inc., WA, USA). Immunolocalization for COX-2 and E-cadherin was
performed using an HRP Plus mouse DAB detection system (Golden
Bridge International, Inc.), and immunolocalization for Slug was
performed using an HRP Plus goat DAB detection system (Golden
Bridge International, Inc.) according to the supplier’s protocol
(LSAB kit; Dako, Carpinteria, CA, USA). Counterstaining was
performed with Mayer’s hematoxylin. An isotype-matched control
antibody was also used. Inflammed granulation tissue was used as
the positive control for IL-1β and COX-2. The positive control for
Slug was colonic adenocarcinoma with strong nuclear staining in a
previous study, and the positive control for E-cadherin was a
normal colonic mucosa adjacent tumor. Instead of the primary
antibody, TBS was used for the negative control.
Analysis and interpretation of
staining
A pathologist, blinded to the clinical course of the
subjects in order to exclude subjectivity, evaluated the staining
results. Staining for IL-1β and COX-2 was determined as positive
when intracytoplasmic staining was identified under an optical
microscope in >5% of the tumor cells in each tissue section.
Positive expression of IL-1β was divided into categories: weakly
positive when 5–20% of tumor cells were stained and strongly
positive when >20% of tumor cells were stained. Positive
expression of COX-2 was divided into categories: weakly positive
when 5–50% of tumor cells were stained and strongly positive when
>50% of tumor cells were stained (18). For the evaluation of Slug
expression, staining intensity was scored as 0 (negative), 1
(weak), 2 (medium) and 3 (strong). Extent of staining was scored as
0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%),
according to the percentage of the positively stained areas in
relation to the entire cancerous area. The sum of the intensity and
extent of staining score was used as the final staining score (0–7)
for Slug. Tumors having a final staining score of ≥6 were
considered to exhibit high expression (19). For the evaluation of E-cadherin,
staining intensity was scored as 0 (negative), 1 (weak), 2 (medium)
and 3 (strong). The extent of membranous E-cadherin expression in
tumor cells was scored as 0 (<5%), 1 (5–25%), 2 (26–50%), 3
(51–75%) and 4 (76–100%). The sum of the intensity and extent of
staining score was used as the final staining score (0–7) for
E-cadherin. Tumors having a final staining score of ≥6 were
considered to exhibit high expression (19).
Statistical analysis
The StatView software package (Abacus Conceptus,
Berkeley, CA, USA) was used for statistical analysis. The
Chi-square test was used to determine the correlation between
clinical stage, histologic tumor grade and lymph node metastasis
and the expression patterns of IL-1β, COX-2, Slug and E-cadherin.
Correlations in the expression patterns between IL-1β and COX-2;
Slug and E-cadherin; IL-1β/COX-2 and Slug/E-cadherin were
identified. Statistical significance was determined at
p<0.05.
Results
IL-1β, COX-2, Slug and E-cadherin
expression according to immunohistochemistry
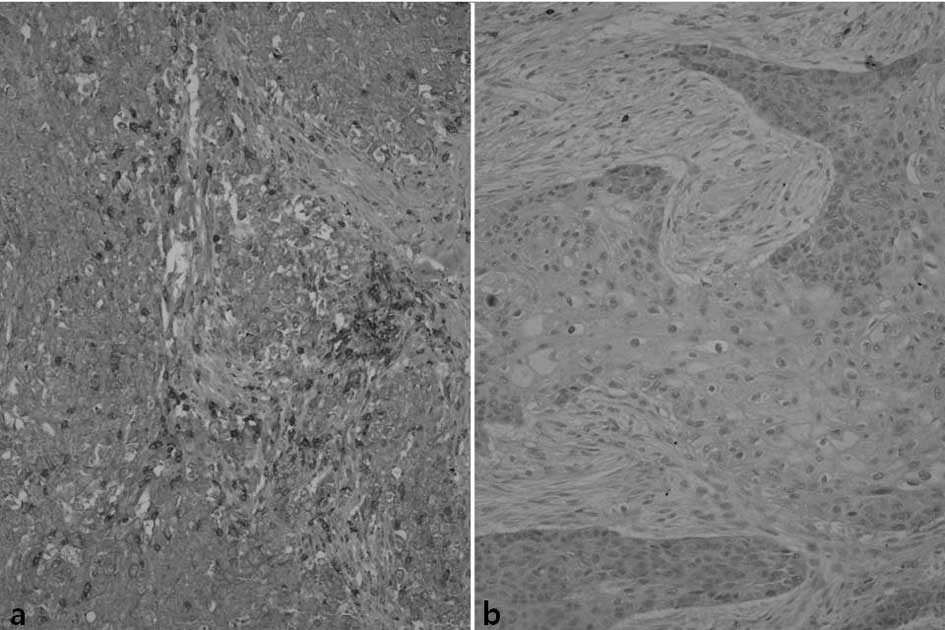
IL-1β staining was positive in some lymphocytes and
macrophages infiltrating the vicinity of tumors, while in some
adjacent fibroblasts or tumor cells weak immunoreactivity was
observed. Among the study subjects, 54 patients (37%) exhibited a
strong reaction to IL-1β, 76 cases (52%) demonstrated a weak
positive reaction and 16 cases (11%) showed a negative reaction
(Fig. 1).
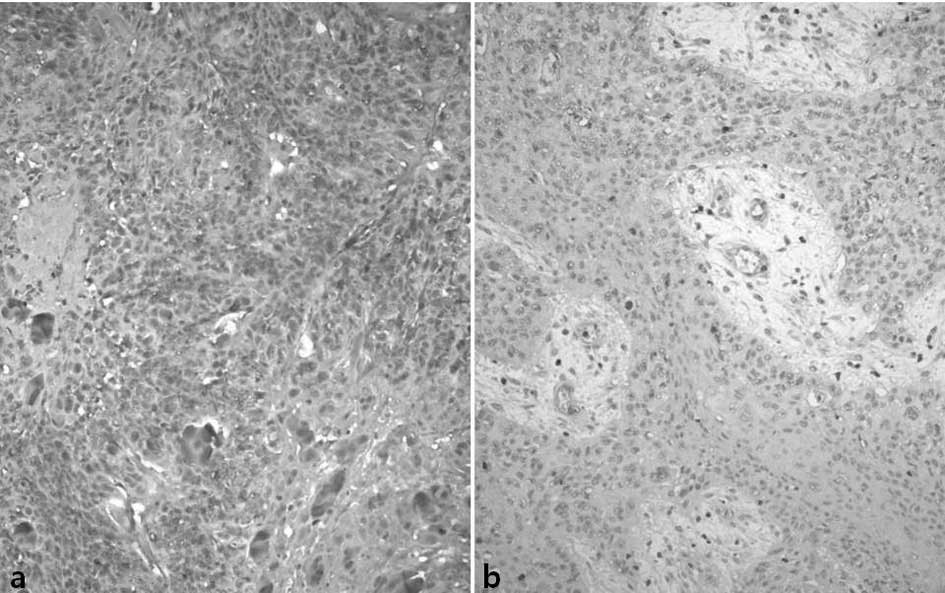
A weak positive reaction for COX-2 was noted in the
mucosal epithelia in the vicinity of the tumors. Macrophages,
vascular endothelial cells and fibroblasts showed a weak positive
cytoplasmic reaction. Regarding COX-2, 96 cases (66%) of the study
subjects showed a strong positive reaction, 43 cases (29%) showed a
weak positive reaction and 7 cases (5%) showed a negative reaction
(Fig. 2).
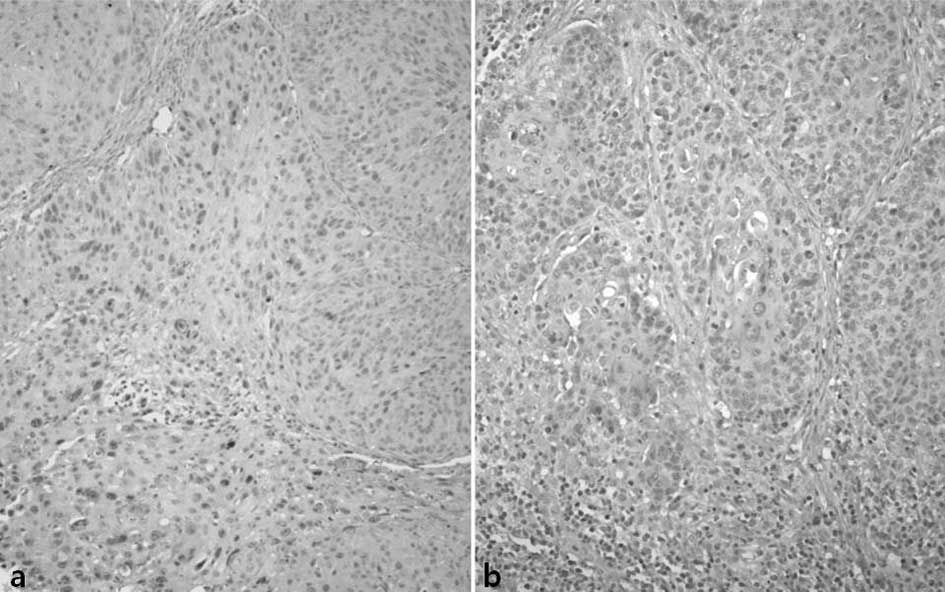
Slug was stained in the nucleus of the tumor cells.
Of the total cases, 79 (54%) showed high expression and 67 (46%)
demonstrated low expression (Fig.
3).
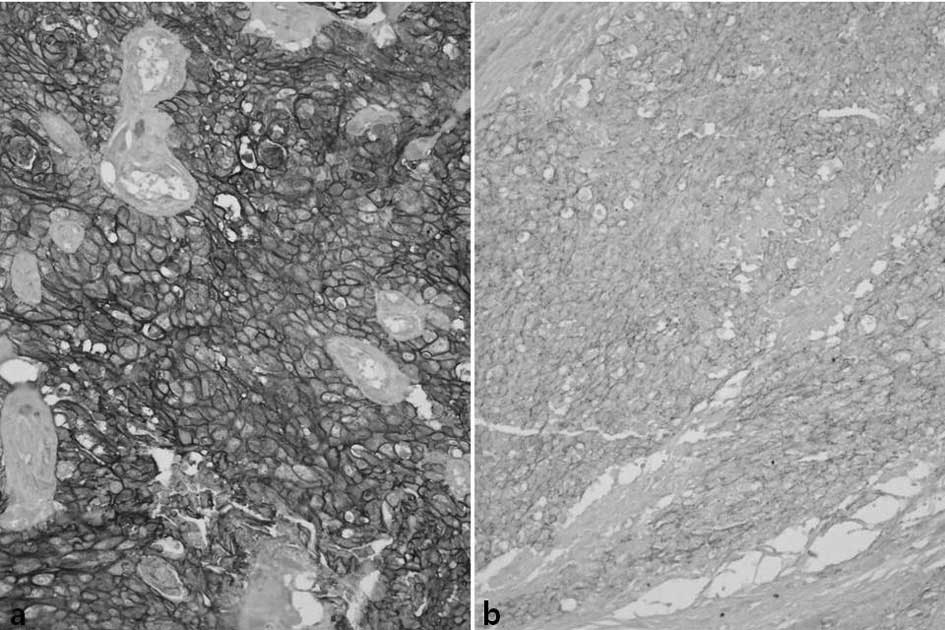
E-cadherin was diversely stained on the cell
membrane of the tumor cells. The entire cell membranes of
epithelial cells forming the normal mucosa in the vicinity of
tumors demonstrated strong and distinct staining. Concerning
E-cadherin, 55 cases showed high expression (38%) and 91 cases
(62%) showed low expression (Fig.
4) (Tables II and III).
 | Table II.Clinicopathologic data according to
the expression pattern of IL-1β and COX-2 in head and neck squamous
cell carcinoma. |
Table II.
Clinicopathologic data according to
the expression pattern of IL-1β and COX-2 in head and neck squamous
cell carcinoma.
| IL-1βa
| COX-2b
|
|---|
| Strong (n=54) | Weak (n=76) | Absent (n=16) | Strong (n=96) | Weak (n=43) | Absent (n=7) |
|---|
|
Differentiation | | | | | | |
| W/D (n=83) | 29 | 45 | 9 | 49 | 31 | 3 |
| M/D (n=53) | 21 | 26 | 6 | 40 | 10 | 3 |
| P/D (n=10) | 4 | 5 | 1 | 7 | 2 | 1 |
| Stage | | | | | | |
| Early (n=91) | 26 | 56 | 9 | 54 | 31 | 6 |
| Advanced
(n=55) | 28 | 20 | 7 | 42 | 12 | 1 |
| Nodal status | | | | | | |
| Negative
(n=88) | 28 | 46 | 14 | 53 | 29 | 6 |
| Positive
(n=58) | 26 | 30 | 2 | 43 | 14 | 1 |
 | Table III.Clinicopathologic data according to
the expression pattern of Slug and E-cadherin in head and neck
squamous cell carcinoma. |
Table III.
Clinicopathologic data according to
the expression pattern of Slug and E-cadherin in head and neck
squamous cell carcinoma.
| Slug a,b
| E-cadherin
a,c
|
|---|
| Low (n=67) | High (n=79) | Low (n=91) | High (n=55) |
|---|
|
Differentiation | | | | |
| W/D (n=83) | 49 | 34 | 44 | 39 |
| M/D (n=53) | 15 | 38 | 39 | 14 |
| P/D (n=10) | 3 | 7 | 8 | 2 |
| Stage | | | | |
| Early (n=91) | 50 | 41 | 59 | 32 |
| Advanced
(n=55) | 17 | 38 | 32 | 23 |
| Nodal status | | | | |
| Negative
(n=88) | 41 | 47 | 51 | 37 |
| Positive
(n=58) | 26 | 32 | 40 | 18 |
Relationship between IL-1β expression and
clinicopathologic parameters
The expression pattern of IL-1β was significantly
correlated with the presence or absence of lymph node metastasis.
As the expression of IL-1β inceased, the rate of lymph node
metastasis was also significantly increased (p<0.05).
Nevertheless, the expression of IL-1β was not significantly
correlated with tumor differentiation and clinical stage (Table II).
Relationship between COX-2 expression and
clinicopathologic parameters
A significant correlation between histological
differentiation, clinical stage and the presence or absence of
lymph node metastasis and the expression level of COX-2 was noted
(p<0.05 each). In other words, as the differentiation grade of
squamous cell carcinoma became poorer and the clinical stage
increased, the strong positive expression of COX-2 was
significantly increased, including in the group with lymph node
metastasis (Table II).
Relationship between Slug expression and
clinicopathologic parameters
A significant correlation was noted between
histological differentiation and clinical stage of the tumors and
the expression of Slug (p<0.001 and <0.05, respectively). As
the differentiation of the squamous cell carcinoma became poorer
and as the clinical stage increased, the strong positive expression
of COX-2 was significantly increased. However, a significant
correlation between Slug and the presence or absence of lymph node
metastasis was not noted (Table
III).
Relationship between E-cadherin
expression and clinicopathologic parameters
The histological differentiation and lymph node
status significantly correlated with the expression level of
E-cadherin (p<0.001 and <0.05, respectively). As the
histological differentiation became poorer, or in the group with
lymph node metastasis, the expression of E-cadherin was
significantly reduced. Nonetheless, E-cadherin expression was not
significantly correlated with clinical stage (Table III).
Relationship between IL-1β and COX-2
expression
The expression of IL-1β and COX-2 showed a
significant correlation (p<0.05). In the cases strongly
expressing IL-1β, COX-2 also showed the tendency to be strongly
expressed. In the cases weakly expressing IL-1β, COX-2 also showed
the tendency to be weakly expressed (Table IV).
 | Table IV.Correlation between the expression of
IL-1β and COX-2 in head and neck squamous cell carcinoma. |
Table IV.
Correlation between the expression of
IL-1β and COX-2 in head and neck squamous cell carcinoma.
| COX-2
| p-value |
|---|
| Strong (n=96) | Weak (n=43) | Absent (n=7) |
|---|
| IL-1β | | | | <0.05 |
| Strong
(n=54) | 45 | 9 | 0 | |
| Weak (n=76) | 51 | 24 | 1 | |
| Absent
(n=16) | 0 | 10 | 6 | |
Relationship between Slug and E-cadherin
expression
The expression of Slug and E-cadherin showed a
significantly inverse correlation (p<0.005). In the cases
strongly expressing Slug, E-cadherin showed the tendency to be
weakly expressed. In the cases weakly expressing Slug, E-cadherin
showed the tendency to be strongly expressed (Table V).
 | Table V.Correlation between the expression of
Slug and E-cadherin in head and neck squamous cell carcinoma. |
Table V.
Correlation between the expression of
Slug and E-cadherin in head and neck squamous cell carcinoma.
| E-cadherin
| p-value |
|---|
| Low (n=91) | High (n=55) |
|---|
| Slug | | | <0.005 |
| Low (n=67) | 29 | 38 | |
| High (n=79) | 62 | 17 | |
Relationship between IL-1β/COX-2 and
Slug/E-cadherin expression
IL-1β was positively correlated with Slug, yet
statistical significance was not achieved. However, IL-1β was
inversely correlated with E-cadherin, with statistical significance
(p<0.05). COX-2 was positively correlated with Slug, and
statistical significance was achieved (p<0.05). COX-2 was
inversely correlated with E-cadherin, but statistical significance
was not achieved (Table VI).
 | Table VI.Correlation between the expression of
IL-1β/COX-2 and Slug/E-cadherin in head and neck squamous cell
carcinoma. |
Table VI.
Correlation between the expression of
IL-1β/COX-2 and Slug/E-cadherin in head and neck squamous cell
carcinoma.
| Slug a
| E-cadherin b
|
|---|
| Low (n=67) | High (n=79) | Low (n=91) | High (n=55) |
|---|
| IL-1β | | | | |
| Strong
(n=54) | 17 | 37 | 46 | 8 |
| Weak (n=76) | 40 | 36 | 41 | 35 |
| Absent
(n=16) | 10 | 6 | 4 | 12 |
| COX-2 | | | | |
| Strong
(n=96) | 28 | 68 | 63 | 33 |
| Weak (n=43) | 34 | 9 | 26 | 17 |
| Absent (n=7) | 6 | 1 | 2 | 5 |
Discussion
EMT is a prerequisite mechanism in developmental
stages, and is a process whereby cells lose polarity and acquire a
mesenchymal phenotype. EMT has been shown to play a central role in
the induction of invasion and metastasis involved in the
progression of tumors (14,20-23).
The major cause of death in HNSCC is lymph node and distant
metastases, such as lung metastases (24,25).
According to studies, a decrease or loss of E-cadherin is generally
associated with aggressiveness in tumors, which results in poor
tumor differentiation, anaplasia and invasive growth (26–29).
The reduction in E-cadherin induced by the binding of Snail to the
E-box of the E-cadherin promoter results in the suppression of
E-cadherin transcription, which induces EMT (30,31).
When the suppression of E-cadherin was induced by Snail, tumors
were found to acquire invasive characteristics and to readily form
metastases (30). In addition,
Snail was found to induce the expression of matrix
metalloproteinase-2 (MMP-2) and to suppress cell-cell adhesion,
contributing to an increase in tumor invasiveness (32). This was confirmed by a previous
study. When the expression of Snail was suppressed by the use of
siRNA, the EMT phenotype disappeared, MMP-2 activity was reduced,
the migration and invasiveness of cells were reduced in
vitro and metastatic potential was elevated in vivo
(33).
In the present study, when the EMT level was
evaluated by the reduction in E-cadherin, an inverse correlation
between the reduction in E-cadherin and the differentiation of
tumors and lymph node metastasis was observed. This confirmed that
EMT plays an crucial role in the invasion, metastasis and the
differentiation of tumors.
Slug, a member of the Snail family, is a molecule
that plays an important role in embryonic development (34). Slug is a Snail transcription factor
and is involved in the progression and metastasis of various types
of tumors (35–37). Thus, Slug plays a role in enhancing
the aggressiveness of tumors by suppressing E-cadherin through
Snail.
In the present study, the expression of Slug was
positively correlated with the differentiation of tumors, and
clinical stage and was inversely correlated with E-cadherin. These
findings corroborate previous theories.
In HNSCC, the elevation of Snail expression is
associated with activation by Akt or other mechanisms. However,
only approximately 30% of cases of metastasis are associated with
Snail. In some of the remaining patients, NBS1 was reported to be
involved. Cancer occurs preferentially in the Nijmegen breakage
syndrome (NBS), the chromosomal-instability syndrome associated
with radiosensitivity and growth retardation. The NBS gene
product is NBS1 (38).
Inflammation is a finding frequently associated with
invasive tumors (3). In HNSCC, an
increase in the levels of various inflammatory mediators has been
observed (39). COX-2 is an
inducible enzyme that is elevated or decreased depending on the
condition and activity (9). COX-2
is regulated by various cytokines and ROS. It is involved in
inflammatory reactions and is in charge of the regulatory action of
cell growth by the involvement in the mitotic reaction. COX-2 is
also involved in the tumorigenic and carcinogenic process (9–12).
The COX-2-dependent up-regulation of Snail leads to a reduction in
E-cadherin and contributes to EMT. IL-1β has been reported to be
associated with the invasion, metastatic potential and treatment
resistance of HNSCC, and to elevate the expression of COX-2
(13,14). Therefore, the expression of IL-1β
and COX-2 may be closely related.
In the present study, the expression of IL-1β was
positively correlated with lymph node metastasis, and COX-2 was
positively correlated with not only histological differentiation,
but also with clinical stage and lymph node metastasis. In
addition, the expression of IL-1β was positively correlated with
the expression of COX-2. Therefore, an increase in IL-1β expression
is anticipated to increase the expression of COX-2, leading to a
reduction in E-cadherin through the up-regulation of
COX-2-dependent Snail, which may induce EMT. This was substantiated
by the results of our study.
Ultimately, the elevation of proinflammatory
mediators may induce EMT, and thus may augment the aggressiveness
of tumors. In contrast, when proinflammatory mediators are blocked
due to the suppression of the Snail axis, the EMT may disappear,
resulting in the suppression of the invasiveness or metastatic
potential of tumors.
The results of our study not only provide useful
information regarding the diagnosis of HNSCC and the prediction of
prognosis, but also present crucial data for the development of
effective treatments to block the invasion and metastasis of
cancer.
Numerous studies have been conducted to characterize
the association of inflammation with the development of cancer
(40,41) and also with inflammation within
tumors. Studies have demonstrated that inflammation induced by
tumor necrosis associated with chemotherapy may mediate effects on
the progression and metastasis of tumors. The theory of the role of
anti-inflammatory agents was proposed, not only for cancer
treatment, but also for its prevention (42).
The result of our study revealed that inflammation
may mediate the effects on the progression and metastasis of cancer
and, specifically, that it may act by the induction of the EMT
process.
The aggressiveness of cancer, such as invasiveness
and metastatic potential, is controlled by a multitude of factors.
Among these, proinflammatory mediators have been suggested to play
a role. Thus, it is timely to consider the administration of
anti-inflammatory agents concurrently with anti-cancer
chemotherapeutics, in other words, the effect of COX-2 blockers
according to the theory elucidated previously (43). COX-2 blockers also inhibit the
release of PGE2, and subsequently block
PGE2-mediated E-cadherin transcription suppressors and
suppress EMT. Hence, a reduction in the metastatic potential of
HNSCC is anticipated.
Collectively, these results further support the
possibility that COX-2 blockers may play an important role in the
effective treatment of HNSCC. In addition, not only COX-2 blockers,
but also various other types of anti-inflammatory agents may be
effective. Additional in vitro and in vivo studies
are required to verify the link between IL-1β/COX-2 and
Slug/E-cadherin expression.
Acknowledgements
This study was supported by a grant
from the National Research Foundation of Korea (NRF) funded by the
Ministry of Education, Science and Technology (MEST) through the
Research Center for Resistant Cells (R13-2003-009).
References
|
1.
|
Zender CA and Petruzzelli GJ: Why do
patients with head and neck squamous cell carcinoma experience
distant metastases: can they be prevented? Curr Opin Otolaryngol
Head Neck Surg. 2:101–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lin DT, Subbaramaiah K, Shah JP,
Dannenberg AJ and Boyle JO: Cyclooxygenase-2: a novel molecular
target for the prevention and treatment of head and neck cancer.
Head Neck. 8:792–799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Wu Y and Zhou BP: Inflammation: a driving
force speeds cancer metastasis. Cell Cycle. 15:3267–3273. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mukhopadhyay P, Ali MA, Nandi A, Carreon
P, Choy H and Saha D: The cyclin-dependent kinase 2 inhibitor
down-regulates interleukin-1β-mediated induction of
cyclooxygenase-2 expression in human lung carcinoma cells. Cancer
Res. 66:1758–1766. 2006.PubMed/NCBI
|
|
5.
|
Teruel A, Romero M, Cacalano NA, Head C
and Jewett A: Potential contribution of naive immune effectors to
oral tumor resistance: role in synergistic induction of VEGF, IL-6,
and IL-8 secretion. Cancer Immunol Immunother. 57:359–366. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bancroft CC, Chen Z, Yeh J, Sunwoo JB, Yeh
NT, Jackson S, Jackson C and van Waes C: Effects of pharmacologic
antagonists of epidermal growth factor receptor, PI3K and MEK
signal kinases on NF-κB and AP-1 activation and IL-8 and VEGF
expression in human head and neck squamous cell carcinoma lines.
Int J Cancer. 99:538–548. 2002.
|
|
7.
|
Wolf JS, Chen Z, Dong G, Sunwoo JB,
Bancroft CC, Capo DE, Yeh NT, Mukaida N and van Waes C: IL
(interleukin)-1α promotes nuclear factor-κB and AP-1-induced IL-8
expression, cell survival, and proliferation in head and neck
squamous cell carcinomas. Clin Cancer Res. 7:1812–1820. 2001.
|
|
8.
|
Tsai CC, Chen CC, Lin CC, Chen CH, Lin TS
and Shieh TY: Interleukin-1β in oral submucous fibrosis, verrucous
hyperplasia and squamous cell carcinoma tissues. Kaohsiung J Med
Sci. 15:513–519. 1999.
|
|
9.
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygenases in inflammation, cancer, and development.
Oncogene. 18:7908–7916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Langenbach R, Loftin CD, Lee C and Tiano
H: Cyclooxygenasedeficient mice. A summary of their characteristics
and susceptibilities to inflammation and carcinogenesis. Ann NY
Acad Sci. 889:52–61. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Callejas NA, Casado M, Diaz-Guerra MJM,
Bosca L and Martin-Sanz P: Expression of cyclooxygenase-2 promotes
the release of matrix metalloproteinase-2 and -9 in fetal rat
hepatocytes. Hepatology. 33:860–867. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Martin-Sanz P, Callejas NA, Casado M,
Diaz-Guerra MJ and Bosca L: Expression of cyclooxygenase-2 in
foetal rat hepatocytes stimulated with lipopolysaccharide and
pro-inflammatory cytokines. Br J Pharmacol. 125:1313–1319. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shioiri M, Shida T, Koda K, Oda K, Seike
K, Nishimura M, Takano S and Miyazaki M: Slug expression is an
independent prognostic parameter for poor survival in colorectal
carcinoma patients. Br J Cancer. 19:1816–1822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Beahrs OH, Henson DE, Hutter RVP and
Kennedy BJ: Manual for Staging for Cancer 4th edition American
Joint Committee on Cancer. JB Lippincott; Philadelphia: pp.
1992
|
|
18.
|
Lim SC, Park SY and Do NY: Correlation of
cyclooxygenase-2 pathway and VEGF expression in head and neck
squamous cell carcinoma. Oncol Rep. 10:1073–1079. 2003.PubMed/NCBI
|
|
19.
|
Kyo S, Sakaguchi J, Ohno S, Mizumoto Y,
Maida Y, Hashimoto M, Nakamura M, Takakura M, Nakajima M, Masutomi
K and Inoue M: High Twist expression is involved in infiltrative
endometrial cancer and affects patient survival. Hum Pathol.
37:431–438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Barbera MJ, Puig I, Domínguez D,
Julien-Grille S, Guaita-Esteruelas S, Peiró S, Baulida J, Francí C,
Dedhar S, Larue L and García de Herreros A: Regulation of Snail
transcription during epithelial to mesenchymal transition of tumor
cells. Oncogene. 23:7345–7354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhou BP, Deng J, Xia W, Xu J, Li YM and
Gunduz M: Dual regulation of Snail by GSK-3beta-mediated
phosphorylation in control of epithelial-mesenchymal transition.
Nat Cell Biol. 6:931–940. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: role
of phosphatidylinositol 3 kinase/ AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: a role for epithelial-esenchymal
transition? Cancer Res. 65:5991–5995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Leemans CR, Tiwari R, Nauta JJ, van der
Waal I and Snow GB: Regional lymph node involvement and its
significance in the development of distant metastases in head and
neck carcinoma. Cancer. 71:452–456. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ferlito A, Rinaldo A, Buckley JG and
Mondin V: General considerations on distant metastases from head
and neck cancer. ORL J Otorhinolaryngol Relat Spec. 63:189–191.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Brabant G, Hoang-Vu C and Cetin Y:
E-cadherin: a differentiation marker in thyroid malignancies.
Cancer Res. 53:4987–4993. 1993.PubMed/NCBI
|
|
27.
|
Naito A, Iwase H, Kuzushima T, Nakamura T
and Kobayashi S: Clinical significance of E-cadherin expression in
thyroid neoplasms. J Surg Oncol. 76:176–180. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Rocha AS, Soares P, Fonseca E,
Cameselle-Teijeiro J, Oliveira MC and Sobrinho-Simoes M: E-cadherin
loss rather than β-catenin alterations is a common feature of
poorly differentiated thyroid carcinomas. Histopathology.
42:580–587. 2003.
|
|
29.
|
Kato N, Tsuchiya T, Tamura G and Motoyama
T: E-cadherin expression in follicular carcinoma of the thyroid.
Pathol Int. 52:13–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Batlle E, Sancho E, Franci C, Dominguez D,
Monfar M and Baulida J: The transcription factor snail is a
repressor of E-cadherin gene expression in epithelial tumour cells.
Nat Cell Biol. 2:84–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yokoyama K, Kamata N, Fujimoto R, Tsutsumi
S, Tomonari M and Taki M: Increased invasion and matrix
metalloproteinase-2 expression by Snail-induced mesenchymal
transition in squamous cell carcinomas. Int J Oncol. 22:891–898.
2003.PubMed/NCBI
|
|
33.
|
Yang MH, Chang SY, Chiou SH, Liu CJ, Chi
CW, Chen PM, Teng SC and Wu KJ: Overexpression of NBS1 induces
epithelial-mesenchymal transition and co-expression of NBS1 and
Snail predicts metastasis of head and neck cancer. Oncogene.
26:1459–1467. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Nieto MA, Sargent MG, Wilkinson DG and
Cooke J: Control of cell behavior during vertebrate development by
Slug, a zinc finger gene. Science. 6:835–859. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hajra KM, Chen DY and Fearon ER: The SLUG
zinc-finger protein represses E-cadherin in breast cancer. Cancer
Res. 15:1613–1618. 2002.PubMed/NCBI
|
|
36.
|
Martin TA, Goyal A, Watkins G and Jiang
WG: Expression of the transcription factors snail, slug, and twist
and their clinical significance in human breast cancer. Ann Surg
Oncol. 12:488–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Uchikado Y, Natsugoe S, Okumura H,
Setoyama T, Matsumoto M, Ishigami S and Aikou T: Slug expression in
the E-cadherin preserved tumors is related to prognosis in patients
with esophageal squamous cell carcinoma. Clin Cancer Res.
11:1174–1180. 2005.PubMed/NCBI
|
|
38.
|
D’Amours D and Jackson SP: The Mre11
complex: at the crossroads of DNA repair and checkpoint signalling.
Nature Rev Mol Cell Biol. 3:317–327. 2002.PubMed/NCBI
|
|
39.
|
Loercher A, Lee TL and Ricker JL: Nuclear
factor-κB is an important modulator of the altered gene expression
profile and malignant phenotype in squamous cell carcinoma. Cancer
Res. 64:6511–6523. 2004.
|
|
40.
|
Chiba T and Marusawa H: A novel mechanism
for inflammation-associated carcinogenesis; an important role of
activation-induced cytidine deaminase (AID) in mutation induction.
J Mol Med. 87:1023–1027. 2009. View Article : Google Scholar
|
|
41.
|
Costa AC, Figueiredo C and Touati E:
Pathogenesis of Helicobacter pylori infection. Helicobacter.
14(Suppl 1): 15–20. 2009.
|
|
42.
|
Lim SC, Kim SM, Choi JE, Kim CH, Duong HQ,
Han SI and Kang HS: Sodium salicylate switches glucose
depletion-induced necrosis to autophagy and inhibits high mobility
group box protein 1 release in A549 lung adenocarcinoma cells.
Oncol Rep. 19:1165–1171. 2008.
|
|
43.
|
Boolbol SK, Dannenberg AJ, Chadburn A,
Martucci C, Guo XJ, Ramonetti JT, Abreu-Goris M, Newmark HL, Lipkin
ML, DeCosse JJ and Bertagnolli MM: Cyclooxygenase-2 overexpression
and tumor formation are blocked by sulindac in a murine model of
familial adenomatous polyposis. Cancer Res. 56:2556–2560.
1996.PubMed/NCBI
|