Introduction
Recently, 2-[18F]-fluoro-2-deoxy-D-glucose (FDG)
positron emission tomography (PET)/computed tomography (CT) has
been used for diagnosing malignant tumors, and many reports have
described its role for predicting the malignant potential of these
tumors. However, the clinical efficacy of PET/CT for diagnosing
hepatocellular carcinoma (HCC) remains controversial, and only a
few reports have described the predictive value of its findings for
pathological malignant potential and prognosis (1–3).
Hepatic resection is performed as a standard therapy
for HCC in Japan (4,5). However, recurrence of HCC after
resection is known to occur at a high rate, and early recurrence is
considered to be a significant prognostic factor for death.
Although macroinvasion of HCC to the portal vein is also a factor
for poor prognosis (6), most
patients with HCC without macro-tumor thrombosis suffer from
recurrence after resection. Previous reports have investigated
prognostic markers for early recurrence and survival, including the
doubling time of pre-operative serum α-fetoprotein (AFP) and
protein induced by vitamin K absence or antagonist II (PIVKA-II)
(7), complication with diabetes
mellitus (8), hepatic steatosis
(9) and tumor node metastasis
(TNM) stage (10), although these
are not adequately sensitive. In the present study, we investigated
the predictive value of PET/CT for the pathological malignant
potential of HCC as a new indicator for early recurrence after
hepatic resection.
Materials and methods
From April 2006 to October 2009, 53 patients with
naïve HCC, examined by PET/CT and treated by hepatic resection,
were enrolled. None had poorly controlled diabetes mellitus. All
were examined using PET/CT (Discovery ST Elite 16; GE Healthcare
Japan Co. Ltd., Tokyo, Japan) within the month prior to resection.
PET/CT was performed 60 min after a bolus injection of F-18 FDG (3
MBGq/kg). Accumulations of FDG [standardized uptake value (SUVmax)]
in HCC and non-HCC areas of the liver as well as the ratio of
SUVmax (R-SUV), which indicated the tumor to non-tumor ratio, were
determined. In cases with multiple HCC, the SUVmax was calculated
for the main nodule. From these findings, we evaluated prognostic
factors for early recurrence, which was defined as recurrence
within 2 years of resection. Moreover, R-SUV values were compared
to the pathological findings, including microvascular invasion
(vp), micro-intrahepatic metastasis (im) and gross type of HCC
(11,12). The patients were divided into two
groups, low R-SUV (n=19) and high R-SUV (n=34), and their clinical
parameters were compared.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Statistical analyses were performed using the Student's
t-test for unpaired data, a χ2 test, Fischer's exact
test, a Mann-Whitney U test and a log-rank test, as appropriate.
All statistical analyses were performed with SPSS 16.0J (SPSS Japan
Inc., Tokyo, Japan). A P-value of <0.05 was considered to
represent statistical significance.
Results
One patient was classified as TNM stage I, 35 as
stage II, 14 as stage III and 3 as stage IV, based on the results
of imaging examinations (abdominal ultrasonography and dynamic CT).
There were no cases with extrahepatic metastasis. R-SUV values
ranged from 1.0 to 6.9. In pathological analyses, all were
diagnosed as typical HCC. PIVKA-II (≥200 mAU/ml), fucosylated AFP
(AFP-L3) (≥15%), tumor size (≥5 cm) and high R-SUV (≥1.5) were
found to be risk factors for early recurrence in a univariate
analysis (P<0.05, respectively) (Table I). In a multivariate analysis, high
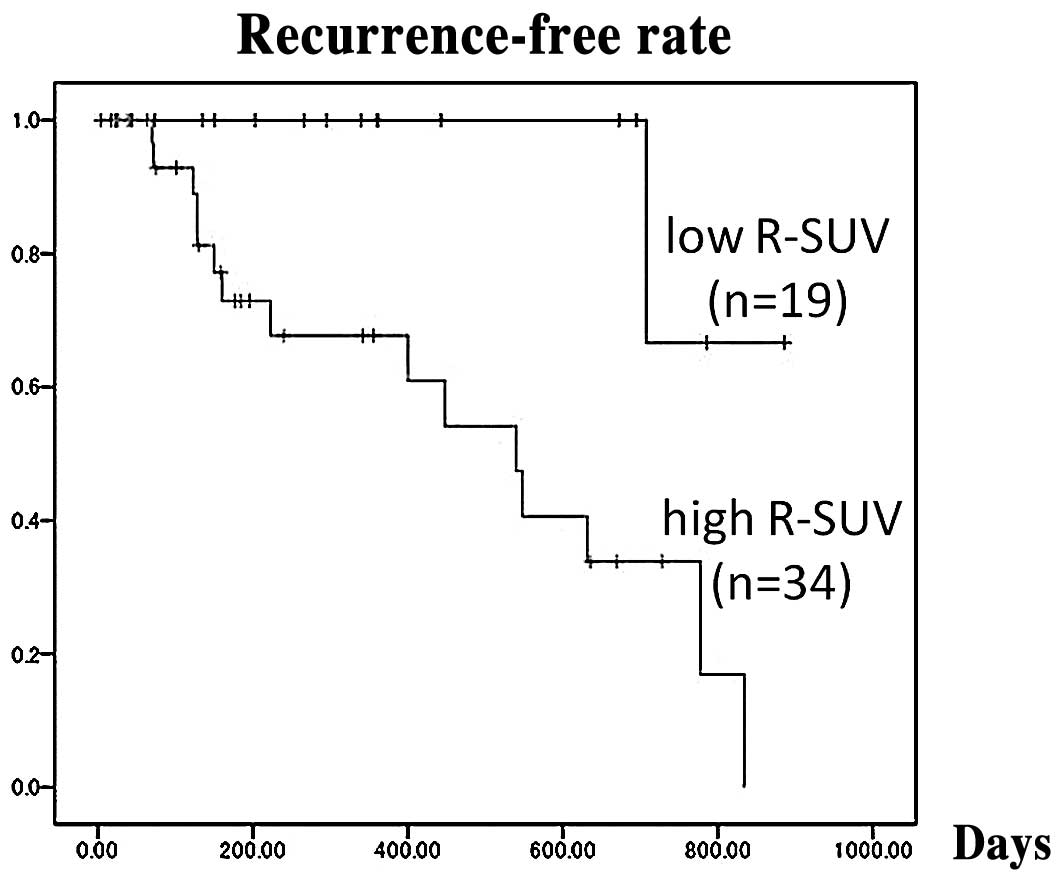
R-SUV (≥1.5) was the only risk factor (P<0.05) (Table II). The recurrence-free rate in the
low R-SUV group was higher than that in the high R-SUV group (1-
and 2-year recurrence-free rates: 100 and 67%, 67 and 17%,
respectively; P<0.01) (Fig. 1).
While the frequencies of high levels of PIVKA-II (≥200 mAU/ml) and
AFP-L3 (≥15%) were greater in the high R-SUV group (52.9 and 38.2%
vs. 21.1 and 10.5%, respectively; P<0.01), there were no
significant differences in regard to the frequencies of high levels
of AFP (≥100 ng/ml), tumor diameter ≥5 cm, Child-Pugh class, number
of tumors and TNM stage between the groups (Table III).
 | Table I.Univariate analysis of clinical
parameters for early recurrence after resection. |
Table I.
Univariate analysis of clinical
parameters for early recurrence after resection.
| Factors | No. | Hazard ratio | 95% CI | P-value |
|---|
| Age in years
(<70:≥70) | 23:30 | 0.662 | 0.234–1.873 | 0.437 |
| Anti-HCV
(positive:negative) | 28:25 | 0.612 | 0.221–1.674 | 0.345 |
| Gender
(male:female) | 40:13 | 0.688 | 0.189–2.507 | 0.571 |
| Aspartate transferase
(IU/l) (<80:≥80) | 44:9 | 2.501 | 0.841–7.437 | 0.099 |
| Alanine transferase
(IU/l) (<80:≥80) | 48:5 | 3.710 | 0.956–14.402 | 0.058 |
| Total bilirubin
(mg/dl) (<2:≥2) | 52:1 | 0.048 | 0.000–2.823 | 0.810 |
| Albumin (g/dl)
(<3.5:≥3.5) | 11:42 | 1.456 | 0.307–6.904 | 0.636 |
| Prothrombin time (%)
(<80:≥80) | 25:28 | 0.923 | 0.341–2.493 | 0.874 |
| Platelets
(x104 cells/μl) (<12:≥12) | 18:35 | 1.453 | 0.515–4.098 | 0.480 |
| Child-Pugh (A:B) | 44:9 | 1.679 | 0.352–8.003 | 0.515 |
| AFP (ng/ml)
(<100:≥100) | 41:12 | 1.996 | 0.619–6.441 | 0.248 |
| AFP-L3 (%)
(<15:≥15) | 38:15 | 3.165 | 1.101–9.095 | 0.032 |
| PIVKA-II (mAU/ml)
(<200:≥200) | 31:22 | 3.805 | 1.285–11.267 | 0.016 |
| Diabetes mellitus
(positive:negative) | 16:37 | 0.371 | 0.102–1.354 | 0.133 |
| No. of HCC
(multiple:single) | 15:38 | 1.578 | 0.540–4.607 | 0.404 |
| Tumor size (<5
cm:≥5 cm) | 31:22 | 3.050 | 1.036–8.983 | 0.043 |
| R-SUV
(<1.5:≥1.5) | 19:34 | 10.581 | 1.394–80.343 | 0.023 |
 | Table II.Multivariate analysis of clinical
parameters for early recurrence after resection. |
Table II.
Multivariate analysis of clinical
parameters for early recurrence after resection.
| Factors | Hazard ratio | 95% CI | P-value |
|---|
| AFP-L3 (≥15%) | 1.644 | 0.510–5.308 | 0.405 |
| PIVKA-II (≥200
mAU/ml) | 2.113 | 0.481–9.275 | 0.322 |
| Tumor size (≥5
cm) | 1.157 | 0.271–4.935 | 0.843 |
| R-SUV (≥1.5) | 8.137 | 1.027–64.466 | 0.047 |
 | Table III.Clinical background of the
patients. |
Table III.
Clinical background of the
patients.
| Low R-SUV group
(n=19) | High R-SUV group
(n=34) | P-value |
|---|
| Age (years) | 68.2±14.1 | 69.5±10.5 | 0.705 |
| Anti-HCV
(positive:negative) | 13:6 | 15:19 | 0.092 |
| Gender
(male:female) | 15:4 | 25:9 | 0.705 |
| Aspartate
transferase (IU/l) | 49.4±32.2 | 50.2±25.8 | 0.308 |
| Alanine transferase
(IU/l) | 40.5±25.4 | 41.5±27.8 | 0.934 |
| Total bilirubin
(mg/dl) | 0.79±0.49 | 0.66±0.28 | 0.085 |
| Albumin (g/dl) | 4.0±0.6 | 4.0±0.5 | 0.555 |
| Prothrombin time
(%) | 80.1±12.2 | 81.2±9.9 | 0.127 |
| Platelets
(x104 cells/μl) | 13.0±4.7 | 16.0±5.7 | 0.696 |
| Child-Pugh class
(A:B) | 15:4 | 29:5 | 0.528 |
| AFP (ng/ml) | 371.2±1,199.4 |
2,306.5±12,657.9 | 0.201 |
| AFP-L3 (%) | 1.6±5.1 | 16.9±23.9 | <0.001 |
| PIVKA-II
(mAU/ml) | 424.4±1,123.6 |
8,436.2±17,683.6 | 0.001 |
| Tumor size (<5
cm:≥5 cm) | 14:5 | 17:17 | 0.077 |
| No. of tumors | 1.4±0.7 | 1.4±0.7 | 0.819 |
| Score of up to 7
criteria | 5.0±1.7 | 7.4±3.4 | 0.013 |
| TNM stage
(I:II:III:IV) | 1:13:5:0 | 0:22:9:3 | 0.320 |
| Mean R-SUV | 1.23±1.45 | 2.73±1.65 | <0.001 |
Patients with HCC nodules rated as Edmondson III
(13) had a higher R-SUV value
(3.0±1.8) than those rated as I and II (1.4±0.3 and 1.9±0.9,
respectively; P<0.01). Patients with nodules showing vp(+) and
im(+), and with non-boundary type of nodules (single nodular type
with extranodular growth, confluent multinodular or invasive type)
had higher R-SUV values than those with vp(−), im(−) or boundary
type (vaguely nodular or single nodular type) (3.6±2.4 vs. 2.0±0.9,
3.5±2.3 vs. 1.9±0.8 and 2.9±1.8 vs. 1.6±0.5, respectively;
P<0.01). Throughout the observation period, extrahepatic
metastasis was observed in 2 cases of stage II; these cases had
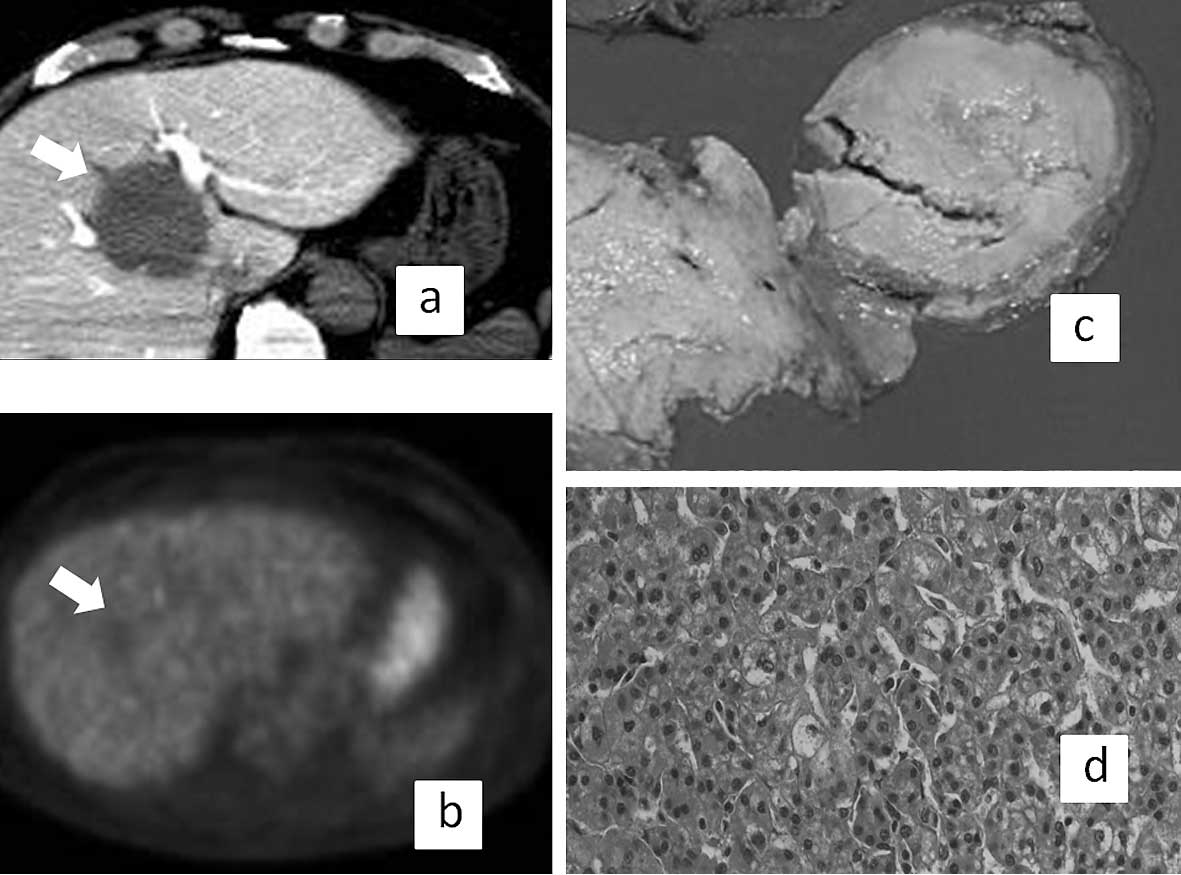
high R-SUV (2.3 and 2.4, respectively). Fig. 2 shows representative results of a
patient with low R-SUV (1.4) whose pathological findings were
single nodular type, Edmondson I, and who was negative for both vp
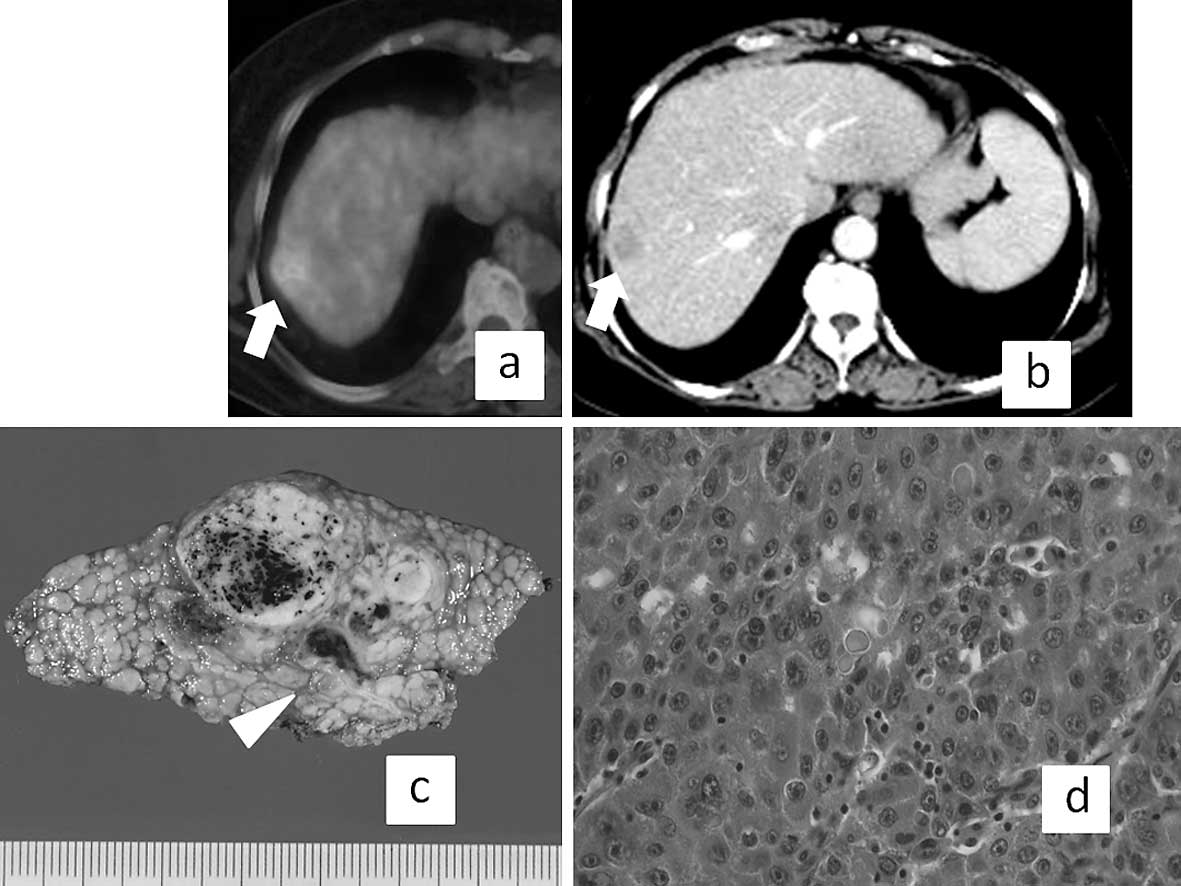
and im. By contrast, Fig. 3
presents the results of a representative patient with high R-SUV
(1.9) whose pathological findings were confluent multinodular type,
Edmondson III and positive for vp, though the tumor size was small
(2.5 cm in diameter).
Discussion
In Japan, the shortage of donors for liver
transplantation is a major obstacle to the treatment of HCC, thus
hepatic resection is often performed as curative therapy (4,5).
FDG-PET/CT is a functional imaging modality that is used to measure
the glucose metabolism of malignant tumors, although its clinical
efficacy has not been established. The ability of PET to detect HCC
in the liver was found to be less effective than that of contrast
enhanced CT (14). On the other
hand, Yoon et al (15) and
Sugiyama et al (16)
reported that PET is useful for the screening of extrahepatic
metastasis from HCC. Recently, the usefulness of FDG-PET for
predicting HCC recurrence following liver transplantation was
proposed (17,18). However, few reports have described
FDG-PET as useful for predicting prognosis after resection
(1,2). Kawamura et al found that even
in patients diagnosed in the early phase of HCC, a high R-SUV value
among other prognostic scores may indicate poor prognosis or the
need for radical treatment (3).
The present results are similar to past reports, which showed that
a high R-SUV value is capable of predicting early recurrence after
resection, and that the relationship between a high R-SUV and
pathological malignant potential is associated with positive
findings for im or vp (19),
higher Edmondson grade and worse gross type (11,12)
in resected specimens. Since the average R-SUV of Edmondson I was
<1.5, we set 1.5 as the cut off; this cut off value predicted
the early recurrence of HCC.
Full body scanning with PET/CT is useful for the
screening of extrahepatic metastasis and staging in patients with
large HCC (15). An FDG-PET/CT
examination is non-invasive and useful for predicting the malignant
pathological potential of HCC before resection without the need for
a biopsy. However, patients with high R-SUV values must be followed
carefully with imaging modalities after resection.
In conclusion, we found that HCC patients with a
high R-SUV value (≥1.5) had an elevated risk of early recurrence
after resection, while R-SUV was also shown to be related with
pathological findings. Thus, R-SUV is proposed as a useful
predictive marker for the early recurrence of HCC before surgical
resection.
References
|
1.
|
Shiomi S, Nishiguchi S, Ishizu H, et al:
Usefulness of positron emission tomography with
fluorine-18-fluorodenoxyglucose for predicting outcome in patients
with hepatocellular carcinoma. Am J Gastroenterol. 96:1877–1880.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hatano E, Ikai I, Higashi T, et al:
Preoperative positron emission tomography with
fluorine-18-fluorodeoxyglucose in predictive of prognosis in
patients with hepatocellular carcinoma after resection. World J
Surg. 30:1736–1741. 2006. View Article : Google Scholar
|
|
3.
|
Kawamura E, Habu D, Ohfuji S, et al:
Clinical role of FDG-PET for HCC: relationship of glucose metabolic
indicator to Japan Integrated Staging (JIS) score.
Hepatogastroenterology. 55:582–586. 2008.PubMed/NCBI
|
|
4.
|
Arii S, Yamaoka Y, Futagawa S, et al:
Results of surgical and nonsurgical treatment for small-sized
hepatocellular carcinomas: a retrospective and nationwide survey in
Japan. The Liver Cancer Study Group of Japan. Hepatology.
32:1224–1229. 2000. View Article : Google Scholar
|
|
5.
|
Ikai I, Arii S, Kojiro M, et al:
Reevaluation of prognostic factors for survival after liver
resection in patients with hepatocellular carcinoma in a Japanese
nationwide survey. Cancer. 101:796–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Arii S, Tanaka J, Yamazoe Y, et al:
Predictive factors for intrahepatic recurrence of hepatocellular
carcinoma after partial hepatectomy. Cancer. 69:913–919. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Masuda T, Beppu T, Horino K, et al:
Preoperative tumor marker doubling time is a useful predictor of
recurrence and prognosis after hepatic resection of hepatocellular
carcinoma. J Surg Oncol. Nov 24–2009.(E-pub ahead of print).
|
|
8.
|
Komura T, Mizukoshi E, Kita Y, et al:
Impact of diabetes on recurrence of hepatocellular carcinoma after
surgical treatment in patients with viral hepatitis. Am J
Gastroenterol. 102:1939–1946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Takuma Y, Nouso K, Makino Y, et al:
Hepatic steatosis correlates with the postoperative recurrence of
hepatitis C virus-associated hepatocellular carcinoma. Liver Int.
27:620–626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Poon RT, Ng IO, Fan ST, et al:
Clinicopathologic features of long-term survivors and disease-free
survivors after resection of hepatocellular carcinoma: a study of a
prospective cohort. J Clin Oncol. 19:3037–3044. 2001.PubMed/NCBI
|
|
11.
|
Stroffolini T, Andreone P, Andriulli A, et
al: Gross pathologic types of hepatocellular carcinoma in Italy.
Oncology. 56:189–192. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Iguchi T, Aishima S, Sanefuji K, et al:
Both fibrous capsule formation and extracapsular penetration are
powerful predictors of poor survival in human hepatocellular
carcinoma: a histological assessment of 365 patients in Japan. Ann
Surg Oncol. 16:2539–2546. 2009. View Article : Google Scholar
|
|
13.
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver. A study of 100 cases among 48900 necrosis
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Trojan J, Schroeder O, Raedle J, et al:
Fluorine-18 FDG positron emission tomography for imaging of
hepatocellular carcinoma. Am J Gastroenterol. 94:3314–3319. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yoon KT, Kim JK, Kim DY, et al: Role of
18F-fluorodeoxyglucose positron emission tomography in detecting
extrahepatic metastasis in pretreatment staging of hepatocellular
carcinoma. Oncology. 72:104–110. 2007. View Article : Google Scholar
|
|
16.
|
Sugiyama M, Sakahara H, Torizuka T, et al:
18F-FDG PET in the detection of extrahepatic metastases from
hepatocellular carcinoma. J Gastroenterol. 39:961–968. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lee JW, Paeng JC, Kang KW, et al:
Prediction of tumor recurrence by 18F-FDG PET in liver
transplantation for hepatocellular carcinoma. J Nucl Med.
50:682–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Komberg A, Freesmeyer M, Barthel E, et al:
18F-FDG-uptake of hepatocellular carcinoma on PET predicts
microvascular tumor invasion in liver transplant patients. Am J
Transplant. 9:592–600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sumie S, Kuromatsu R, Okuda K, et al:
Microvascular invasion in patients with hepatocellular carcinoma
and its predictable clinicopathological factors. Ann Surg Oncol.
15:1375–1382. 2008. View Article : Google Scholar : PubMed/NCBI
|