Introduction
Urothelial carcinoma (UC), also known as
transitional cell carcinoma, is a malignant tumor arising from a
transitional type of stratified epithelium, the urothelium, and
affects the renal collecting system (1). It is the most common cancer of the
urinary bladder and ureter (1) and
the second leading cause of death among malignancies of the
genitourinary tract system (2). In
the case of bladder cancer, most diagnosed patients have non-muscle
invasive disease with a high risk of recurrence (3). Therefore, approaches for the
determination of bladder cancer recurrence are important in medical
care (4). At present, cystoscopic
examination is recommended every 3–6 months for 3 years after
surgical treatment (5). Cystoscopy
in combination with urinary cytology is the standard protocol for
the detection and surveillance of bladder cancer.
Recently, Dickkopf-1 (DKK1) was found to be
associated with the development of several types of cancer,
including Wilm’s tumor, hepatoblastoma, hepatocellular carcinoma,
colorectal cancer, lung and esophageal carcinomas, breast,
cervical, endometrial and kidney cancers (6–10).
DKK1 is a secreted protein that plays a crucial role in head
formation during vertebrate development and specifically inhibits
Wnt/β-catenin signaling (11).
DKK1 binds to the LRP5/6 and Kremen proteins leading to LRP
endocytosis, which prevents the formation of the
Wnt-Frizzled-LRP5/6 receptor complex (12). Furthermore, the Wnt signaling
pathway was found to be involved in the pathogenesis of UC
(13–19). Therefore, an association of DKK1
expression with UC is a reasonable speculation. To date, the
significance of DKK1 activation in human UC is still not clear. In
this study, the correlation between DKK1 expression and UC
progression was investigated.
Materials and methods
Subjects
Seventy-five UC patients who underwent surgery at
the Department of Urology, Chiayi Christian Hospital, were enrolled
in the study. Seventy-five age-matched cancer-free volunteers were
recruited as control individuals. Tumor specimens, blood samples
and demographic information (including age, gender and smoking
status) were collected at the time of enrollment. Informed consent
was obtained prior to the collection of specimens and demographic
information. The specimens were collected and stored according to
the protocols approved by the Institutional Review Board of the
Chiayi Christian Hospital. The clinical features of the UC patients
are listed in Table I.
 | Table I.Characteristics of the 75 patients
with urothelial carcinoma. |
Table I.
Characteristics of the 75 patients
with urothelial carcinoma.
| Characteristic | | No. of patients
(%) |
|---|
| Age, years | | |
| Median | 73 | |
| Range | 33–88 | |
| Gender | | |
| Male | | 59 (78.7) |
| Female | | 16 (21.3) |
| Surgery | | |
| TURBT | | |
| Cigarette
smoking | | |
| Never | | 59 (78.7) |
| Ever | | 16 (21.3) |
| Clinical stage | | |
| Ta/T1 | | 35 (46.7) |
| T2 | | 19 (25.3) |
| T3 | | 16 (21.3) |
| T4 | | 5 (6.7) |
| Histological
grade | | |
| Low (G1 or G2
low) | | 16 (21.3) |
| High (G2 high and
above) | | 59 (78.7) |
| Serum DKK1
(pg/ml) | | |
| Median | 741.53 | |
| Range | 10.86-3098.77 | |
Detection of serum DKK1 by ELISA
Serum DKK1 was detected using the DuoSet ELISA
Development System (R&D System, Minneapolis, MN, USA) according
to the manufacturer’s instructions. Briefly, a rabbit polyclonal
antibody against DKK1 was coated onto a 96-well microplate and
incubated overnight at room temperature. After washing, 300 μl of
Reagent Diluent was added to each well and incubated at room
temperature for 1 h. After further washing, 100 μl of serum or
standard in the Reagent Diluent was added to each well and
incubated for 2 h at room temperature. After further washing, 100
μl of a biotinylated goat anti-human DKK1 antibody diluted in
Reagent Diluent (50 ng/ml) was added to each well and incubated for
2 h. After subsequent washing, 100 μl of the working solution
(1:200) containing horseradish peroxidase (HRP)-streptavidin was
added to each well and incubated for 20 min. After subsequent
washing, 100 μl of substrate solution was added to each well for 20
min. The reaction was terminated by adding 50 μl of Stop Solution.
Each well was assayed using a microplate reader at a 450-nm
wavelength.
Statistical analysis
Statistical analysis was performed using the SPSS
software version 11 for Windows (SPSS Inc., Chicago, IL, USA). In
the statistical analysis, clinical disease stage and tumor grade
were stratified as a binary variable, with non-muscle invasive
disease (pT1 or pTa) compared to muscle-invasive disease (pT2, pT3
or pT4), and with low grade (G1 or G2 low) compared to high grade
(G2 high or above) tumors. The serum DKK1 level was defined as a
binary variable, with a serum DKK1 cut-off level of <1,049 pg/ml
being compared to a serum DKK1 ≥1,049 pg/ml. Demographic and
clinical information was compared across serum DKK1 levels using
the Pearson test. The difference in the levels of DKK1 between the
tumor and control groups was analyzed by the Mann-Whitney U test.
Disease-free survival (DFS) was calculated from the date of surgery
to the date of recurrence or last known date without recurrence.
Overall survival (OS) was calculated from the date of surgery to
the date of death or last known date that the patient was alive.
DFS and OS were calculated by the Kaplan-Meier method. The Cox
proportional hazards model was used to evaluate the effect of serum
DKK1 level on DFS and OS. Two-sided p-values of <0.05 were
considered significant.
Detection of DKK1 expression in UC
tissues by RT-PCR
The DKK1 gene product was detected by
semi-quantitative reverse transcription-PCR (RT-PCR). Total RNA was
extracted from tumor tissues with TRIzol (Invitrogen, Carlsbad, CA,
USA) following the manufacturer’s instructions. Reverse
transcription of mRNA to cDNA was performed using random hexamers
as reaction primers (Roche Diagnostics, Indianapolis, IN, USA) and
SuperScript II (Invitrogen). Semi-quantitative RT-PCR was carried
out with two sets of primers, 5′-TAGAGTCTAGAACGCAAGGATCTC-3′ and
5′-CAA AAACTATCACAGCCTAAAGGG-3′, specific to DKK1; and a set
of primers, 5′-GAGGTGATAGCATTGCTTTCG-3′ and
5′-CAAGTCAGTGTACAGGTAAGC-3′, specific to β-actin as an
internal control.
Detection of DKK1 protein in UC tissues
by Western blotting
Expression of DKK1 protein in UC tissues was
detected by Western blotting. Total protein of the tissues was
extracted and determined usingthea Bio-Rad protein assay (Bio-Rad,
Hercules, CA, USA). The tissue extracts were resolved on denaturing
polyacrylamide gels and then transferred to a PVDF membrane. After
blocking with 3% blocker (Bio-Rad) in TBS Tween-20 (TBST), the
membrane was incubated with a rabbit polyclonal antibody against
human DKK1 (hDKK1; Santa Cruz Biotechnology, Santa Cruz, CA, USA)
for 2 h at room temperature. After washing, the membrane was
incubated with an HRP-conjugated secondary antibody (Santa Cruz
Biotechnology) for 1 h at room temperature. The membrane was washed
with TBST and developed using the enhanced OPTI-4CN Colorimetric
Detection kit (Bio-Rad).
Results
Serum DKK1 levels in UC patients
The DKK1 levels from the sera of 75 UC patients and
75 cancer-free control individuals were examined by ELISA. Serum
DKK1 levels in the UC patients were significantly higher than those
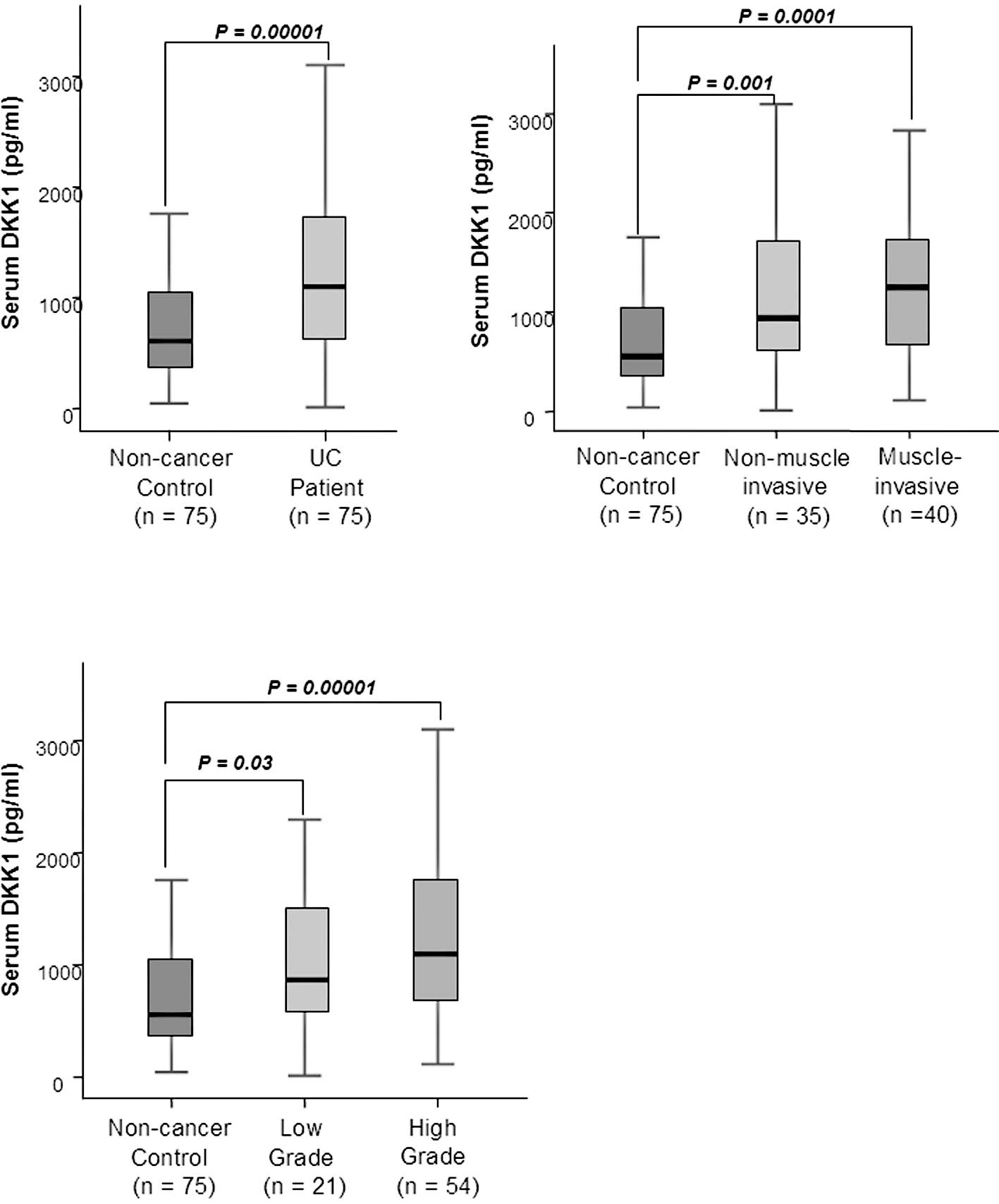
in the control individuals (p=0.00001) (Fig. 1A). The Spearman correlation study
revealed that none of the prognostic parameters correlated with the
serum DKK1 levels, except that both clinical stage and histological
grade showed mild correlations with serum DKK1 levels (r=0.357 and
0.392, respectively) (Table II).
Furthermore, in the subgroup analyses the serum DKK1 levels in the
non-muscle invasive and muscle-invasive groups of UC patients were
significantly higher than the levels in the cancer-free control
group (p=0.01 and 0.001, respectively) (Fig. 1B). In addition, when the
histological grades of the UC tumors were considered, the serum
DKK1 levels in patients with low-grade and high-grade tumors were
higher than those in the non-cancer control individuals (p=0.03 and
0.0001, respectively) (Fig.
1C).
 | Table II.Spearman correlation coefficients
(rs) for the relationships between serum DKK1 and
different variables in patients with urothelial carcinoma. |
Table II.
Spearman correlation coefficients
(rs) for the relationships between serum DKK1 and
different variables in patients with urothelial carcinoma.
| Variable | rs | p-value |
|---|
| Age (1 year) | 0.104 | 0.206000 |
| Gender (male→0,
female→1) | −0.027 | 0.738000 |
| Cigarette smoking
(never smoker→0, smoker→1) | −0.040 | 0.626000 |
| Clinical stage
(control→0, non-muscle invasive→1, muscle invasive→2) | 0.357 | 0.000010 |
| Histological grade
(control→0, low→1, high→2) | 0.392 | 0.000001 |
Correlation of serum DKK1 levels with
overall survival of UC patients
To define high DKK1 expression, the cut-off value
for a high expression level was determined from the dataset
covering the 75 UC patients and 75 control individuals using ROC
curve analysis. The optimum cut-off value in this study was
determined to be 1,049 pg/ml, which gave a sensitivity of 52% and a
specificity of 80%. Based on this definition, a serum DKK1 level
≥1,049 pg/ml was considered high expression. Analyses of the
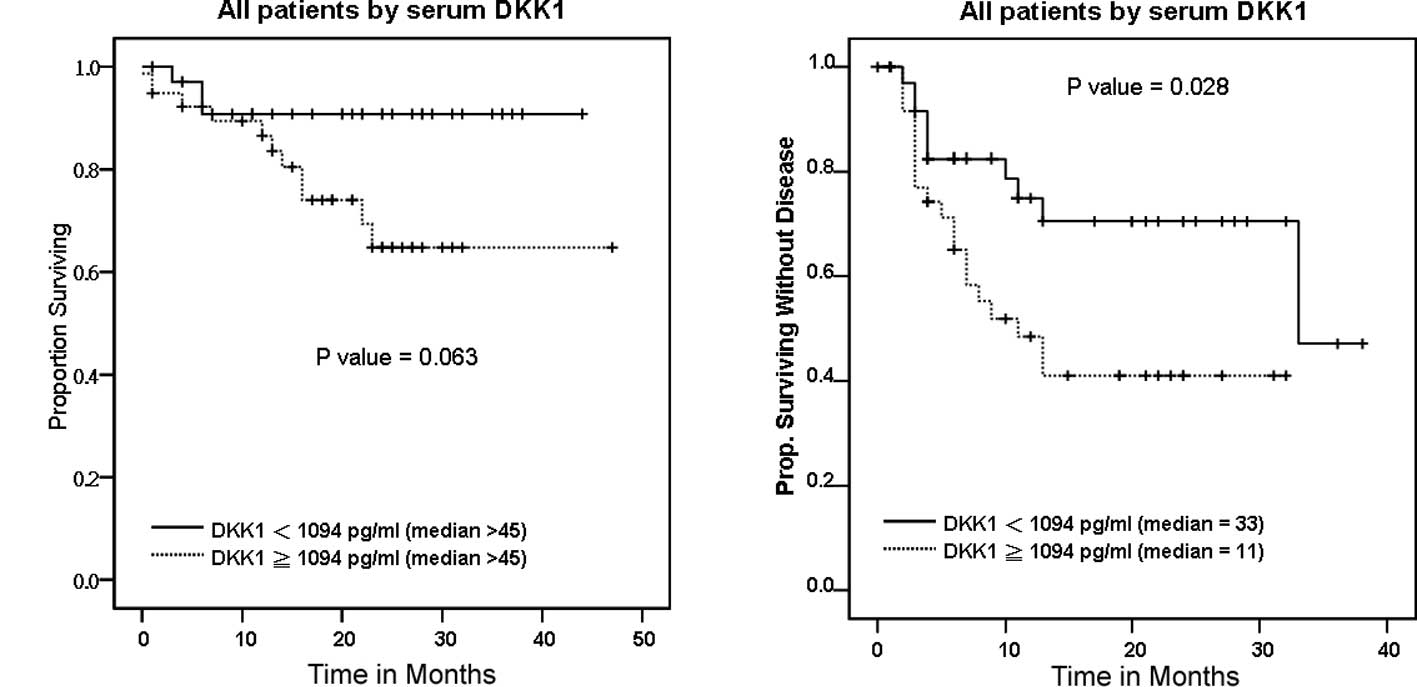
influence of serum DKK1 on OS showed that the survival time of UC
patients with high-serum DKK1 expression was shorter than that of
patients with low-serum DKK1 expression. However, this difference
showed only a marginal significance (p=0.063) (Fig. 2A). Univariate and multivariate
analyses showed that age, gender, cigarette smoking status,
clinical stage and histological grade were not significantly
associated with OS (Table
III).
 | Table III.Univariate and multivariate analyses
for the disease-free survival (DFS) and overall survival (OS) of
the urothelial carcinoma (UC) patients. |
Table III.
Univariate and multivariate analyses
for the disease-free survival (DFS) and overall survival (OS) of
the urothelial carcinoma (UC) patients.
| DFS of UC patients
| OS of UC patients
|
|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|
| Univariate
analysis | | | | | | |
| Age (1 year) | 1.00 | 0.97–1.03 | 0.970 | 1.06 | 0.99–1.12 | 0.06 |
| Gender (male vs.
female) | 1.24 | 0.47–3.25 | 0.670 | 0.48 | 0.16–1.42 | 0.18 |
| Cigarette smoking
(never vs. ever) | 0.77 | 0.31–1.89 | 0.570 | 0.55 | 0.12–2.46 | 0.43 |
| Clinical stage
(non-muscle invasive vs. muscle invasive) | 1.12 | 0.54–2.33 | 0.760 | 1.02 | 0.36–2.93 | 0.96 |
| Histological
grade (high vs. low) | 1.55 | 0.66–3.65 | 0.310 | N/A | N/A | N/A |
| Serum DKK1 level
(1 pg/ml) | 1.00 | 1.000–1.001 | 0.007 | 1.00 | 0.99–1.00 | 0.07 |
| Serum DKK1 level
(≥1,049 vs. <1,049 pg/ml) | 2.44 | 1.10–5.40 | 0.028 | 3.37 | 0.94–12.11 | 0.06 |
| Multivariate
analysis | | | | | | |
| Age (1 year) | 0.99 | 0.97–1.03 | 0.900 | 1.05 | 0.96–1.12 | 0.14 |
| Gender (male vs.
female) | 1.47 | 0.52–4.13 | 0.470 | 0.94 | 0.30–2.99 | 0.92 |
| Cigarette smoking
(never vs. ever) | 0.72 | 0.28–1.81 | 0.480 | 0.90 | 0.18–4.58 | 0.90 |
| Clinical stage
(non-muscle invasive vs. muscle invasive) | 1.06 | 0.51–2.20 | 0.878 | 0.39 | 0.12–1.28 | 0.12 |
| Histological
grade (high vs. low) | 1.47 | 0.60–3.64 | 0.410 | N/A | N/A | N/A |
| Serum DKK1 level
(≥1,049 vs. <1,049 pg/ml) | 2.36 | 1.06–5.25 | 0.035 | 2.70 | 0.74–9.90 | 0.13 |
Correlation of serum DKK1 levels with
disease-free survival of UC patients
Univariate analyses was used to assess the influence
of serum DKK1 levels on DFS among the UC patients. The median DFS
was found to be 11 months [95% confidence interval (CI) 5–17] for
patients with high expression of serum DKK1 vs. 33 months (95% CI
0–90) for those with low expression of serum DKK1 (Fig. 2B). The difference in DFS was
statistically significant (p=0.028) (Table III). When serum DKK1 was defined as
a continuous variable, the risk of recurrence was found to increase
as the level of serum DKK1 increased (p=0.007; Table III). Univariate and multivariate
analyses showed that age, gender, cigarette smoking status,
clinical stage and histological grade were not associated with DFS
(Table III).
Expression of DKK1 in UC tissues
The results described above demonstrated that serum
DKK1 levels in UC patients were higher than those in the control
individuals. Yet, whether the expression of DKK1 was also higher in
UC tissues compared with normal tissues was still undetermined.
Since previous studies revealed that the expression of DKK1 was
elevated in lung and esophageal cancer tissues (20–25),
the expression of DKK1 in UC tissues was examined. Forty-four UC
tissue samples from the 75 UC patients were available for
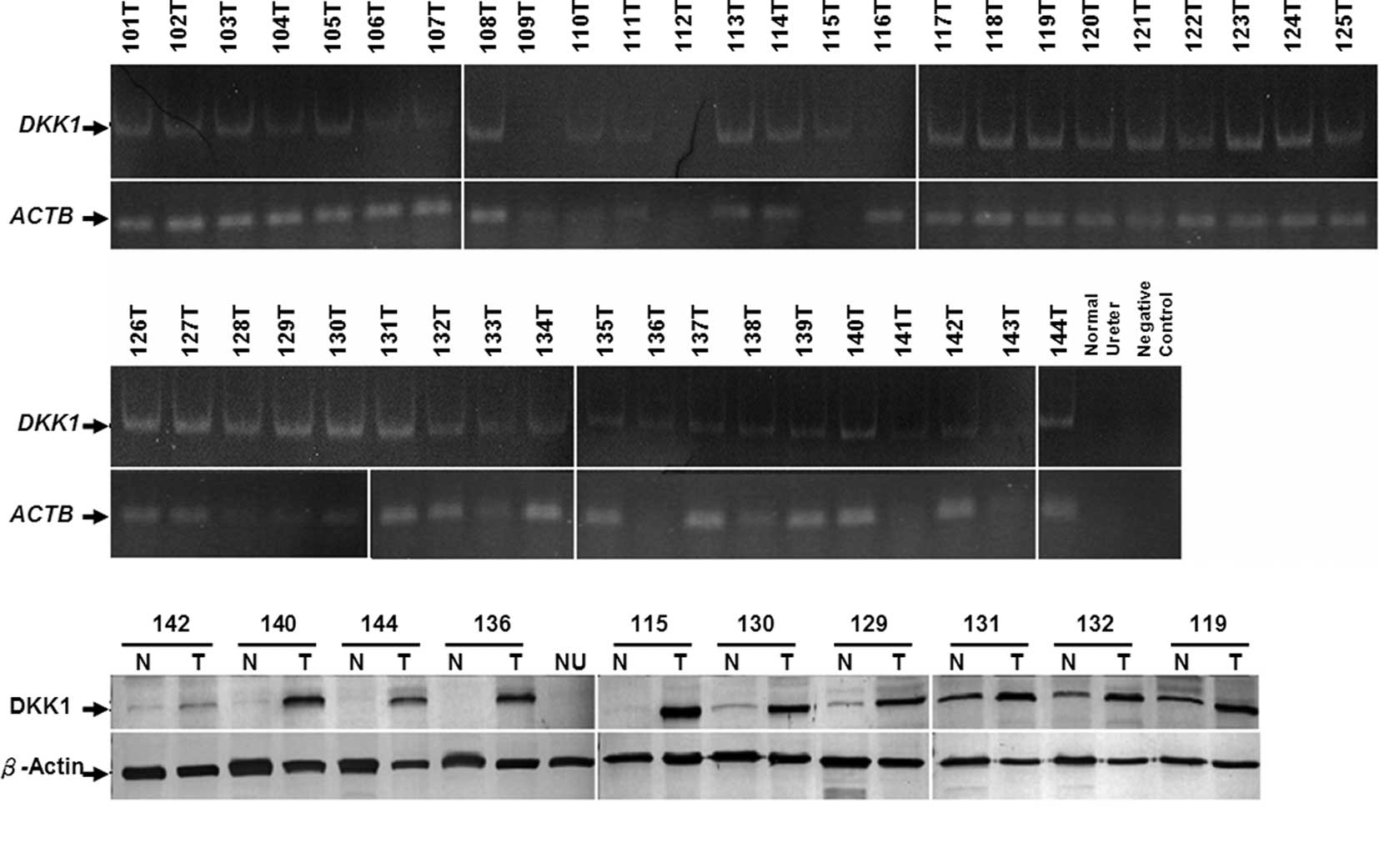
examination. Results of the RT-PCR analysis showed that high
expression of DKK1 was found in 93% (41/44) of the examined UC
tissues (Fig. 3A). Furthermore, 10
UC tissues with adjacent normal tissues were examined for DKK1
protein expression by Western blotting. The results showed that the
expression of DKK1 was higher in the UC tissues when compared to
the normal tissues (Fig. 3B).
Discussion
In the present study, the expression of DKK1 in UC
patients was investigated. Serum DKK1 levels in UC patients were
higher than those in the control individuals. Serum DKK1 levels
were also higher in the UC patients with muscle-invasive and
high-grade tumors than those in the controls. A high serum DKK1
level also appeared to be correlated with poor DFS in the UC
patients. In addition, high expression of DKK1 mRNA was also
demonstrated in UC tissues using RT-PCR, and the expression of DKK1
protein in UC tissues was higher than that in the adjacent normal
tissues as analyzed by Western blotting. This is the first report
of high DKK1 expression in serum and in UC tissue from patients
with UC. In addition, the elevation in DKK1 expression appeared to
be correlated with clinical stage and histological grade.
DKK1 is a secreted protein that functions as a
negative regulator of the Wnt/β-catenin signaling, which is
aberrantly activated in most human colon cancers and a number of
other carcinomas (26,27). In human urothelia carcinoma, Wnt
antogonists, Wif-1 and negative regulators of Wnt/β-catenin
signaling, sFRP1, sFRP2, sFRP4, sFRP5 and APC, have been reported
to be down-regulated, suggesting that their functional loss might
play an important role in cancer pathogenesis through aberrant
canonical Wnt/β-catenin pathway activation. In addition, DKK1 has
been shown to be a downstream target of the β-catenin/TCF pathway,
to participate in a negative feedback loop in Wnt signaling and to
be strongly localized in a zone directly adjacent to the region
where Wnt/β-catenin signaling is active (28,29).
Taken together, these findings imply a mechanism in the zone of the
tumor and adjacent cells whereby Wnt/β-catenin pathway activation
restricts itself through the induction of DKK1, resulting in an
elevated DKK1 expression level. In agreement with this, previous
studies on multiple myeloma (30),
lung, esophageal (10) and
hepatocellular carcinomas (31),
estrogen and progesterone receptor-negative [ER(−)/PR(−)] breast
cancer (7), and cervical,
endometrial (8) and kidney cancers
(7) have revealed that DKK1 is
preferentially expressed in a great majority of cancer cases. These
studies have established DKK1 as a potential prognostic and
diagnostic marker for cancer patient cohorts with poor
prognosis.
Although high expression of DKK1 appeared to be
associated with DFS in this study it only showed a marginal
association with OS in UC patients. This may be due to the short
follow-up time (<3 years) in the present study. Given that
individuals with non-muscle invasive disease are at low risk of
death and those who have muscle-invasive disease benefit from the
successful therapy of radical surgery and urinary diversion,
numerous clinical series have demonstrated a favorable 5-year
prognosis for UC patients (32).
Therefore, a possible reason for the lack of statistical
significance in this setting may be the small number of deaths that
were confirmed during the limited 3-year follow-up of these UC
patients. In addition, notably, the present study failed to find
any statistically significant relationship between the serum DKK1
level and any of the other variables measured, including age,
gender and smoking status.
Although most UC patients are non-muscle invasive at
the time of being diagnosed and are able to be treated by
aggressive management such as surgical removal of the tumor, the
recurrence and progression of UC are not easily diagnosed often
resulting in negative consequences for patients. In the present
study, DKK1 was found to be elevated in the sera and UC tissues of
UC patients. Therefore, the detection of DKK1 expression in serum
is a potential index for the recurrence or progression of UC. In
addition, the inhibition of DKK1 may be a possible therapeutic
target for UC in the future.
Acknowledgements
This study was supported by the Chiayi
Christian Hospital grant R96-12.
References
|
1.
|
Arrizabalaga M, Navarro J, Mora M, Castro
M, Extramiana J, Manas A, Diez J and Paniagua P: [Transitional
carcinomas of the urinary tract: synchronous and metachronous
lesions]. Actas Urol Esp. 18:782–796. 1994.
|
|
2.
|
Shen CH, Wang YH, Wang WC, Jou YC, Hsu HS,
Hsieh HY and Chiou HY: Inducible nitric oxide synthase promoter
polymorphism, cigarette smoking, and urothelial carcinoma risk.
Urology. 69:1001–1006. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Herr HW: Natural history of superficial
bladder tumors: 10- to 20-year follow-up of treated patients. World
J Urol. 15:84–88. 1997.PubMed/NCBI
|
|
4.
|
Pashos CL, Botteman MF, Laskin BL and
Redaelli A: Bladder cancer: epidemiology, diagnosis, and
management. Cancer Pract. 10:311–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Morey SS: American Urological Association
issues guidelines on the management of bladder cancer. Am Fam
Physician. 61:3734–3736. 2000.PubMed/NCBI
|
|
6.
|
Aguilera O, Fraga MF, Ballestar E, Paz MF,
Herranz M, Espada J, Garcia JM, Munoz A, Esteller M and
Gonzalez-Sancho JM: Epigenetic inactivation of the Wnt antagonist
DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene.
25:4116–4121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Forget MA, Turcotte S, Beauseigle D,
Godin-Ethier J, Pelletier S, Martin J, Tanguay S and Lapointe R:
The Wnt pathway regulator DKK1 is preferentially expressed in
hormone-resistant breast tumours and in some common cancer types.
Br J Cancer. 96:646–653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jiang T, Wang S, Huang L and Zhang S:
Clinical significance of serum DKK-1 in patients with gynecological
cancer. Int J Gynecol Cancer. 19:1177–1181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Patil MA, Chua MS, Pan KH, Lin R, Lih CJ,
Cheung ST, Ho C, Li R, Fan ST, Cohen SN, Chen X and So S: An
integrated data analysis approach to characterize genes highly
expressed in hepatocellular carcinoma. Oncogene. 24:3737–3747.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yamabuki T, Takano A, Hayama S, Ishikawa
N, Kato T, Miyamoto M, Ito T, Ito H, Miyagi Y, Nakayama H, Fujita
M, Hosokawa M, Tsuchiya E, Kohno N, Kondo S, Nakamura Y and Daigo
Y: Dikkopf-1 as a novel serologic and prognostic biomarker for lung
and esophageal carcinomas. Cancer Res. 67:2517–2525. 2007.
View Article : Google Scholar
|
|
11.
|
Niida A, Hiroko T, Kasai M, Furukawa Y,
Nakamura Y, Suzuki Y, Sugano S and Akiyama T: DKK1, a negative
regulator of Wnt signaling, is a target of the beta-catenin/TCF
pathway. Oncogene. 23:8520–8526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Gonzalez-Sancho JM, Aguilera O, Garcia JM,
Pendas-Franco N, Pena C, Cal S, Garcia de Herreros A, Bonilla F and
Munoz A: The Wnt antagonist DICKKOPF-1 gene is a downstream target
of beta-catenin/TCF and is downregulated in human colon cancer.
Oncogene. 24:1098–1103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Dulaimi E, Uzzo RG, Greenberg RE,
Al-Saleem T and Cairns P: Detection of bladder cancer in urine by a
tumor suppressor gene hypermethylation panel. Clin Cancer Res.
10:1887–1893. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kastritis E, Murray S, Kyriakou F, Horti
M, Tamvakis N, Kavantzas N, Patsouris ES, Noni A, Legaki S,
Dimopoulos MA and Bamias A: Somatic mutations of adenomatous
polyposis coli gene and nuclear b-catenin accumulation have
prognostic significance in invasive urothelial carcinomas: evidence
for Wnt pathway implication. Int J Cancer. 124:103–108. 2009.
View Article : Google Scholar
|
|
15.
|
Marsit CJ, Karagas MR, Andrew A, Liu M,
Danaee H, Schned AR, Nelson HH and Kelsey KT: Epigenetic
inactivation of SFRP genes and TP53 alteration act jointly as
markers of invasive bladder cancer. Cancer Res. 65:7081–7085. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Maruyama R, Toyooka S, Toyooka KO, Harada
K, Virmani AK, Zochbauer-Muller S, Farinas AJ, Vakar-Lopez F, Minna
JD, Sagalowsky A, Czerniak B and Gazdar AF: Aberrant promoter
methylation profile of bladder cancer and its relationship to
clinicopathological features. Cancer Res. 61:8659–8663.
2001.PubMed/NCBI
|
|
17.
|
Stoehr R, Wissmann C, Suzuki H, Knuechel
R, Krieg RC, Klopocki E, Dahl E, Wild P, Blaszyk H, Sauter G, Simon
R, Schmitt R, Zaak D, Hofstaedter F, Rosenthal A, Baylin SB,
Pilarsky C and Hartmann A: Deletions of chromosome 8p and loss of
sFRP1 expression are progression markers of papillary bladder
cancer. Lab Invest. 84:465–478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Urakami S, Shiina H, Enokida H, Kawakami
T, Kawamoto K, Hirata H, Tanaka Y, Kikuno N, Nakagawa M, Igawa M
and Dahiya R: Combination analysis of hypermethylated
Wnt-antagonist family genes as a novel epigenetic biomarker panel
for bladder cancer detection. Clin Cancer Res. 12:2109–2116. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Urakami S, Shiina H, Enokida H, Kawakami
T, Tokizane T, Ogishima T, Tanaka Y, Li LC, Ribeiro-Filho LA,
Terashima M, Kikuno N, Adachi H, Yoneda T, Kishi H, Shigeno K,
Konety BR, Igawa M and Dahiya R: Epigenetic inactivation of Wnt
inhibitory factor-1 plays an important role in bladder cancer
through aberrant canonical Wnt/beta-catenin signaling pathway. Clin
Cancer Res. 12:383–391. 2006. View Article : Google Scholar
|
|
20.
|
Kakiuchi S, Daigo Y, Ishikawa N, Furukawa
C, Tsunoda T, Yano S, Nakagawa K, Tsuruo T, Kohno N, Fukuoka M,
Sone S and Nakamura Y: Prediction of sensitivity of advanced
non-small cell lung cancers to gefitinib (Iressa, ZD1839). Hum Mol
Genet. 13:3029–3043. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kakiuchi S, Daigo Y, Tsunoda T, Yano S,
Sone S and Nakamura Y: Genome-wide analysis of organ-preferential
metastasis of human small cell lung cancer in mice. Mol Cancer Res.
1:485–499. 2003.PubMed/NCBI
|
|
22.
|
Kikuchi T, Daigo Y, Ishikawa N, Katagiri
T, Tsunoda T, Yoshida S and Nakamura Y: Expression profiles of
metastatic brain tumor from lung adenocarcinomas on cDNA
microarray. Int J Oncol. 28:799–805. 2006.PubMed/NCBI
|
|
23.
|
Kikuchi T, Daigo Y, Katagiri T, Tsunoda T,
Okada K, Kakiuchi S, Zembutsu H, Furukawa Y, Kawamura M, Kobayashi
K, Imai K and Nakamura Y: Expression profiles of non-small cell
lung cancers on cDNA microarrays: identification of genes for
prediction of lymph-node metastasis and sensitivity to anti-cancer
drugs. Oncogene. 22:2192–2205. 2003. View Article : Google Scholar
|
|
24.
|
Taniwaki M, Daigo Y, Ishikawa N, Takano A,
Tsunoda T, Yasui W, Inai K, Kohno N and Nakamura Y: Gene expression
profiles of small-cell lung cancers: molecular signatures of lung
cancer. Int J Oncol. 29:567–575. 2006.PubMed/NCBI
|
|
25.
|
Yamabuki T, Daigo Y, Kato T, Hayama S,
Tsunoda T, Miyamoto M, Ito T, Fujita M, Hosokawa M, Kondo S and
Nakamura Y: Genome-wide gene expression profile analysis of
esophageal squamous cell carcinomas. Int J Oncol. 28:1375–1384.
2006.PubMed/NCBI
|
|
26.
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Aman A and Piotrowski T: Wnt/beta-catenin
and Fgf signaling control collective cell migration by restricting
chemokine receptor expression. Dev Cell. 15:749–761. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chamorro MN, Schwartz DR, Vonica A,
Brivanlou AH, Cho KR and Varmus HE: FGF-20 and DKK1 are
transcriptional targets of beta-catenin and FGF-20 is implicated in
cancer and development. EMBO J. 24:73–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Politou MC, Heath DJ, Rahemtulla A, Szydlo
R, Anagnostopoulos A, Dimopoulos MA, Croucher PI and Terpos E:
Serum concentrations of Dickkopf-1 protein are increased in
patients with multiple myeloma and reduced after autologous stem
cell transplantation. Int J Cancer. 119:1728–1731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Yu B, Yang X, Xu Y, Yao G, Shu H, Lin B,
Hood L, Wang H, Yang S, Gu J, Fan J and Qin W: Elevated expression
of DKK1 is associated with cytoplasmic/nuclear beta-catenin
accumulation and poor prognosis in hepatocellular carcinomas. J
Hepatol. 50:948–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
Raghavan D and Skinner DG: Radical cystectomy in the treatment of
invasive bladder cancer: long-term results in 1,054 patients. J
Clin Oncol. 19:666–675. 2001.PubMed/NCBI
|