Introduction
Despite the earlier diagnosis due to improved
screening procedures and the progress regarding adjuvant
treatments, young breast cancer patients still have a poorer
prognosis compared to their older counterparts (1,2). A
comprehensive genomic analysis of 784 young patients with
early-stage breast cancers established that age at breast cancer
diagnosis remains the most important variable in determining
outcome (3). However, the
fundamental causes of the aggressive behavior of breast cancer in
young patients with locoregional and distant recurrence have not
yet been elucidated (4). Estrogen
plays a key role in the pathogenesis and development of breast
cancer, and the risk for this disease is associated with the length
of exposure to endogenous and exogenous estrogens (5). In addition, 17β-estradiol modulates
vascular endothelial growth factor (VEGF) expression in breast
cancer cells (6) and is a
physiological regulatory factor for the peripheral development of
T-regulatory cells (T-regs) (CD4+CD25+
T-regs) (7). It has been suggested
that the tumor cytosolic content of VEGF is a possible predictor of
survival in patients with node-positive breast cancer (8), and T-regs may be dominantly
responsible for the immunosuppression in tumor immunity and a
potential predictor of the poor prognosis of young breast cancer
patients (9).
Current standard options for the adjuvant treatment
of pre-menopausal women include chemotherapy followed by
anti-estrogen therapy, predominantly with tamoxifen, and ovarian
ablation by surgery, radiotherapy or by luteinizing
hormone-releasing hormone (LH-RH) agonists (10). Moreover, the benefits of
chemotherapy in pre-menopausal breast cancer patients have been
attributed, in part, to the phenomenon of chemotherapy-related
amenorrhea (11).
To date, the data have shown that there is a trend
towards improved recurrence-free and overall survival (OS) in
premenopausal patients who receive an LH-RH agonist plus
chemotherapy and tamoxifen combination in comparison to
chemotherapy alone (12,13).
In recent years, an important change in the adjuvant
treatment of postmenopausal patients has occurred, after the
publication of the results of adjuvant trials that used
third-generation aromatase inhibitors (14–17).
In addition, one study demonstrated that an aromatase inhibitor
added to an LH-RH analogue was more effective than tamoxifen for
the inhibition of estradiol secretion (18). This suggests that aromatase
inhibitors are more effective than tamoxifen as adjuvant hormonal
treatment for pre-menopausal patients with endocrine-responsive
breast cancer.
Since September 1993, over 200 pre-menopausal women
with high-risk early breast carcinoma were treated by our group
with a gonadotropin-releasing hormone (Gn-RH) analogue for ovarian
protection before and during adjuvant chemotherapy, which was
tailored to the specific biological characteristrics of each
patient tumor (19,20). The present study prospectively
evaluated 63 female patients under 40 years of age treated with an
LH-RH analogue for 5 years, tailored chemotherapy and by an
aromatase inhibitor for estrogen receptor (ER)-positive tumors.
Patients and methods
Study design and patient selection
Sixty-three women with histologically confirmed
breast cancer who had undergone complete or segmental mastectomy
plus axillary node dissection or sentinel node biopsy were
recruited for the study. All patients were pre-menopausal, between
26 and 40 years of age, with normal menstruation and normal
gonadotropins, estradiol and progesterone values. Patients with
pathological T1–4, N0–2 and M0 tumors were eligible. Women with
high-risk node-negative tumors (≥1 cm in diameter with a high
histological grade, lymphovascular invasion or both) were also
included. The levels of hormone receptors were determined by an
immunohistochemical assay, with a cutoff point of 10%. Patients
were excluded if they had distant metastases, residual disease in
the breast or axilla, other serious medical illnesses or a previous
occurrence of cancer. Women considering pregnancy in a period of 5
years or using hormones were excluded.
The following laboratory parameters were required:
granulocyte count ≥2,000/μl, platelet count ≥100,000/μl, hematocrit
≥30%, total bilirubin and AST levels ≤1.5 times the upper limit of
normal, serum creatinine concentration ≤1.8 mg/dl and a left
ventricular ejection fraction ≥50%. Bilateral bone marrow aspirates
and biopsies immunostained for cytokeratin were performed routinely
in patients with >5 positive axillary nodes and having
radiographic or scintigraphic pelvic bone abnormalities present.
VEGF was determined by the Human VEGF Colorimetric ELISA kit
(Pierce Endogen) as previously described (21), while flow cytometric analysis was
used to measure T-regs.
Treatment plan
The study was performed according to the Declaration
of Helsinki following local ethics committee approval, and the
patients provided their written informed consent. Staging
assessment included patient history, physical examination, complete
blood count and liver function tests before chemotherapy, and chest
radiograph and bone scan within 8 weeks of starting chemotherapy.
The patients were required to start chemotherapy within 4–6 weeks
after the first histological diagnosis of breast cancer. They
received 11.25 mg of the LH-RH analogue subcutaneously 1 week
before starting chemotherapy, and every 84 days for 5 years. All
patients received cyclophosphamide 600 mg/m2, epirubicin
75 mg/m2 on day 1 and 5-fluorouracil 600 mg/m2 on days 1 and 8 for
four courses. Forty-four patients were treated with segmental
mastectomies and 10 patients with modified radical mastectomy;
patients with more than 10 positive axillary nodes received
radiation therapy to the breast and supraclavicular nodes, with
concurrent cyclophosphamide 600 mg/m2, 5-fluorouracil
600 mg/m2 and methotrexate 40 mg/m2 (CMF) on
day 1, every 3 weeks, for four courses. In addition, the patients
received further adjuvant chemotherapy with the appropriate regimen
being selected according to the biologically relevant
characteristics of the tumor subpopulation.
Ten patients with more than 10 positive axillary
nodes and 8 patients with triple negative tumors received a
high-dose platinum-based chemotherapy with (22) or without (23) bone marrow (PBPC) transplantation,
respectively. Fifteen patients with CERB-2+ tumors
received four courses of 100 mg/m2 docetaxel, after the
end of the anthracycline-based chemotherapy, and/or CMF and
radiation therapy. All 43 patients with ER+ tumors
received an aromatase inhibitor daily for 5 years following the
completion of chemotherapy. During the study, all patients were
supplemented with vitamin D, calcium and bisphosphonates and
received continuous psychological support.
Statistical considerations
The study was designed to test the hypothesis that
the administered regimen decreases VEGF and T-regs. A positive
response was defined as a ≥50% decrease from baseline values of
VEGF and T-regs in the absence of any clinical or radiological
evidence of disease progression for at least 12 months. Simon's
optimal two-stage design was used (24). The first stage required that 8 or
more patients out of 24 had a confirmed ≥50% decrease in VEGF and
T-regs to rule out an undesirably low response probability of 0.30
(P0), in favor of a desirable response probability of 0.50 (P1),
with a 5% probability of accepting a poor agent (α=0.05) and a 10%
probability of rejecting a good agent (β=0.1) before proceeding to
the second stage. In the second stage, accrual of a total of 63
assessable patients provided the desired outcome when a total of 10
or more patients showed a confirmed decrease in VEGF and T-regs,
then the primary endpoint was met.
Secondary end points were the determination of
disease-free survival (DFS), OS and fertility preservation rate.
The results were expressed as the mean ± standard deviation of
determinations made in three different experiments. Post hoc
comparisons were performed by Turkey's honestly significant
difference test. For variables not normally distributed
(CD4+/CD8+), the Friedman repeated measures
ANOVA by ranks was used. Post hoc comparisons were performed
using the Wilcoxon's rank sign test, with a downward adjustment of
a level to compensate for multiple comparisons. The time to relapse
was defined as the time between the start of therapy and any
relapse or any appearance of a second primary cancer or death,
whichever occurred first. OS was measured from study entry to
death, or study entry to February 2010, for censored patients.
Statistical analysis was performed with SAS Statistical software
(version 8.12, 2000; SAS Institute Inc., Cary, NC, USA).
Progression-free survival (PFS) and OS were determined using the
Kaplan-Meier method (25). Adverse
events were evaluated using Standard World Health Organization
criteria (26). Analysis of data
was performed in February 2010.
Results
Patient characteristics
The baseline demographics and tumor characteristics
of the patients are shown in Table
I. Sixty-three consecutive patients diagnosed with unilateral
adenocarcinoma of the breast, stage PT2–3a, N−/+, M0, who had
undergone modified radical mastectomy or breast conserving surgery
plus full axillary node dissection or sentinel node biopsy, were
recruited into the study. These patients were also fully evaluated
for ovarian function protection. All patients had a good
performance status, with a median age of 36 years (range 26–40).
Treatment compliance was satisfactory. All patients completed
chemotherapy and treatment with an LH-RH analogue after a median
follow-up of 110 months.
 | Table I.Patients and tumor
characteristics. |
Table I.
Patients and tumor
characteristics.
| Characteristics | No. | % |
|---|
| No. of patients | 63 | 100 |
| Age (years) | | |
| Median | 36 | |
| Range | 26–40 | |
| Hormone receptor
status | | |
| ER+ | 41 | 65 |
| ER− | 22 | 35 |
| Tumor histology | | |
| Ductal
infiltrating | 51 | 81 |
| Lobular
infiltrating | 5 | 8 |
| Medullary | 5 | 8 |
| Other | 2 | 3 |
| Grading | | |
| G2 | 25 | 40 |
| G3 | 38 | 60 |
| Clinical stage | | |
| IIA | 43 | 68 |
| IIB | 7 | 11 |
| IIIA | 1 | 2 |
| IIIB | 4 | 6 |
| IIIC | 8 | 13 |
| Nodes | | |
| 0 | 15 | 24 |
| 1–3 | 33 | 52 |
| 4–9 | 7 | 11 |
| >10 | 8 | 13 |
| Type of primary
surgery | | |
| Mastectomy | 19 | 30 |
|
Quadrantectomy | 44 | 70 |
Biomarker results
Serial assessment of plasma biomarkers was available
for 44 patients at baseline, for 40 patients after 1 year and for
38 patients after 5 and 6 years. After 1 year, there was a 67%
relative decrease in serum VEGF levels (95% CI 63–71; P<0.0001)
and a 56% relative decrease in Fox-P3 T-regs (95% CI 46–66;
P<0.0001) from baseline. The measurements performed yearly
showed a progressive decrease in VEGF and Fox-P3 values (Table II). One year after the
discontinuation of LH-RH analogues, there was a 59% decrease in
VEGF (95% CI 53–65), with respect to the 5th year value and a 36%
increase in T-regs (95% CI 27–46) with respect to the values
obtained at the end of the 5th year. In addition, changes in VEGF
could not be attributed to chemotherapy-induced thrombocytopenia
and successive rebound increase in platelet count (27). In fact, VEGF was measured at
baseline, before chemotherapy and 1 year after, when chemotherapy
was completed.
 | Table II.Vascular endothelial growth factor
(VEGF) and T-regulatory cell (T-reg) measurement at baseline and at
1, 5 and 6 years. |
Table II.
Vascular endothelial growth factor
(VEGF) and T-regulatory cell (T-reg) measurement at baseline and at
1, 5 and 6 years.
| VEGF (pg/mla) | T-regs
(mm3 ± SD) |
|---|
| Baseline | 533.7±258 | 101±20 |
| 1 year | 359.6±174 | 58±13 |
| 5 years | 272.6±146 | 33±21 |
| 6 years | 216.6±92 | 90±11 |
Fertility outcome
One year after the last dose of the LH-RH analogue,
91% of the patients exhibited normal gender steroid hormones and
menses, including 5 women treated with high-dose chemotherapy and
PBPC transplantation. One of the patients that had been treated
with high-dose chemotherapy and PBPC transplantation became
pregnant and had a voluntary abortion. Two patients completed a
normal pregnancy, which resulted in the birth of 3 healthy children
at term, 5 years after the completion of chemotherapy and
radiotherapy.
Outcome: Disease-free survival and
overall survival
At data cut-off for this analysis, February 2010,
median follow-up for living patients was 110 months (range 60–120).
The 10-year PFS was 86.1%, with 8 patients exhibiting disease
progression (12.7%, 95% CI 6–23) and 55 patients being disease
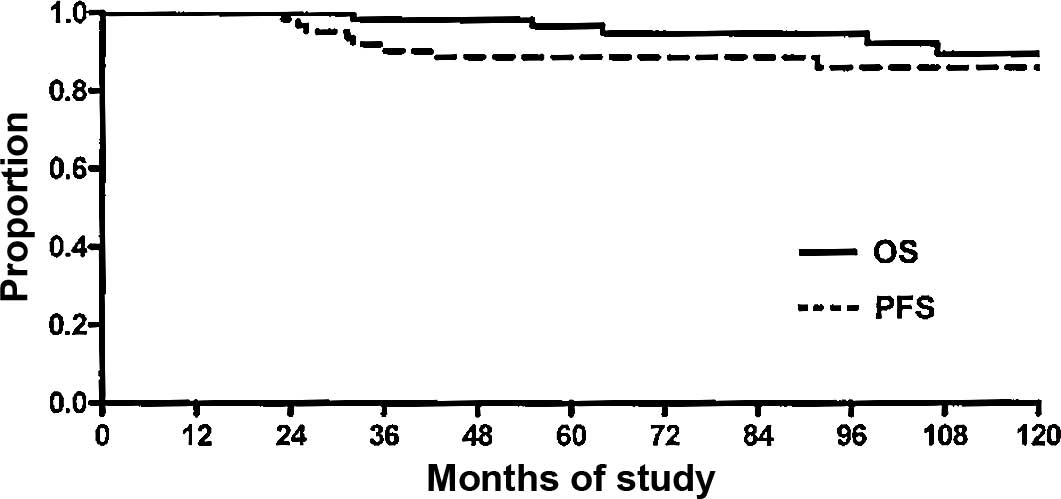
progression-free (87.3%, 95% CI 76–94) (Fig. 1). The 10-year OS rate was 89.7%,
with 5 death events (7.9%, 95% CI 3–17) and 58 censored patients
(92%, 95% CI 82–97) (Fig. 1).
The effect of this therapy was explored in subgroups
defined by overall hormone receptor status. As expected by this
total estrogen blockade, there was a statistically significant
difference (P<0.05) in DFS, favoring the 41 patients with
ER+ with respect to the 22 patients with ER-. OS did not
show any statistically significant difference between the two
groups (P=0.9).
Toxicity
Adverse events reported during the chemotherapeutic
treatment are shown in Table III.
Forty patients (63%) complained of hot flushes. No unexpected
toxicity occurred during the administration of anthracyclne and
taxane-based chemotherapy, and all 63 patients completed the
scheduled treatment. Hematological toxicity occurred in all paients
treated with high-dose chemotherapy with or without PBPC
transplantation. Three of these patients, with a platelet count
<20×104/mm3 required a platelet
transfusion (median 2 units). Diarrhea occurred in 15 patients
(24%), while 16 patients (25%) reported nausea and vomiting. An
infection was reported in 3 patients (17%). Grade 3 alopecia was
universal. No significant reduction in the left ventricular
ejection fraction was observed in any patient. Anemia, which was
infrequent due to the use of erythropoietin, occurred in 17
patients (27%). Mucositis occurred in 22 patients (35%). Bone pain
with median duration of 2 days was reported by 4 patients after
PBPC transplantation. There were no chemotherapy-related
deaths.
 | Table III.Toxicity. |
Table III.
Toxicity.
| Type of therapy
|
|---|
| LH-RH analogue (63
patients)a | Anthracycline-based
CT (63 patients)a | HD-CT (18
patients)a |
|---|
| Hematologic | | | |
| Leukopenia | 0 | 40 (63) | 18 (100) |
|
Thrombocytopenia | 0 | 18 (29) | 18 (100) |
| Anemia | 0 | 17 (27) | 7 (39) |
|
Gastrointestinal | | | |
|
Nausea-vomiting | 0 | 16 (25) | 6 (33) |
| Diarrhea | 0 | 15 (24) | 2 (11) |
| Mucositis | 0 | 22 (35) | 7 (39) |
| Infection | 0 | 9 (14) | 3 (17) |
| Neurotoxicity | 0 | 2 (3) | 2 (11) |
| Alopecia | 0 | 63 (100) | 18 (100) |
| Hot flushes | 40 (63) | 0 | 0 |
Discussion
No consensus exists regarding the causes and
treatment of the aggressive behavior of breast cancer in patients
younger than 40 years of age. In these patients, the role of
chemotherapy, tamoxifen and LH-RH analogues, alone or in various
combinations, has been the subject of intense investigation. A
meta-analysis of randomized adjuvant trials of LH-RH analogues as
adjuvant treatment in pre-menopausal patients with hormone
receptor-positive breast cancer, combined with chemotherapy and
tamoxifen, demonstrated a significant 32.3% reduction in the odds
of recurrence in patients 40 years of age or less (13). To the best of our knowledge, no
study has been conducted using the three modalities used in our
study in the following sequence: ovarian suppression for 5 years,
tailored chemotherapy and hormonal therapy with an aromatase
inhibitor. After surgery, all of the patients received the LH-RH
analogue with the aim of ‘putting to sleep’ the ovaries and thus
protecting rapidly proliferating oocytes from the damage of
chemotherapy. The objective of the ovarian protection was partially
achieved; only 9% (95% CI 3–19) of the patients had
chemotherapy-related amenorrhea (CRA). These findings were
favorable when compared to data reported from the literature that
described a 40% CRA (95% CI 36–44) for patients less than 40 years
of age treated with chemotherapy (28).
We hypothesized that immunologic-mediated
mechanisms, the decrease in VEGF and T-regs, could explain the
improved clinical outcome observed in our patients. Both of these
factors play a fundamental role in reproduction and are mediated by
estrogens, which also regulate the expression of the oncogenic
signaling pathways that characterize premenopausal breast
cancer.
Estrogens mediate several reproductive functions
through the secretion of VEGF (29); inducing neoangyogenesis at the
implantation site, VEGF creates an environment necessary for the
embryo survival (30). In the
regulation of normal menstruation, inhibition of VEGF at the time
of follicle recruitment or selection prevents endothelial cell
proliferation, leading to the inhibition of follicular development
(29).
Apart from physiological conditions, VEGF also has a
fundamental role in the development of breast cancer. VEGF is a
target gene for the ER and contributes to breast cancer progression
(31). Estrogen induction of free
extracellular VEGF may be one mechanism involved in gender
steroid-dependent breast carcinogenesis; tumor development may be
facilitated by a deregulation of angiogenic factors (32). Estradiol modulates VEGF expression
in breast cancer cells, involving transcriptional activation
through the ER (33). Other in
vivo findings showed that overexpression of VEGF significantly
increased tumor growth and angiogenesis in a murine model of breast
cancer (31). In addition,
patients with early-stage breast cancer who have tumors with
elevated levels of VEGF have a higher likelihood of recurrence or
death compared to patients with low-angiogenic tumors, even when
treated with conventional adjuvant therapy (34).
Another function of estrogens, directly related to
reproduction, is the increase in T-regs in order to allow tolerance
of the eterologous embryo. In fact, estradiol modulates the
function of human T-reg cells by regulating their numbers (35). Previous studies in mouse models
showed that T-regs are essential for maternal tolerance of the
conceptus and that the expansion of the T-reg cell pool through
antigen-specific and antigen non-specific pathways allows their
suppressive actions to be exerted in the critical peri-implantation
phase of pregnancy (30). It has
been observed that estradiol, at physiological doses, not only
expanded T-reg cells in different tissues, but also increased the
expression of the Foxp3 gene, a hallmark for
CD4+CD25+ T-reg cell function, and the IL-10
gene as well (36). In fertile
non-pregnant women, an expansion in T-regs is tightly correlated
with serum levels of estradiol, and reproductive failure may result
from the inability of T-regs to expand during the pre-implantatory
phase (37).
The suppressive effect of T-reg cells facilitates
breast cancer development; in fact, T-regs prevent effector T-cell
activation, facilitating immune escape and, ultimately, tumor
progression (38).
One study suggested that T-regs are reversely
correlated with the survival of patients with breast cancer; a new
subset of tumor-resident T-regs, CCR6(+) T-regs, may be dominantly
responsible for the immunosuppression in tumor immunity and a
potential predictor of poor prognosis in breast cancer (9).
All of these estrogen-mediated functions correspond
to patterns exhibited by breast cancer for its progression. In the
present study, patients treated with the LH-RH analogue exhibited
an early decrease in VEGF and T-regs. This decrease was stable over
time, and the values of both VEGF and T-regs were maintained at low
values over a 5-year period. After the discontinuation of the LH-RH
analogue a normal ovarian function was observed in 91% of the
patients who enjoyed a normal sexual and reproductive life. Our
9-year DFS and OS for patients with ER+ and positive
lymph nodes were both 92%. Our data compared favorably to data from
the literature that report a PFS and OS of 64 and 69%,
respectively, in the same category of patients (39).
In conclusion, the administration of an LH-RH
analogue for 5 years, followed by tailored chemotherapy and an
aromatase inhibitor for ER+ patients seems to be
feasible, well tolerated and appears to improve the expected
outcome of patients less than 40 years of age with high-risk early
breast cancer.
References
|
1.
|
Varga D, Koenig J, Kuhr K, et al:
Comparison of early onset breast cancer patients to older
premenopausal breast cancer patients. Arch Gynecol Obstet.
Jan;2010.(E-pub ahead of print).
|
|
2.
|
El Saghir NS, Seoud M, Khalil MK, et al:
Effects of young age at presentation on survival in breast cancer.
BMC Cancer. 6:1942006.PubMed/NCBI
|
|
3.
|
Anders CK, Hsu DS, Broadwater G, et al:
Young age at diagnosis correlates with worse prognosis and defines
a subset of breast cancers with shared patterns of gene expression.
J Clin Oncol. 26:3324–3330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bollet MA, Sigal-Zafrani B, Mazeau V, et
al: Age remains the first prognostic factor for loco-regional
breast cancer recurrence in young (<40 years) women treated with
breast conserving surgery first. Radiother Oncol. 82:272–280.
2007.PubMed/NCBI
|
|
5.
|
Wendy Y and Chen MD: MPH. Exogenous and
endogenous hormones and breast cancer. Best Pract Res Clin
Endocrinol Metab. 22:573–585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Buteau-Lozano H, Ancelin M, Lardeux B,
Milanini J and Perrot-Applanat M: Transcriptional regulation of
vascular endothelial growth factor by estradiol and tamoxifen in
breast cancer cells: a complex interplay between estrogen receptors
alpha and beta. Cancer Res. 62:4977–4984. 2002.
|
|
7.
|
Tai P, Wang J, Jin H, et al: Induction of
regulatory T cells by physiological level estrogen. J Cell Physiol.
214:456–464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Linderholm BK, Gruvberger-Saal S, Fernö M,
Bendahl PO and Malmström P: Vascular endothelial growth factor is a
strong predictor of early distant recurrences in a prospective
study of premenopausal women with lymph-node negative breast
cancer. Breast. 7:484–491. 2008. View Article : Google Scholar
|
|
9.
|
Xu L, Xu W, Qiu S and Xiong S: Enrichment
of CCR6(+) Foxp3(+) regulatory T cells in the tumor mass correlates
with impaired CD8(+) T cell function and poor prognosis of breast
cancer. Clin Immunol. 135:466–475. 2010.
|
|
10.
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomized trials. Lancet. 365:1687–1717.
2005.PubMed/NCBI
|
|
11.
|
Parulekar WR, Day AG, Ottaway JA, et al:
National Cancer Institute of Canada Clinical Trials Group.
Incidence and prognostic impact of amenorrhea during adjuvant
therapy in high-risk premenopausal breast cancer: analysis of a
National Cancer Institute of Canada Clinical Trials Group Study –
NCIC CTG MA5. J Clin Oncol. 23:6002–6008. 2005.PubMed/NCBI
|
|
12.
|
Goel S, Sharma R, Hamilton A and Beith J:
LH-RH agonists for adjuvant therapy of early breast cancer in
premenopausal women. Cochrane Database Syst Rev.
4:CD0045622009.
|
|
13.
|
LH-RH-agonists in Early Breast Cancer
Overview Group; Cuzick J, Ambroisine L, Davidson N, et al: Use of
luteinising-hormone-releasing hormone agonists as adjuvant
treatment in premenopausal patients with hormone-receptor-positive
breast cancer: a meta-analysis of individual patient data from
randomised adjuvant trials. Lancet. 369:1711–1723. 2007. View Article : Google Scholar
|
|
14.
|
Howell A, Cuzick J, Baum M, et al: Results
of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial
after completion of 5 years' adjuvant treatment for breast cancer.
Lancet. 365:60–62. 2005.
|
|
15.
|
Coombes RC, Hall E, Gibson LJ, et al: A
randomized trial of exemestane after two or three years of
tamoxifen therapy in postmenopausal women with primary breast
cancer. N Engl J Med. 350:1081–1092. 2004.PubMed/NCBI
|
|
16.
|
Thürlimann B, Keshaviah A, Coates AS, et
al: A comparison of letrozole and tamoxifen in postmenopausal women
with early breast cancer. N Engl J Med. 353:2747–2757.
2005.PubMed/NCBI
|
|
17.
|
Goss PE, Ingle JN, Martino S, et al:
Randomized trial of letrozole following tamoxifen as extended
adjuvant therapy in receptor-positive breast cancer: updated
findings from NCIC CTG MA. 17. J Natl Cancer Inst. 97:1262–1271.
2005. View Article : Google Scholar
|
|
18.
|
Rossi E, Morabito A, Di Rella F, et al:
Endocrine effects of adjuvant letrozole compared with tamoxifen in
hormone-responsive postmenopausal patients with early breast
cancer: the HOBOE trial. Clin Oncol. 27:3192–3197. 2009. View Article : Google Scholar
|
|
19.
|
Recchia F, Sica G, De Filippis S, Rosselli
M and Rea S: Goserelin as ovarian protection in the adjuvant
treatment of premenopausal breast cancer. A phase II pilot study.
Anticancer Drugs. 13:417–424. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Recchia F, Saggio G, Amiconi G, et al:
Gonadotropin-releasing hormone analogues added to adjuvant
chemotherapy protect ovarian function and improve clinical outcomes
in young women with early breast carcinoma. Cancer. 106:514–523.
2006. View Article : Google Scholar
|
|
21.
|
Recchia F, Saggio G, Cesta A, et al: Phase
II randomized study of interleukin-2 with or without 13-cis
retinoic acid as maintenance therapy in patients with advanced
cancer responsive to chemotherapy. Anticancer Res. 25:3149–3157.
2005.PubMed/NCBI
|
|
22.
|
Recchia F, Accorsi P, Bonfini T, et al:
Randomized trial of sequential administration of G-CSF and GM-CSF
vs. G-CSF alone following peripheral blood progenitor cell
autograft in solid tumors. J Interferon Cytokine Res. 20:171–177.
2000. View Article : Google Scholar
|
|
23.
|
Recchia F, De Fillipis S, Piccinini M and
Rea S: High-dose carboplatin, cyclophosphamide, etoposide with
hematological growth factors, without stem cell support in patients
with advanced cancer. Anticancer Res. 23:4141–4147. 2003.PubMed/NCBI
|
|
24.
|
Simon R: Optimal two-stage designs for
phase II clinical trials. Control Clin Trials. 10:1–10. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
26.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors: European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Verheul HM, Hoekman K, Luykx-de Bakker S,
et al: Platelet: transporter of vascular endothelial growth factor.
Clin Cancer Res. 3:2187–2190. 1997.PubMed/NCBI
|
|
28.
|
Bines J, Oleske DM and Cobleigh MA:
Ovarian function in premenopausal women treated with adjuvant
chemotherapy for breast cancer. J Clin Oncol. 5:1718–1729.
1996.PubMed/NCBI
|
|
29.
|
Fraser HM and Duncan WC: SRB Reproduction,
Fertility and Development Award Lecture 2008. Regulation and
manipulation of angiogenesis in the ovary and endometrium. Reprod
Fertil Dev. 21:377–392. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Krüssel J, Behr B, Hirchenhain J, et al:
Expression of vascular endothelial growth factor mRNA in human
preimplantation embryos derived from tripronuclear zygotes. Fertil
Steril. 74:1220–1226. 2000.PubMed/NCBI
|
|
31.
|
Applanat MP, Buteau-Lozano H, Herve MA and
Corpet A: Vascular endothelial growth factor is a target gene for
estrogen receptor and contributes to breast cancer progression. Adv
Exp Med Biol. 617:437–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Dabrosin C: Positive correlation between
estradiol and vascular endothelial growth factor but not fibroblast
growth factor-2 in normal human breast tissue in vivo. Clin Cancer
Res. 11:8036–8041. 2005. View Article : Google Scholar
|
|
33.
|
Buteau-Lozano H, Ancelin M, Lardeux B,
Milanini J and Perrot-Applanat M: Transcriptional regulation of
vascular endothelial growth factor by estradiol and tamoxifen in
breast cancer cells: a complex interplay between estrogen receptors
alpha and beta. Cancer Res. 62:4977–4984. 2002.
|
|
34.
|
Gasparini G: Prognostic value of vascular
endothelial growth factor in breast cancer. Oncologist. 5(Suppl 1):
37–44. 2000. View Article : Google Scholar
|
|
35.
|
Prieto GA and Rosenstein Y: Oestradiol
potentiates the suppressive function of human CD4 CD25 regulatory T
cells by promoting their proliferation. Immunology. 118:58–65.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Tai P, Wang J, Jin H, et al: Induction of
regulatory T cells by physiological level estrogen. J Cell Physiol.
214:456–464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Arruvito L, Sanz M, Banham AH and Fainboim
L: Expansion of CD4+CD25+ and FOXP3+
regulatory T cells during the follicular phase of the menstrual
cycle: implications for human reproduction. J Immunol.
178:2572–2578. 2007.PubMed/NCBI
|
|
38.
|
Gobert M, Treilleux I, Bendriss-Vermare N,
et al: Regulatory T cells recruited through CCL22/CCR4 are
selectively activated in lymphoid infiltrates surrounding primary
breast tumors and lead to an adverse clinical outcome. Cancer Res.
69:2000–2009. 2009. View Article : Google Scholar
|
|
39.
|
Davidson NE, O'Neill AM, Vukov AM, et al:
Chemoendocrine therapy for premenopausal women with axillary lymph
node-positive, steroid hormone receptor-positive breast cancer:
results from INT 0101 (E5188). J Clin Oncol. 23:5973–5882. 2005.
View Article : Google Scholar
|