Contents
Introduction
Active species for sterilization by gas plasma
Current uses of plasma for decontamination
Speculated mechanism of the action of gas plasma
Sporicidal test and the importance of spore
clumping
Perspectives for the future
Introduction
Plasma is defined as an ionized (or energized) gas
with an equal number of positively and negatively charged
particles. Plasma is often regarded as the ‘fourth state of matter’
(the other three being solids, liquids and gases) because, while
plasma is neither a gas nor a liquid, its properties are similar to
those of both gases and liquids. Plasma can be categorized as
either high-temperature- or low-temperature-based. A good example
of a naturally occurring high-temperature plasma is lightning. This
type of plasma can be artificially generated using a high-voltage
high-temperature arc, which is the basis for the corona discharge
process and for plasma torches used to vaporize and redeposit
metals. Low-temperature plasmas, used in surface modification,
cleaning, decontamination and sterilization applications, are
ionized gases generated under deep vacuum (low-pressure)
conditions. These types of plasmas operate within a vacuum chamber
in which atmospheric gases have been introduced into the chamber
typically evacuated below 0.1 Torr. Such low pressures allow for a
relatively long free path of accelerated electrons and ions within
the chamber. Since these ions and neutral particles are at or near
ambient temperatures and the long free path of electrons at a high
temperature or at electron volt levels have relatively few
collisions with molecules at this pressure, the overall exposure
conditions remain at a low temperature.
For some applications, deep vacuum pressure
sterilization cannot be used, and atmospheric plasmas have been
proposed. The lifetime of the reactive plasma species under
atmospheric conditions is much shorter than that of low-pressure
plasma. As a result, most of the plasma active species, with the
exception of the metastables (long-life electronically excited
atoms), lose their reactivity dramatically in a remote position.
According to one report, atmospheric or one third atmospheric
pulsed plasma equipment may be more desirable (1).
It is important to note that ‘true’ plasma
decontamination processes have not been widely used to date. A
variety of hydrogen peroxide gas-based processes that use plasma as
part of the sterilization process are instead commonly regarded as
‘plasma sterilization’. However, it is now known that the
mechanisms of antimicrobial action in these processes are primarily
due to hydrogen peroxide gas (or condensed gas) and not plasma,
although the plasma generation phase of these processes is known to
assist in the breakdown of residual peroxide in the chamber load
(2).
There are various reports regarding the potential
use of plasmas for sterilization applications, in particular the
demonstration of broad spectrum antimicrobial (particularly
sporidical) activity and the kinetics of this activity over time.
This is an important requirement for the demonstration of any
sterilization process as described in international standards
(3). Several types of source gases
and production procedures of gas plasma have been reported and
discussed (4,5). As a focus in this review article, we
consider a number of variables that may have been overlooked and
misunderstood in previous studies. One is the tailing phenomenon
observed in microbial inactivation studies. This is an important
variable in the design of a typical overkill sterilization process,
and may be due to a number of factors, including artifacts in the
test method, such as spore clumping in a biological indicator (BI).
Spore clumping may have the same effects as organic matter in
interfering with the sterilization efficiency of gas plasma under
such investigations. The tailing phenomenon commonly reported for
plasma sterilization investigations has been further investigated
and clarified using clumping-free BIs. A further consideration is
the use of so-called ‘inert gas plasma sterilization’ and
differences in its mechanisms of action. The use of inert gases is
quite desirable, since they are believed to attain the required
overkill in the sterilization process [such as a sterility
assurance level (SAL) of at least 10−6] in parallel with
material and functional compatibility. This is an important balance
to achieve for sterilization validation in medical and
manufacturing applications. Inert gas plasma applications are the
focus of further investigation, patenting and discussion
worldwide.
Active species for sterilization by gas
plasma
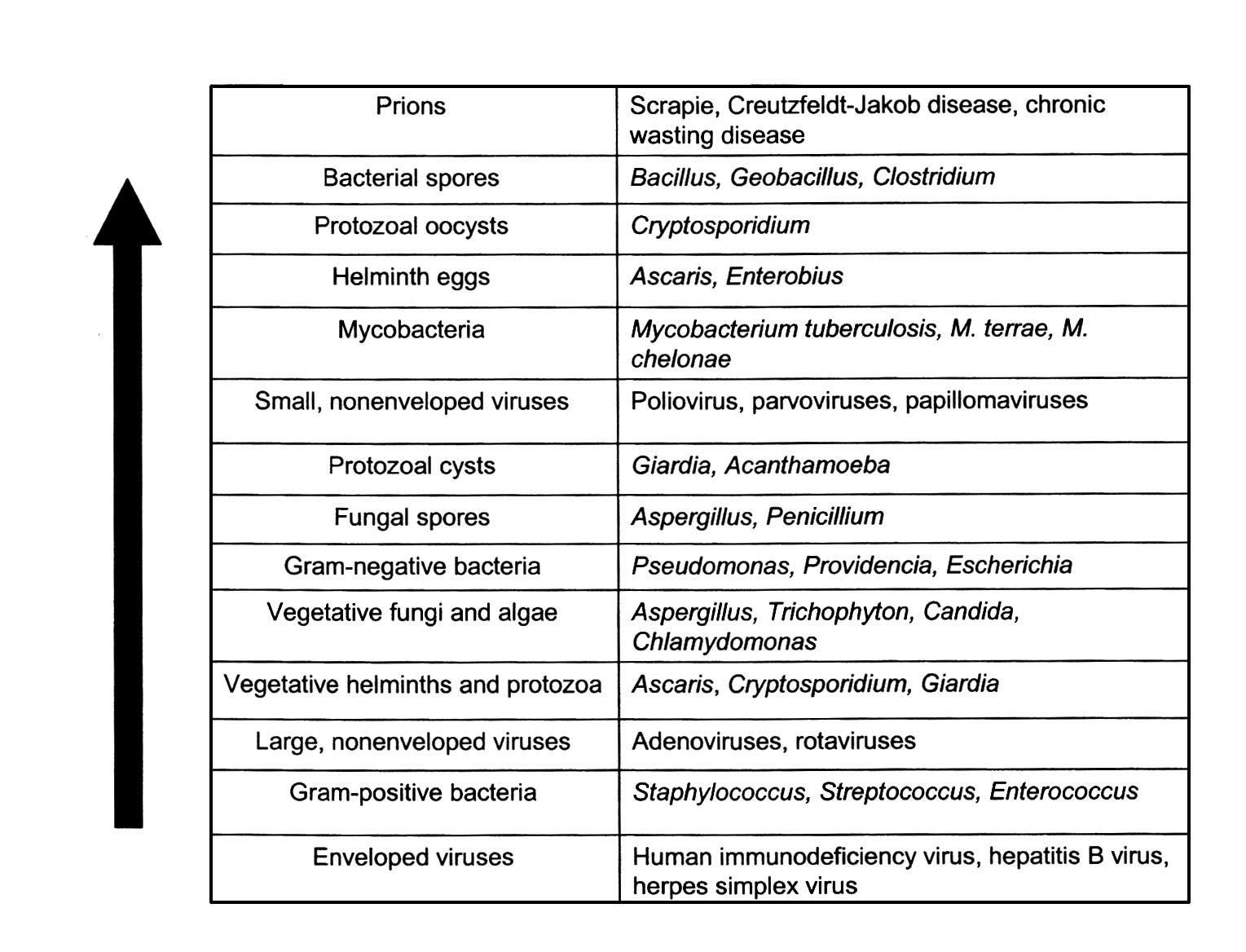
Several types of active species are considered to
contribute to the antimicrobial effects of gas plasma. They include
various types of ions, electrons, radicals, UV light, vacuum UV
(VUV), electric fields and metastables (Figs. 1 and 2) (http://www.astp.com/plasma-equipment/applications). It
is important to note that these active species may differ depending
on the types of gas used for plasma formation and the methods used
to generate the plasma. These are further discussed below, based on
the various types of gas commonly used.
Oxygen
The most popular gas investigated to date for plasma
sterilization is oxygen (O2). O2 produces O,
OH, OOH and a mixture of other radicals that contribute to the
sterilization effects (6). Among
these, the OH radical is often considered the most efficient for
microbial inactivation, despite its extremely short lifetime
(6) (Fig. 3).
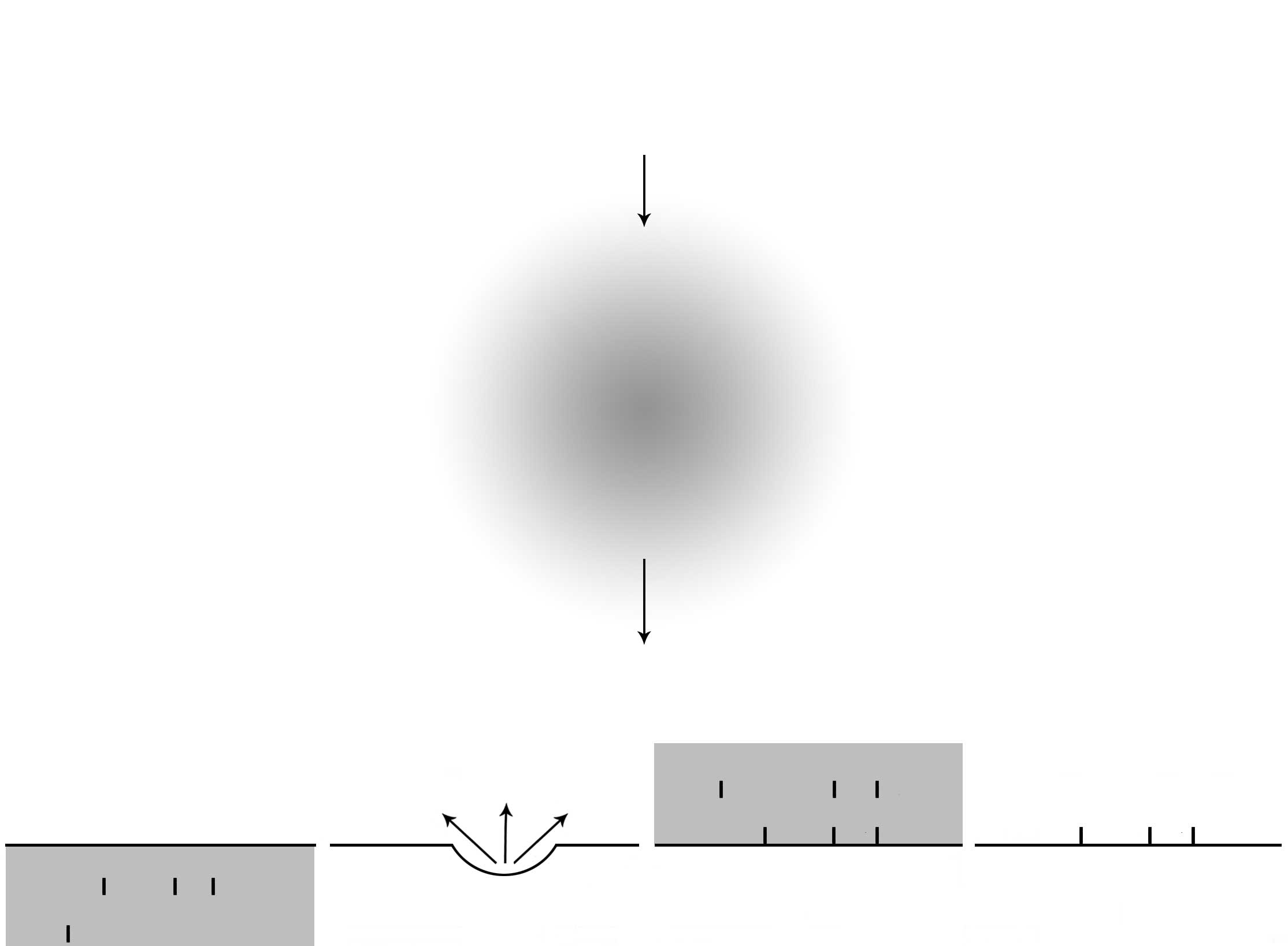
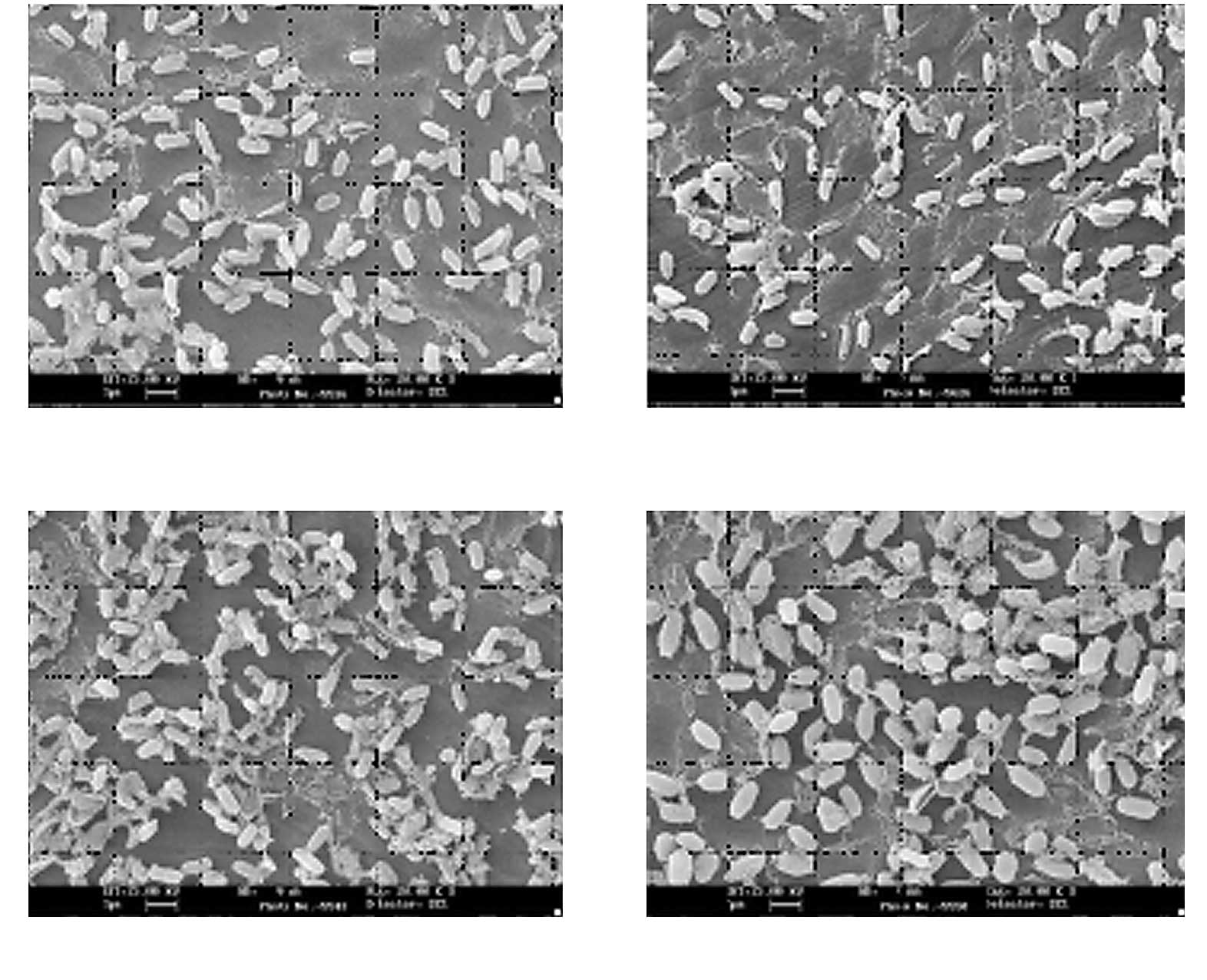
The mechanism of various O2 radicals is
believed to be due to etching effects (Fig. 2). Based on direct visualization of
exposed bacterial endospores, the greater the amount of
O2 plasma exposure, the more shrinkage of spores is
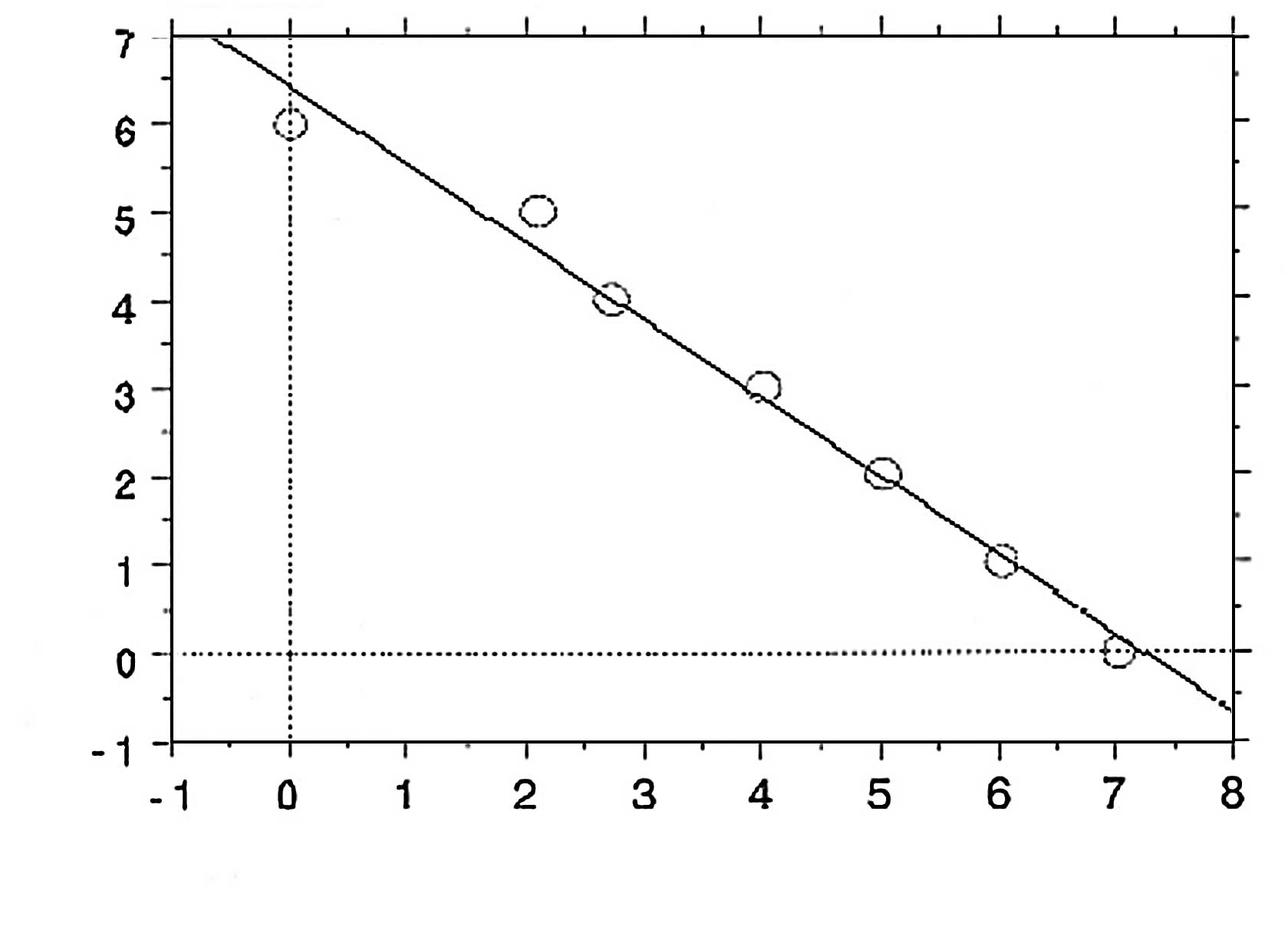
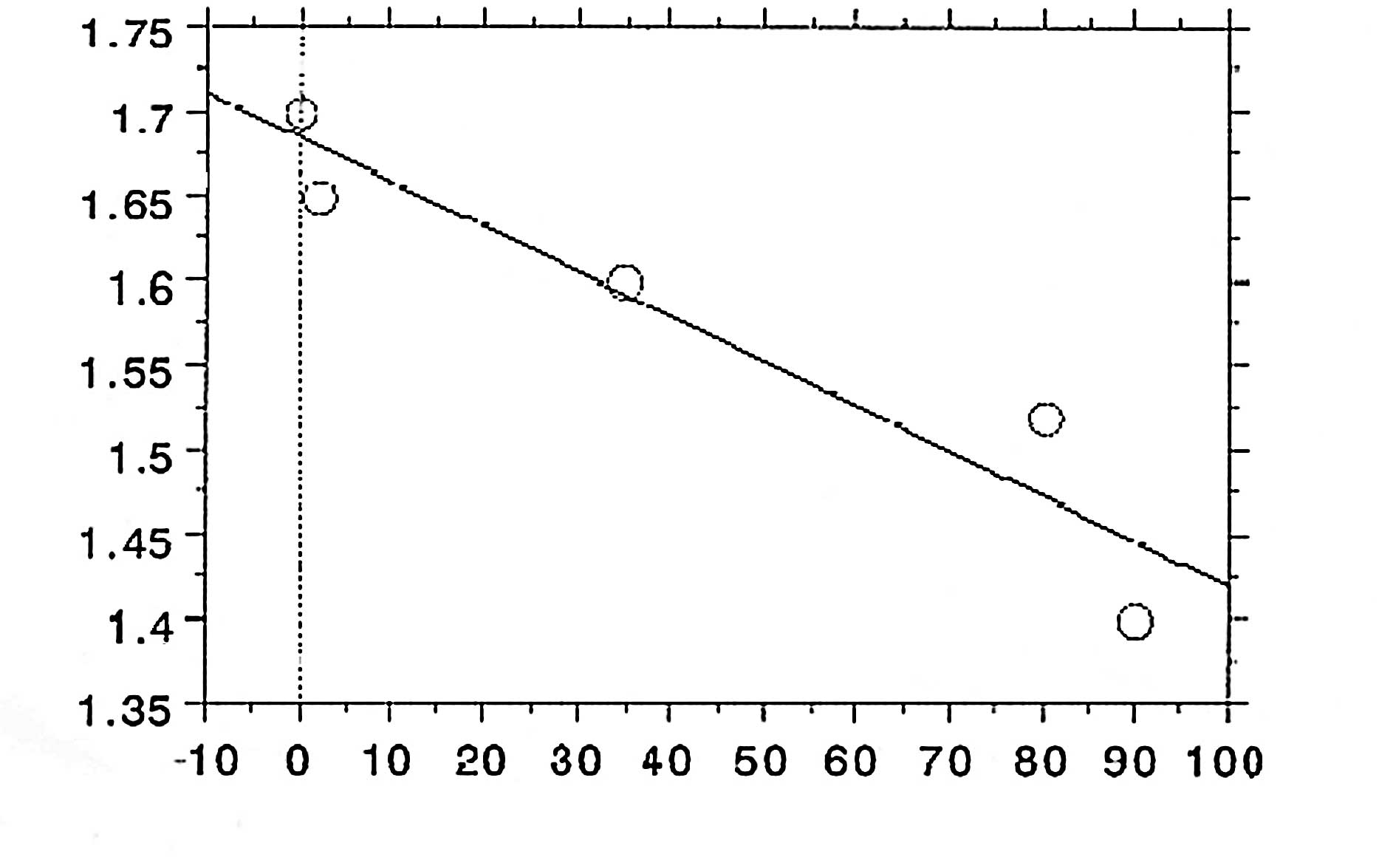
observed (Fig. 4) (5). There is a significant relationship
between the degree of shrinkage and spore death (Fig. 5) (7); the coefficient relationship of the
regression line as shown in Fig. 5
is 0.949. By contrast, N2 plasma does not present any
shrinkage (7,8). The difference in the mechanisms of
O2 and N2 gases has yet to be clarified, and
should be seriously considered from an antimicrobial and
compatibility point of view.
Nitrogen
As demonstrated by Rossi et al (7,8),
upon N2 gas plasma generation the active species had no
or insufficient ability to shrink bacterial endospores, suggesting
a different mechanism of action than that at play with oxygen.
There are currently no specific theories as to why this is the
case, but it may be due to the difficulty of ionizing
N2. Chemically, N2 is a molecule with a
triple bond, so the dissociation energy is high – approximately
9.91 eV (1) – suggesting it is
resistant to ionization (Table I).
From this, it can be speculated that N+ or N•
ions are hard to practically attain in comparison to oxygen.
However, it is not known which active species are present to cause
the sterilization effects observed with N2 gas plasmas.
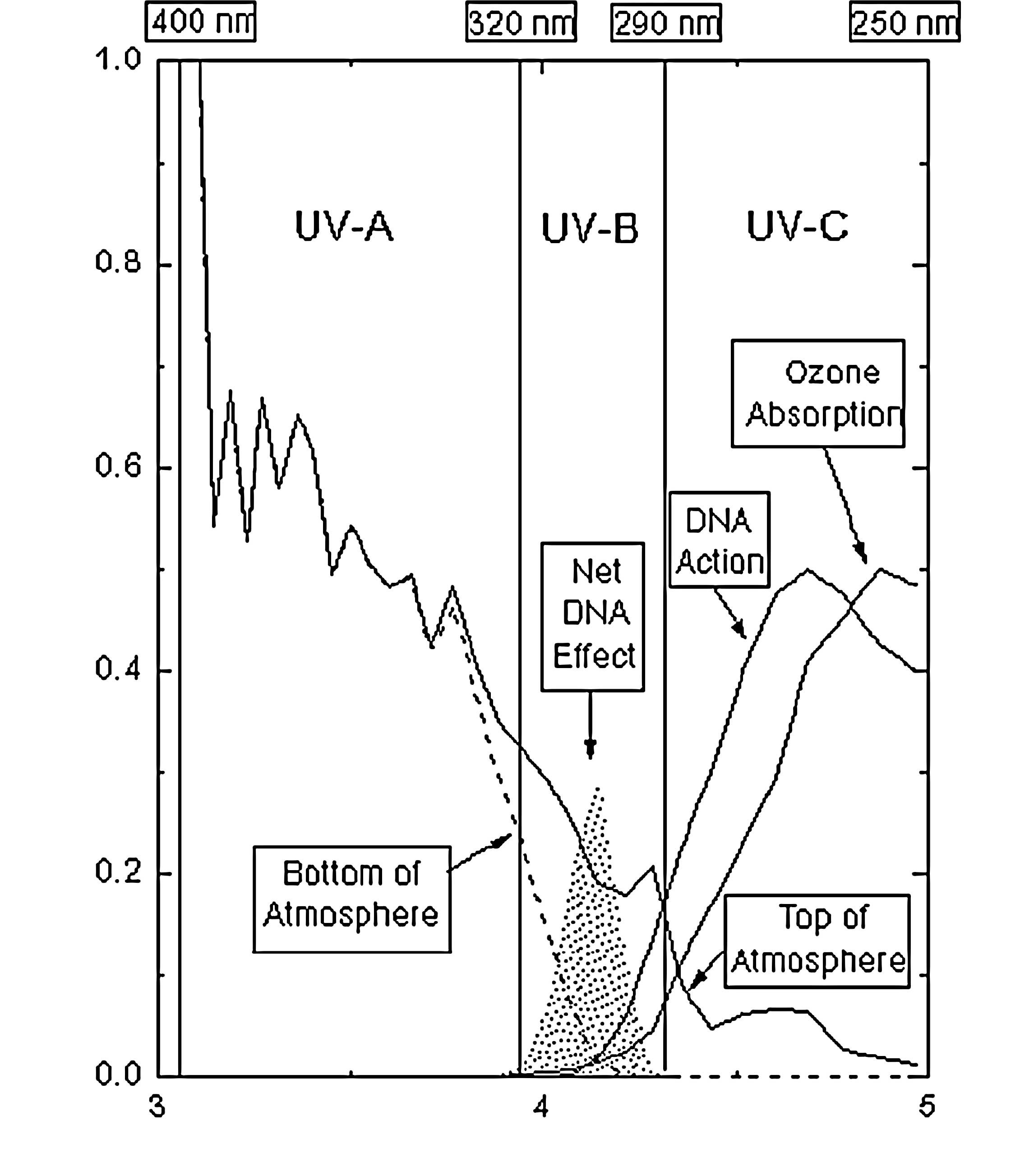
UV-C (254 nm) (Fig. 6) (http://www.phys.ksu.edu/gene/f_5.html)
may be present, but in amounts that are considered to be low
(1); VUV (<200 nm) may also be
present, but this remains to be definitively confirmed.
 | Table I.Dissociation energy of several sorts
of gases. |
Table I.
Dissociation energy of several sorts
of gases.
| Gas | Dissociation energy
(eV) |
|---|
| N2 | 9.91 |
| O2 | 5.21 |
| H2O | 5.11 |
| NO | 6.50 |
| SO2 | 5.60 |
| N2O | 4.93 |
| CO2 | 5.52 |
| O3 | 1.05 |
|
H2O2 | 2.21 |
If we consider the effects observed with pulsed
plasma processes (1), the direct
or contributory effects of the pulsed voltage may lead to direct
structural changes in spores, where many cracks are observed on
microscopic analysis (1) (Fig. 7). Overall, whether such physical
factors significantly contribute to these effects is difficult to
assess, and further research is needed to elucidate the true
antimicrobial effects and active species involved in N2
gas plasmas.
Penetration depth of active species in
O2 and N2 gas plasmas
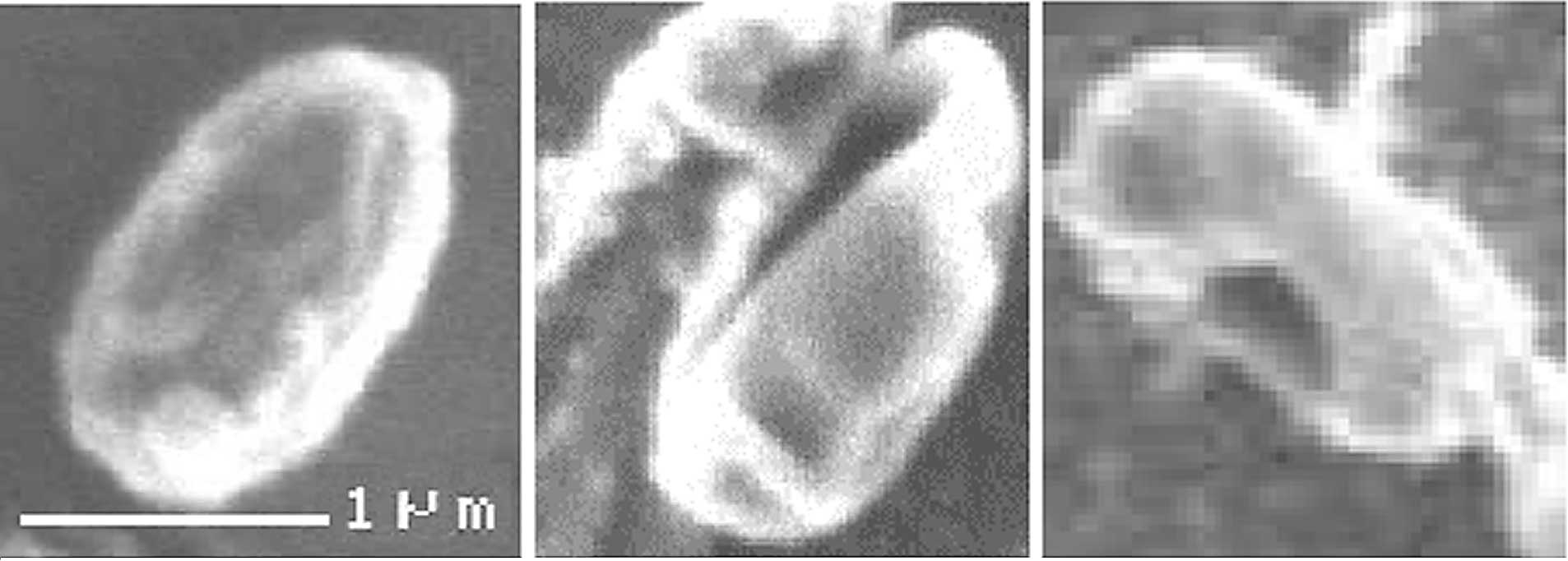
The penetration depth of active species (ions and
radicals; Figs. 1 and 2) in the case of N2 gas
appears to be approximately 10–40 nm from the surface of spores, as
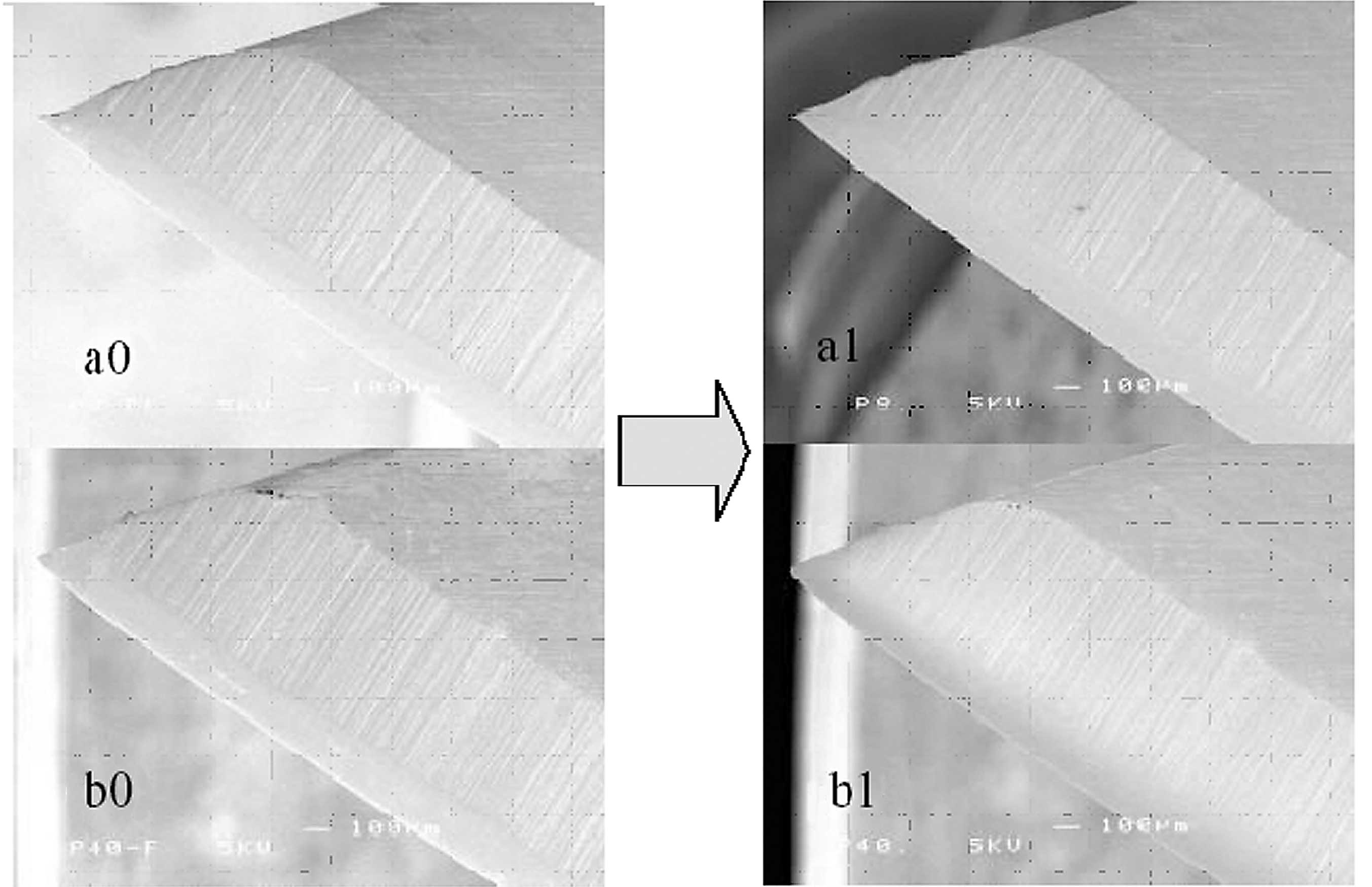
observed by atomic force microscopy (Fig. 8) (1).
Fig. 8 displays the
effect of N2 gas plasma exposure in contrast to
O2 gas plasma exposure, which appears to have a somewhat
deeper penetrating ability and more extensive effects on bacterial
spores (7,8). Since the scale of a typical spore
structure is 1,000 × 3,000 nm, the penetration depth of
O2 gas plasma was predicted to be <1,000 nm. This
suggests that it penetrates ten times deeper than N2 gas
plasma.
Rare or noble gases, such as helium,
neon, argon and xenon
Plasma generated from other rare or noble gases,
such as helium (He), neon (Ne), argon (Ar) and xenon (Xe), has also
been studied (8–13). In a report (8) that presented a case for the use of
Ar, the direct effects of Ar on exposed spores were similar to
those observed with N2 gas plasma. Similar results were
observed regarding He gas plasma, as shown in Figs. 9 and 10 (9)
.
As rare gases are quite difficult to ionize, it is
unlikely that the various ions, electrons, radicals or even UV
contribute significantly as the active species in gas plasma
sterilization exposures (9). In
addition, a direct comparison to UV alone did not show any
sporicidal effects (Fig. 10)
(9).
The effects of VUV should be considered (14–16),
and indeed relevant research of the effects of exposure with VUV
(at a 174-nm emission using an Ekishima UV apparatus from Iwasaki
Electronic Co. Ltd., Japan) is underway (14).
As a whole, during O2 gas plasma
exposure, it is not difficult to propose and define the active
species present and responsible for the observed activity. However,
the active species present in N2 and rare gas plasmas
are more difficult to pinpoint and elucidate. It would appear that
the O2 gas plasmas tested to date may have a significant
disadvantage in terms of the deterioration of materials due to an
etching phenomenon. By contrast, inert gases such as N2
or rare gases inactivate spores by a different mechanism, and are
thus more desirable as they may achieve both the SAL efficacy
requirements and material/functional compatibility required for
validated sterilization processes. These may therefore prove to be
useful alternatives to traditional sterilization methods such as
humidified ethylene oxide gas, γ-ray, electron beam, steam
sterilization and dry heat.
Current uses of plasma for
decontamination
Decontamination is a general term that can be
defined as any physical and/or chemical means to render a surface,
instrument, tissue, liquid, air or indeed any item safe for
handling, use or disposal (17).
One typical example of cleaning by gas plasma is shown in Fig. 11 (1). ‘Safe’ in decontamination refers to
reducing or completely removing/inactivating microorganisms that
could lead to infection or other complications when inadvertently
introduced into an individual. This includes numerous types of
cleaning, disinfection and sterilization applications that are
widely used in diverse medical, dental, agricultural, veterinarian,
industrial and manufacturing practices. Various forms of plasma may
have potential use in these applications. Currently, a variety of
methods are used (17). These
include physical methods, such as heat and radiation sources, as
well as a range of chemicals, including hydrogen peroxide,
glutaraldehyde and alcohols. Plasmas can be considered
physico-chemical as they include a gas that in its own right may
have some antimicrobial effects, but are also a physical source of
energy.
To date, plasma decontamination applications have
been limited to some industrial uses, and have not been widely
adopted. There are currently no true commercially developed
plasma-based decontamination processes, despite many investigations
and patents issued since the 1970s on various types of
disinfection, sterilization and even cleaning applications.
Notwithstanding the considerable research, the only processes that
have been extensively used do not involve ‘true’ plasmas. In such
processes, various types of gaseous oxidants, such as hydrogen
peroxide, peracetic acid and ozone, are used to generate plasmas,
but the plasma form is not used for antimicrobial purposes. In the
case of the widely used hydrogen peroxide gas plasma sterilizers
(STERRAD® systems), the cycle conditions clearly show
that the plasma is only generated following exposure and removal of
the gas (by vacuum) (2) and has
little antimicrobial effect. However, in such processes, the plasma
is important as it aids in the removal of peroxide (gas and liquid)
residuals from the sterilizer load in order to make the contents
safe for handling and use. Similarly, to date products that claim
to use plasma to ‘activate’ water or other liquids and for
decontamination simply use plasma as an alternative energy form to
activate the various chemicals already present in (or added to) the
water. Examples include the addition of NaCl to water, which
activates the water to make active free chlorine or hypochlorous
acid. Indeed, in some investigations the mechanism of action has
been clearly demonstrated to be chlorine rather than any true
direct plasma effects (18).
A particularly active area of research over the last
5–10 years has been the elucidation and optimization of the various
types and applications of true plasma-based antimicrobial
processes. Potential applications have included, as an optimal
cleaning process, combined cleaning-disinfection, terminal
disinfection, and sterilization processes. In addition to
industrial applications, these may have medical uses, such as in
devices, drug-device combinations, tissues and air/water handling
systems.
Once effective processes have been developed to
apply plasma technology safely, effectively and practically, then
commercial success can be achieved (19). Plasma technology has many
advantages, such as broad spectrum antimicrobial activity
(including effects against problematic biomolecules, such as prions
and endotoxins), ease of generation, and environmental safety
profiles in comparison to other thermal- and chemical-based
technologies.
Speculated mechanism of the action of gas
plasma
The mechanisms of action of gas plasma are clearly
an area for further research. It has been suggested that they are
related to various oxidation and reduction effects on the
macromolecules that make up microbial structures (such as proteins,
lipids and nucleic acids) (20).
No studies published to date have directly demonstrated or
clarified the definite mechanisms of gas plasma. Furthermore, as
mentioned in this review, differences in the mechanisms of action
have been reported between oxygen-based plasmas in comparison to
other inert gases, such as N2, He and Ar.
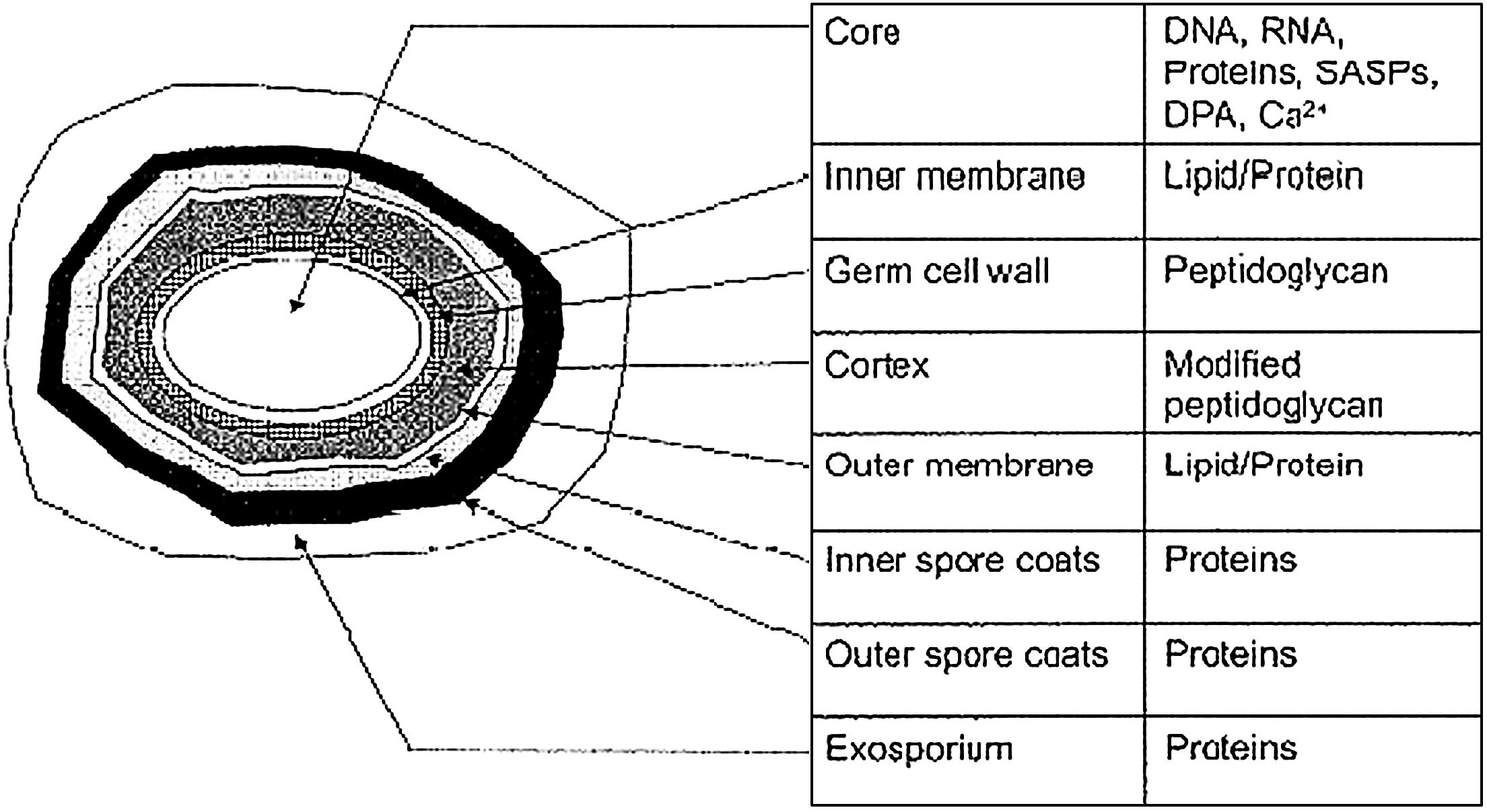
Fig. 12 shows a
representation of the external and internal structure of a
bacterial endospore (20).
Although these structures vary depending on the bacterial species
and growth conditions, it is a useful representation for the
purpose of this discussion. The inner spore membranes are quite
rigid structures, where only molecules <200 daltons are capable
of passing though. These membranes are important as they contain
receptors for various germinants that promote germination and the
outgrowth of the spore. Despite their importance, if the inner
membrane is damaged, dipicolic acid (DPA) leaks out (as a clear
indicator of both structural spore damage and initial germination),
and germination is subsequently not promoted (21,22).
Access to these membranes is limited due to the various spore coats
at the more external part of the spore structure, which require
further damage/penetration in order for these inner membranes to be
accessed. The inner core of the spore itself consists of various
protection mechanisms, including the small acid soluble proteins
that are tightly associated with and protect DNA from
physical/chemical attack. Thus, DNA damage is also an important
factor to consider. Inert gases are inactive, but excited inert gas
(metastable gas) molecules and radicals are expected to be capable
of penetrating into the interior of the spore, without interfering
with charged proteins and lipids surrounding the inner membrane and
core. Excited molecules and radicals may therefore attack and pass
though the inner membrane in order to attack the DNA at the core.
In that sense, cations, anions, electrons, photons or UV-C
(Figs. 1 and 2) are not speculated to be candidates for
attacking the interior of the spore. Whether the inner membrane is
damaged or not can be clarified by observation using a
phase-contrast microscope or scanning electron microscopy (23), and DPA release is determined in the
culture medium of the surrounding area of the spore. Further
experiments will be discussed in detail elsewhere (24).
By contrast, as proposed with other gas plasmas, the
sporicidal effects are predominantly observed at the spore surface,
leading to etching. These effects may initially cause spore surface
damage (by oxidation-reduction reactions), but the affected
proteins and other macromolecules then cross-link each other,
leading to the loss of spore viability. Similar differences in
efficacy have been reported for other sporicidal agents, even
though they may be using the same biocide. For example, in the case
of hydrogen peroxide, the mechanisms of action of hydrogen peroxide
in a true gas form appear to be different from those of liquid or
condensed peroxide (18,19). The gas form appears to primarily
target the peptide bonds of proteins, leading to protein
degradation; by contrast, the liquid form preferably oxidizes the
amino acid side chains of the same proteins, which appears to
result in cross-linking and protein clumping. These effects are
further complicated in the presence of other (inert) chemistries
(as is common when using hydrogen peroxide), and there has also
been a report of other oxidizing agents.
Overall, the true mechanisms of action of most
biocides against bacterial spores are not very well described in
the literature, while an area for future research should include
the study of these mechanisms with various plasmas. Experiments
that investigate the direct effects of plasma on the macromolecules
that make up microbial structures may be an initial and significant
way to enhance the understanding of these effects under controlled
conditions.
Sporicidal testing and the importance of
spore clumping
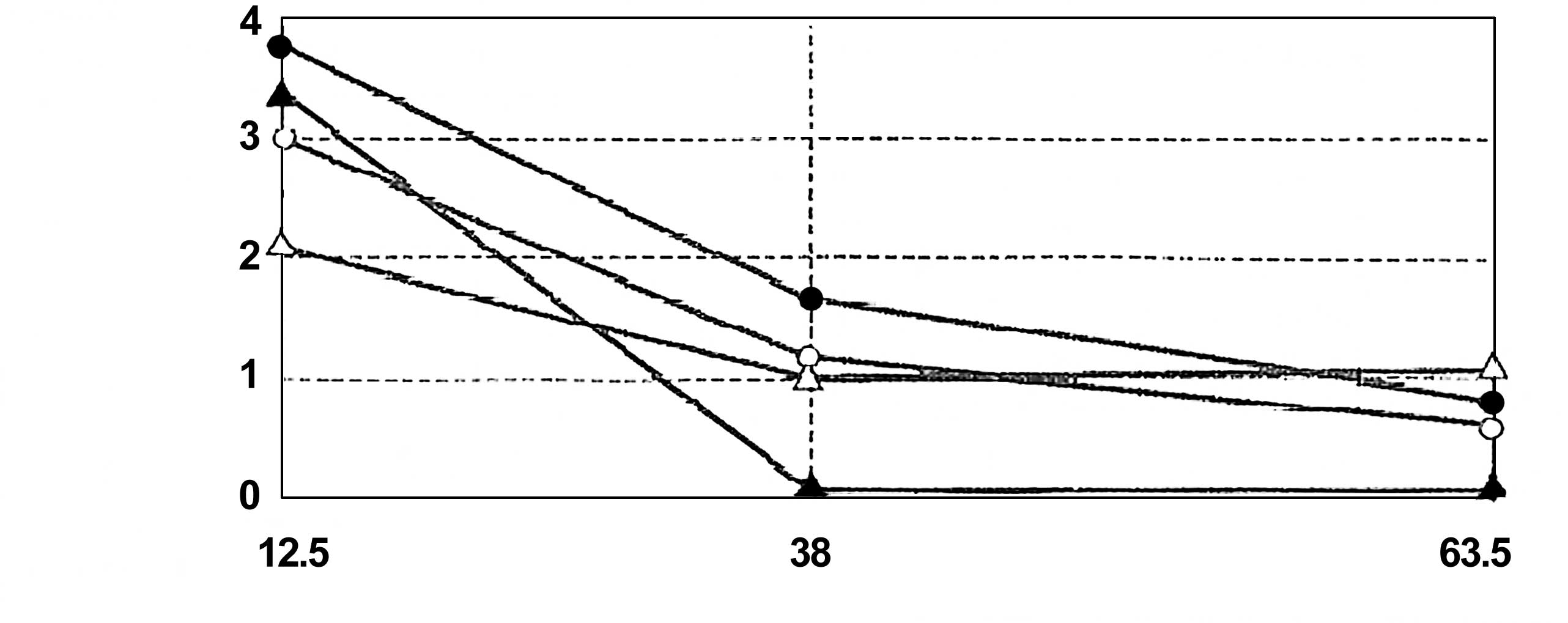
Almost all studies investigating the kinetics of
sporicidal activity with plasmas published to date present the same
tailing phenomenon in survivor curves (9,25).
The structure of such survivor curves is an important consideration
in differentiating a disinfection process from a sterilization
process, and particularly in allowing the extrapolation of
sterility assurance levels. Tailing in plasma survivor curves is
shown by an initial linear relationship between log reduction of
spore populations over time, followed by a non-linear relationship
in the quantal region of the kill curve (Fig. 10). This would prevent the
extrapolation of an SAL of 10−6 at an initial population
of 106 CFU required for an overkill sterilization
process. This can be interpreted as a limitation in the
antimicrobial process under testing and/or an artifact of the test
method. The most common cause of artifacts is clumping of the spore
preparations used for testing (7).
The most recognized resistant organism for steam
sterilization, hydrogen peroxide gas, peroxide gas plasma and
potentially other plasmas (to date) are the endospores of
Geobacillus stearothermophilus ATCC 7953 or 12980. During
sterilization investigations, spore suspensions of G.
stearothermophilus at a population of 106 CFU are
inoculated onto appropriate carrier materials, such as stainless
steel or polystyrene. These are commercially available as BIs
(Figs. 13 and 14). In plasma experiments, BIs from one
manufacturer (A) demonstrated a reproducible tailing phenomenon,
which was assumed to be due to clumping (due to spores being
present in multilayers; Fig. 13).
By contrast, the survival curve observed by another manufacturer
(B) (Fig. 14) using the same
plasma process presented a reproducible linear relationship
(Fig. 15), allowing for the
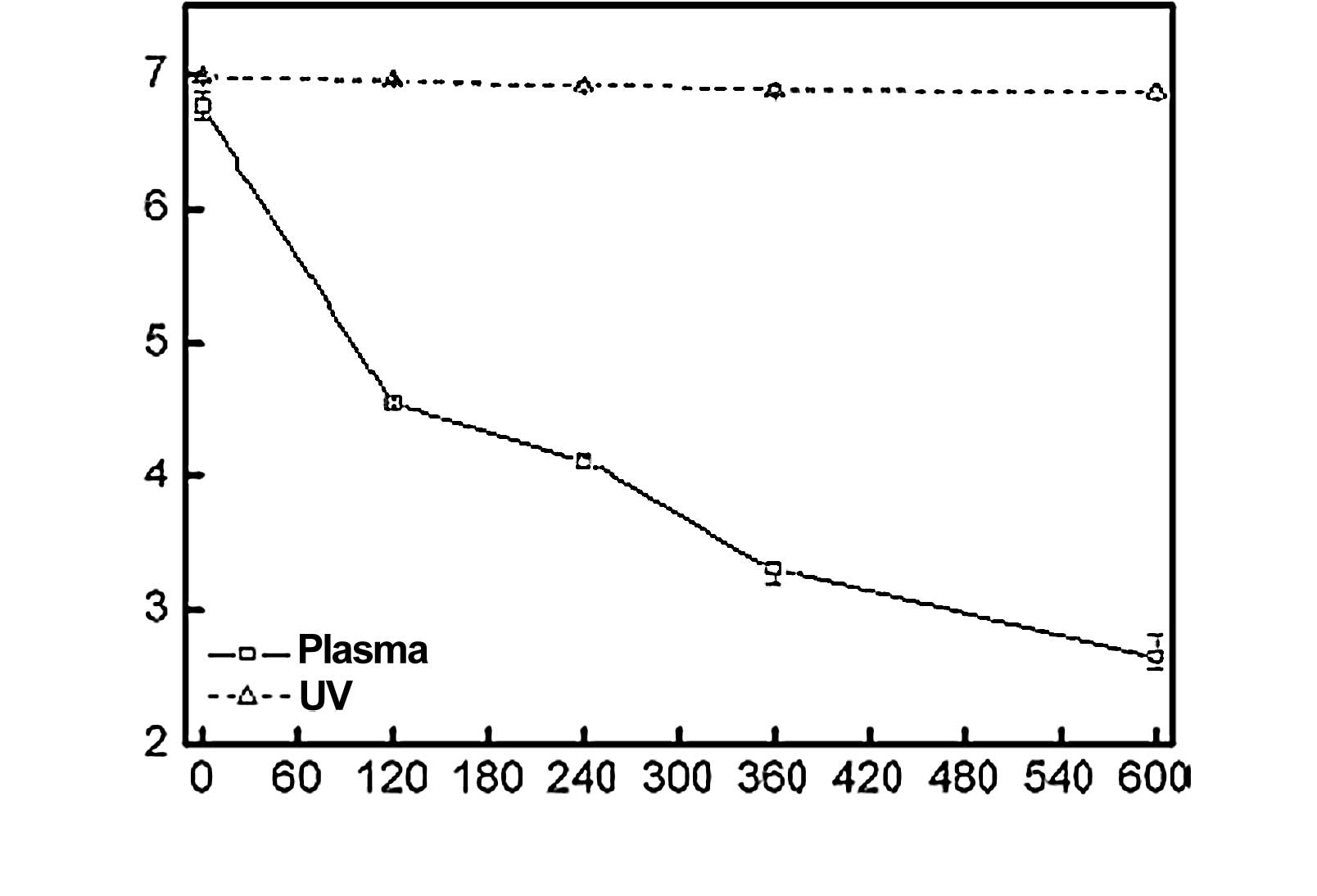
extrapolation of a SAL. The D-value (the average time to obtain a
log reduction of the test microorganism) calculated from Fig. 15 was approximately 1 min.
Therefore, a minimum sterilization time of 12 min for overkill
method under these exposure conditions would provide a SAL of
10−6, as required by the international standards for
sterilization processes (ISO 14937) (3). The coefficient correlation between
log reduction of the test microorganism and exposure time was 0.992
(Fig. 15). This relationship is
quite satisfactory for the extrapolation of a SAL.
The overkill method of demonstrating a sterilization
process is the most widely used method, but not the only one.
Sterilization is also demonstrated by an understanding of the
natural bioburden (numbers and types of viable microorganisms)
present on/in the product to be sterilized; this is indeed the real
target for sterilization. When the bioburden is low, the
sterilization challenge is not great, but when it is high and
associated with significant clumping (such as observed with viruses
and various types of bacteria, including the presence of biofilm)
(26), this provides a significant
challenge to the sterilization process. However, in many cases
these vegetative bacterial cells (27) and other microorganisms are
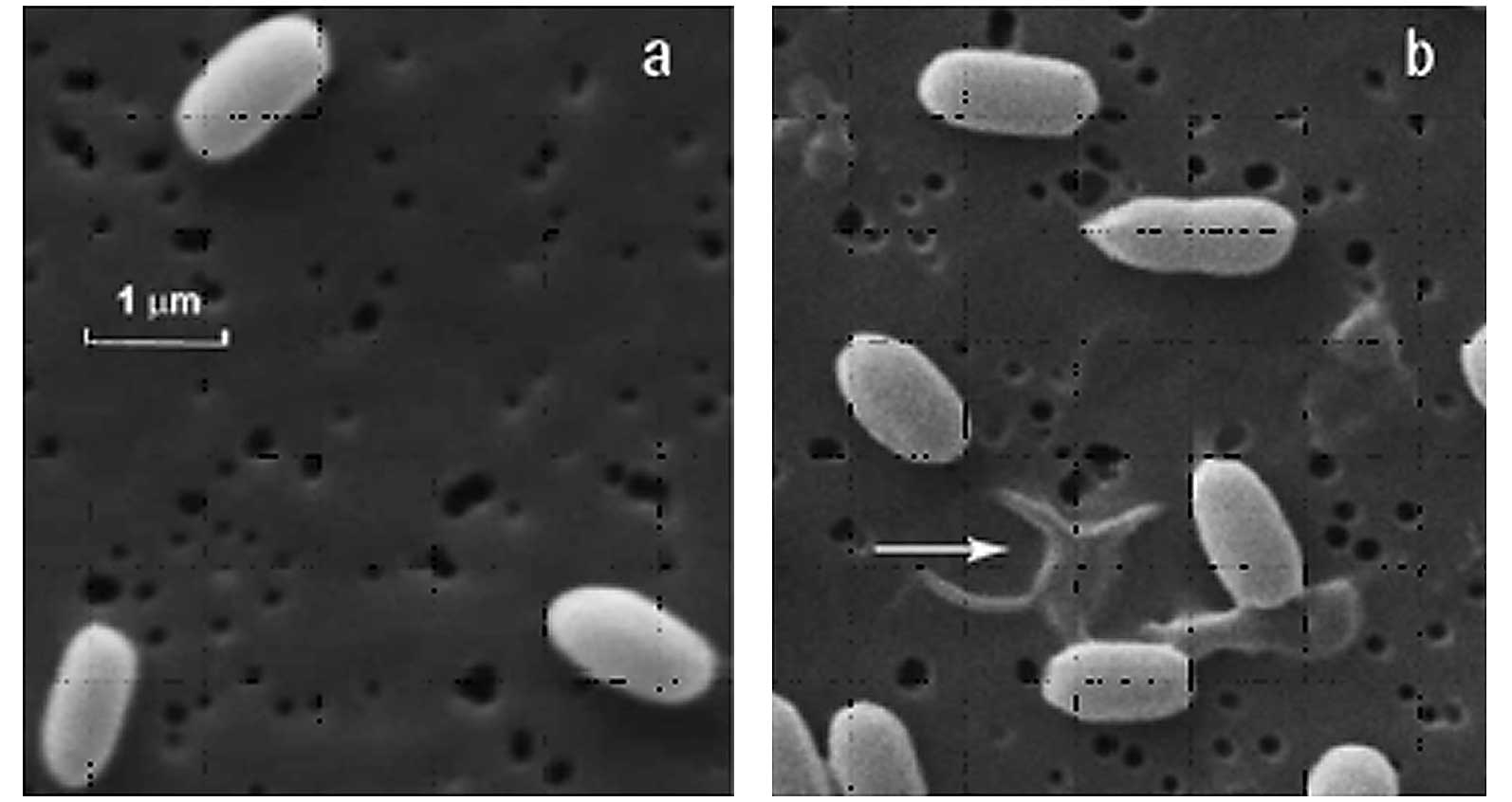
generally much less resistant to gas plasmas (20) (Fig.
16). Therefore, the gas plasma penetration ability, as
previously discussed, should be sufficient to penetrate and
sterilize any such bioburden present and to simultaneously attain
an appropriate SAL of 10−6 together with material and
functional compatibility. This needs to be demonstrated for any
potential plasma sterilization process.
Perspectives for the future
It seems likely that gas plasma disinfection and/or
sterilization methods can be developed to provide the required
antimicrobial reduction (as defined by an appropriate SAL, e.g.,
10−6 for sterilization) and material/functional
compatibility. Plasma may have important applications in medical
and industrial settings. In this sense, perspectives on plasma
sterilization methods require further investigation. To clarify the
mechanisms and optimize the development of gas plasma
sterilization, close collaboration between microbiologists and
physical chemists will provide the best opportunity for the future
success of this technology.
References
|
1.
|
Shintani H, Shimizu N, Imanishi Y, Sekiya
T, Tamazawa K, Taniguchi A and Kido N: Inactivation of
microorganisms and endotoxins by low temperature nitrogen gas
plasma exposure. Biocontrol Sci. 12:131–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Krebs MC, Becasse P, Verjat D, et al: Gas
plasma sterilization: relative efficiency of the hydrogen peroxide
phase as compared to that of the plasma phase. Int J Pharm.
160:75–81. 1988. View Article : Google Scholar
|
|
3.
|
ISO 14937: 2009, Sterilization of health
care products. General requirements for characterization of a
sterilizing agent and the development, validation and routine
control of a sterilization process for medical devices.
|
|
4.
|
Sato N: Basic approach to plasma
production and control. Advanced Plasma Technology. D'Agostino R,
Favia P, Kawai Y, Ikegami H, Sato N and Arefi-Khonsari F:
Wiley-VCH, Verlag GmbH & Co; Weinheim, Germany: pp. 1–16.
2008
|
|
5.
|
Kylian O, Sasaki T and Rossi F: Plasma
sterilization of Geobacillus stearothermophilus by
O2:N2 RF inductively coupled plasma. Eur Phys
J Appl Phys. 34:139–142. 2006.
|
|
6.
|
Jacobs PT and Lin S-M: Sterilization
processes utilizing low-temperature plasmas. Disinfection,
Sterilization and Preservation. Block SS: Lippincott Williams &
Wilkins; New York: pp. 747–765. 2001
|
|
7.
|
Rossi F, Kylian O and Hasiwa M: Mechanisms
of sterilization and decontamination of surfaces by low-pressure
plasma. Advanced Plasma Technology. D'Agostino R, Favia P, Kawai Y,
Ikegami H, Sato N and Arefi-Khonsari F: Wiley-VCH Verlag GmbH &
Co; Weinheim, Germany: pp. 319–340. 2008
|
|
8.
|
Rossi F, Kylian O and Hasiwa M:
Decontamination of surfaces by low pressure plasma discharges.
Plasma Process Polym. 3:431–442. 2006. View Article : Google Scholar
|
|
9.
|
Deng X, Shi J and Kong MG: Physical
mechanisms of inactivation of Bacillus subtilis spores using
cold atmospheric plasmas. IEEE Trans Plasma Sco. 34:1310–1316.
2006.
|
|
10.
|
Kim S-M and Kim J-I: Decomposition of
biological macromolecules by plasma generated with helium and
oxygen. J Microbiol. 44:466–471. 2006.PubMed/NCBI
|
|
11.
|
Lee K, Paek K-H, Ju W-T and Lee Y:
Sterilization of bacteria, yeast, and bacterial endospores by
atmospheric-pressure cold plasma using helium and oxygen. J
Microbiol. 44:269–275. 2006.PubMed/NCBI
|
|
12.
|
Yu QS, Huang C, Hsieh F-H, Huff H and Duan
Y: Bacterial inactivation using a low-temperature atmospheric
plasma brush sustained with argon gas. J Biomed Mater Res Part B:
Appl Biomater. 80B:211–219. 2007. View Article : Google Scholar
|
|
13.
|
Purevdorj D, Igura N, Ariyada O and
Hayakawa I: Effect of feed gas composition of gas charge plasmas on
Bacillus pumillus spore mortality. Lett Appl Microbiol.
37:31–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kinoshita S: Examination of UV and VUV
effect on sterilization. Bohkin Bobai. (In press).
|
|
15.
|
Halfmann H, Bibinov N, Wunderlich J and
Awakowicz P: Correlation between VUV radiation and sterilization
efficiency in a double inductively coupled plasma. In: Presented at
the 28th ICPIG Congress; Prague. pp. 2007
|
|
16.
|
Lerouge S, Fozza AC, Wertheimer MR,
Marchand R and Yahia L: Sterilization by low-pressure plasma: the
role of vacuum-ultraviolet radiation. Plasma Polymers. 5:31–46.
2000. View Article : Google Scholar
|
|
17.
|
Güçeri S and Fridman A: Plasma Assisted
Decontamination of Biological and Chemical Agents. Springer; The
Netherlands: pp. 2008
|
|
18.
|
McDonnell G: Peroxygens and other forms of
oxygen: their use for effective cleaning, disinfection and
sterilization. New Biocides Development: the Combined Approach of
Chemistry and Microbiology. Zhu PC: Oxford University Press; New
York: pp. 292–308. 2006
|
|
19.
|
Finnegan M, Linley E, Denyer SP, McDonnell
G, Simons C and Maillard J-Y: The mode of action of hydrogen
peroxide and other oxidizing agents: differences in liquid and gas
form. J Pharm Sci. (In press).
|
|
20.
|
McDonnell GE: Antisepsis, Disinfection,
and Sterilization. ASM Press; Washington DC: pp. 33pp. 2822007
|
|
21.
|
Cortezzo DE, Koziol-Dube K, Setlow B and
Setlow P: Treatment with oxidizing agents damages the inner
membrane of spores of Bacillus subtilis and sensitizes
spores to subsequent stress. J Appl Microbiol. 97:838–852. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Setlow P: Spores of bacillus
subtilis: their resistance to and killing by radiation, heat
and chemicals. J Appl Microbiol. 101:514–525. 2006.
|
|
23.
|
Imae Y, Strominger MB and Strominger J:
Electron microscope studies of conditional spore cortexless mutants
of Bacillus sphaericus. J Bacteriol. 127:1568–1570.
1976.PubMed/NCBI
|
|
24.
|
Shintani H and McDonnell G: Inactivation
of microorganisms (spore type and vegetative cells) and the
mechanism by gas plasma. Sterilization and Disinfection by Plasma:
Sterilization Mechanism, Biological and Medical Applications.
Sakudo A and Shintani H: NOVA Science Publishers; New York: (In
press).
|
|
25.
|
Moisan M, Barbeau J, Moreau S, Pelletier
J, Tabrizian M and Yahia LH: Low-temperature sterilization using
gas plasmas: a review of the experiments and an analysis of the
inactivation mechanism. Int J Pharm. 226:1–21. 2001. View Article : Google Scholar
|
|
26.
|
Joaquin JC, Kwan C, Abramzon N,
Vandervoort K and Breiles-Marino G: Is gas-discharge plasma a new
solution to the old problem of biofilm inactivation? Microbiology.
155:724–732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Shintani H, Taniai E, Miki A, Kurosu S and
Hayashi F: Comparison of the collecting efficiency of
microbiological air samplers. J Hosp Infect. 56:42–48. 2004.
View Article : Google Scholar : PubMed/NCBI
|