Introduction
Radiotherapy is a crucial treatment method in the
management of human laryngeal carcinoma. Unfortunately, in some
cases, little tumor-controlling efficacy is achieved by radiation
alone (1,2), due to the inherent radioresistance of
this tumor. The development of new strategies to improve the
sensitivity to radiotherapy of cancer is absolutely necessary. For
this purpose, in recent years, potential targets for therapeutic
intervention of therapy-resistant cancers have been extensively
studied. Among the various related factors investigated, signal
transducer and activator of transcription 3 (STAT3) is an important
potential therapeutic target.
Physiologically, STAT3 has diverse biological
functions, including the modulation of cell growth and cell
differentiation, and the regulation of apoptosis (3–5).
Moreover, STAT3 has been shown to be constitutively activated in
various types of human cancer and to be necessary for tumor cell
growth (6). In human cancer, STAT3
participates in oncogenesis through the modulation of p53
expression (7), the regulation of
cell cycle control genes, including C-Myc (8–10)
and cyclinD1/D2 (11), the
up-regulation of genes encoding apoptosis inhibitors, such as B
cell lymphoma 2 (Bcl-2), Bcl-xL, survivin and Mcl-1 (5,12–15),
and the induction of angiogenesis by vascular endothelial growth
factor (VEGF) (13,16).
Radiosensitivity is affected by changes in the cell
cycle, programmed cell death, DNA injury repair, and other
mechanisms regulated by oncogenes after radiation. In view of the
fact that many factors related to the tumor cell cycle, apoptosis
and DNA injury repair are downstream of the STAT3 pathway, we
hypothesized that STAT3 may be involved in cancer
radioresistance.
In our previous study (17), we successfully demonstrated that
the apoptosis of hep-2 cells is significantly increased by blocking
the STAT3 pathway with short hairpin RNA (shRNA) combined with
radiation in vitro, indicating the potential
radiosensitization effects of STAT3 shRNA-based RNA interference
(RNAi). This is a new technique that inhibits mRNA expression by
inducing the sequence-specific destruction of homologous mRNA in
cells with small interference RNA (siRNA). Here, to confirm our
previous findings, a xenograft model of human laryngeal squamous
cell carcinoma was established in nude mice, and a constructed
recombinant plasmid vector carrying human STAT3 shRNA was
transfected into the tumor-bearing mice followed by radiation, with
the aim of examining its efficacy in the inhibition of tumor
growth. The results demonstrate that shRNA targeting STAT3
potentiate the radiosensitivity of human laryngeal carcinoma
xenografts in vivo.
Materials and methods
Reagents
RPMI-1640 media, Opti-MEMI and Lipofectamine 2000
were purchased from Invitrogen Corp. (Carlsbad, CA, USA). Plasmid
pGPU6/GFP/Neo and siRNA oligonucleotides were provided by Gene
Pharma Corporation (Shanghai, China). The target region of STAT3
siRNA was selected and recombinant plasmids were constructed
according to our previous study (17). The Wizard® Plus
Megapreps DNA Purification System was purchased from Promega (USA).
The AMV First Strand cDNA Synthesis kit and primers were products
of Sangon Biological Engineering Technology and Services Corp.
(Shanghai, China). STAT3, p-STAT3 and Bcl-2 mouse monoclonal
antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA,
USA). Rabbit anti-human p53 polyclonal antibody was obtained from
Seitz Biological Technology Co., Ltd. (Beijing, China) and Rabbit
anti-human VEGF polyclonal antibody was from Boster Biological
Technology, Ltd. (Wuhan, China). CD34 mouse monoclonal antibody,
the SP kit and DAB developer were from Zhongshan Goldenbridge
Bio-technology Co., Ltd. (Beijing, China). Propidium iodide was
purchased from Sigma (USA).
Tumor transfection and treatment
Male BALB/c nude mice, aged 4–5 weeks and weighing
12–18 g, were obtained from the Medical Department of Peking
University Laboratory Animal Center (Beijing, China) and housed
under specific pathogen-free conditions at the Bethune
International Peace Hospital (Shijiazhuang, China) in accordance
with the National Regulations on Animal Experiments and Animal
Welfare. To generate the tumor xenografts, 2×106 viable
hep-2 cells were subcutaneously injected into the right-side back
of the mice. When tumors reached a volume of ∼150 mm3
(on day 14), 28 tumor-bearing mice were randomly divided into four
groups: the negative control group (pshNeg), tumors injected with
negative plasmid; the STAT3 siRNA group (pshSTAT3), tumors treated
with STAT3 shRNA recombinant plasmid alone, without irradiation;
the irradiation group (IR), tumors exposed to radiation alone, with
5 Gy of γ-rays per irradiation; the combination group (pshSTAT3
plus IR), tumors treated with STAT3 shRNA recombinant plasmid
combined with irradiation as above.
To perform the in vivo gene transfection, the
mice in the pshNeg, pshSTAT3, and pshSTAT3 plus IR groups were
intratu-morally injected with a 200 μl mixture of plasmid (20 μg/20
μl plasmid + 50 μl Lipofectamine + 130 μl serum-free RPMI-1640
culture medium). The mice in the IR group were injected with the
same amount of control mixture (50 μl Lipofectamine + 150 μl
serum-free RPMI-1640 culture medium). Intratumoral injection was
performed on days 0, 3, 6, 9, 12, 15, 18 and 21, with day 0
representing the first day of injection. To test the transfection
efficiency, fresh tumor cells were obtained at 48 h
post-transfection from a separate group of animals in a parallel
single transfection experiment, and the transfection efficiency was
measured by flow cytometry (FCM). On days 2, 5, 8 and 11 of the
planned treatment scheme, radiotherapy was performed with γ-rays on
the tumors only using a 60Co unit, with the animals
immobilized and biologically isolated from ambient air using a
special device. The remaining parts of the animals were protected
by a 1-cm thick stereotype. Projectile’s Ueno Area was 4×4 cm, with
a source tumor distance of 100 cm and an absorbed dose rate of
78.79 cGy/min. Tumor volumes were estimated four times weekly
according to the formula v = a2b/2, where a and b are
the shortest and longest diameter, respectively (18). Upon termination of the experiment,
the mice were sacrificed by cervical dislocation, and the tumors
were excised for weighing, immunohistochemistry and FCM. Tumor
growth inhibition rates were calculated using the formula (1 -
average tumor weight of experimental group/average tumor weight of
control group) × 100%.
Semi-quantitative RT-PCR analysis
The cell suspension was prepared from fresh tumor
tissues. Total RNA was extracted from 1×106 fresh tumor
cells using TRIzol reagent according to the manufacturer’s
instructions. RT-PCR was performed using the two-step method. cDNA
was synthesized according to the protocol of the AMV First Strand
cDNA Synthesis kit. STAT3 gene primers were: forward,
5′-gtcagatgccaaatgc-3′; reverse, 5′-cctggaggcttagtgc-3′. β-actin
primers were: forward, 5′-GCATGGGTGCCCCGACGTTG-3′; reverse,
5′-GCTCCG GCCAGAGGCCTCAA-3′. The PCR reaction was performed using a
PCR instrument (UNOII, Biometra, Germany). The reaction conditions
were: 95°C for 5 min, followed by 30 cycles at 95°C for 30 sec,
55°C for 45 sec, 72°C for 60 sec, and a final elongation at 72°C
for 10 min. PCR products were separated on a 2% agarose gel, and
visualized by ethidium bromide staining.
Gene expression analysis and intratumoral
microvessel density (MVD)
Tumor tissues were fixed in 4% paraformalde-hyde and
embedded in paraffin, then 4-mm sections were cut and prepared.
Immunohistochemical stainning was performed according to the
standard protocol of the SP kit. In brief, after dewaxing and
rehydration, the sections were subjected to heat-induced antigen
retrieval in a high pressure cooker, quenched in reagent A (3%
H2O2 methanol) for 10 min to remove
endogenous peroxidase activity, washed in PBS, and then incubated
with normal goat serum for 30 min to block non-specific binding
sites. Subsequently, the sections were incubated with primary
antibodies (STAT3, p-STAT3, Bcl-2, p53, VEGF and CD34) overnight at
4°C. After the primary antibody was removed, the slides were washed
with PBS and incubated with reagent C (biotin-conjugated
goat-anti-mouse IgG) for 30 min. Sections were rinsed with PBS and
developed with reagent D (horseradish-peroxidase-labeled pronase
avidin) for 15 min, then counterstained for 3–5 min with
hematoxylin and coloured by 3,3′-diaminobenzidine (DAB). Sections
from human laryngeal carcinoma known to have abundant STAT3,
p-STAT3 and related protein (Bcl-2, p53 and VEGF) expression served
as the positive control. For the negative controls, PBS was used
rather than the primary antibodies. Protein staining was quantified
using computer-assisted image analysis with Image Pro Plus software
(Media Cybernetics) (19). MVD was
assessed by the hot spot method (20).
Analysis of apoptosis by flow
cytometry
Single cell suspensions were prepared from fresh
tumor tissues. Cell viability was assessed by typan blue exclusion,
and the samples were fixed in 70% ethanol at 4°C for 24 h. Cells
were resuspended in PBS and stained with propidium iodide (50 mg/l)
according to the manufacturer’s instructions, then analyzed by FCM
(Epics-XLII; Beckman Coulter, USA). For DNA staining, a total of
10,000 cells were counted and analyzed by Muticycle AV software.
The stained cells were analyzed by FCM. Forward light scatter
characteristics were used to exclude cell debris from the analysis.
Apoptotic cells were determined by their hypochromic subdiploid
staining profiles.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). Statistical analyses were performed using SPSS15.0 software.
One-way ANOVA was used to determine statistical differences between
the experimental groups. After normalization, two-sided variance
were analyzed by Pearson’s correlation coefficient. P<0.05 was
considered statistically significant.
Results
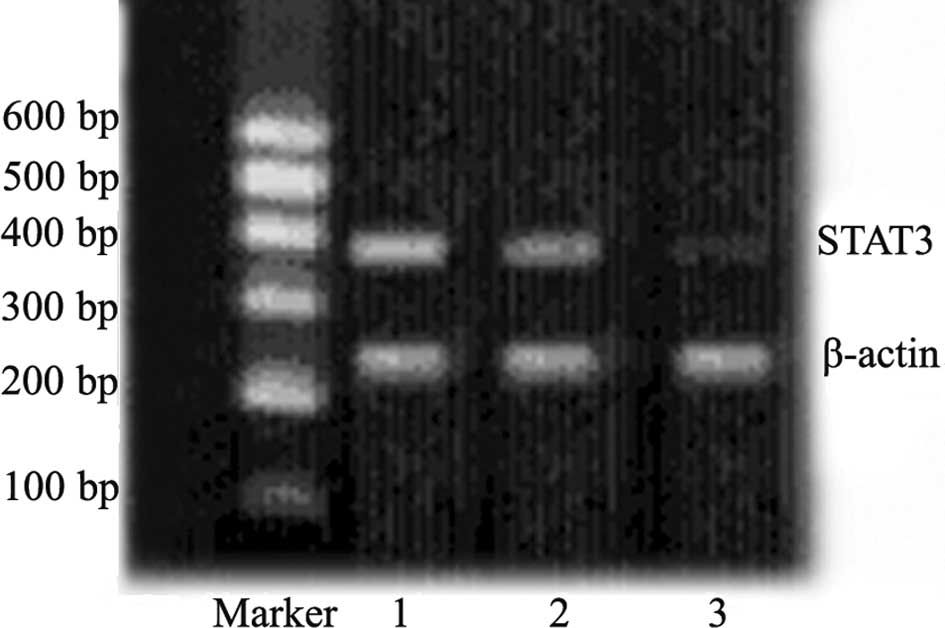
Effects of STAT3 shRNA on mRNA expression
in vivo
As shown in Fig. 1,
the expression of STAT3 mRNA was significantly down-regulated 48 h
after transfection in tumor cells from animals transfected with
plasmid carrying STAT3 shRNA as compared to the negative controls.
This confirmed the transfection efficiency of the experimental
scheme applied for the in vivo transfection.
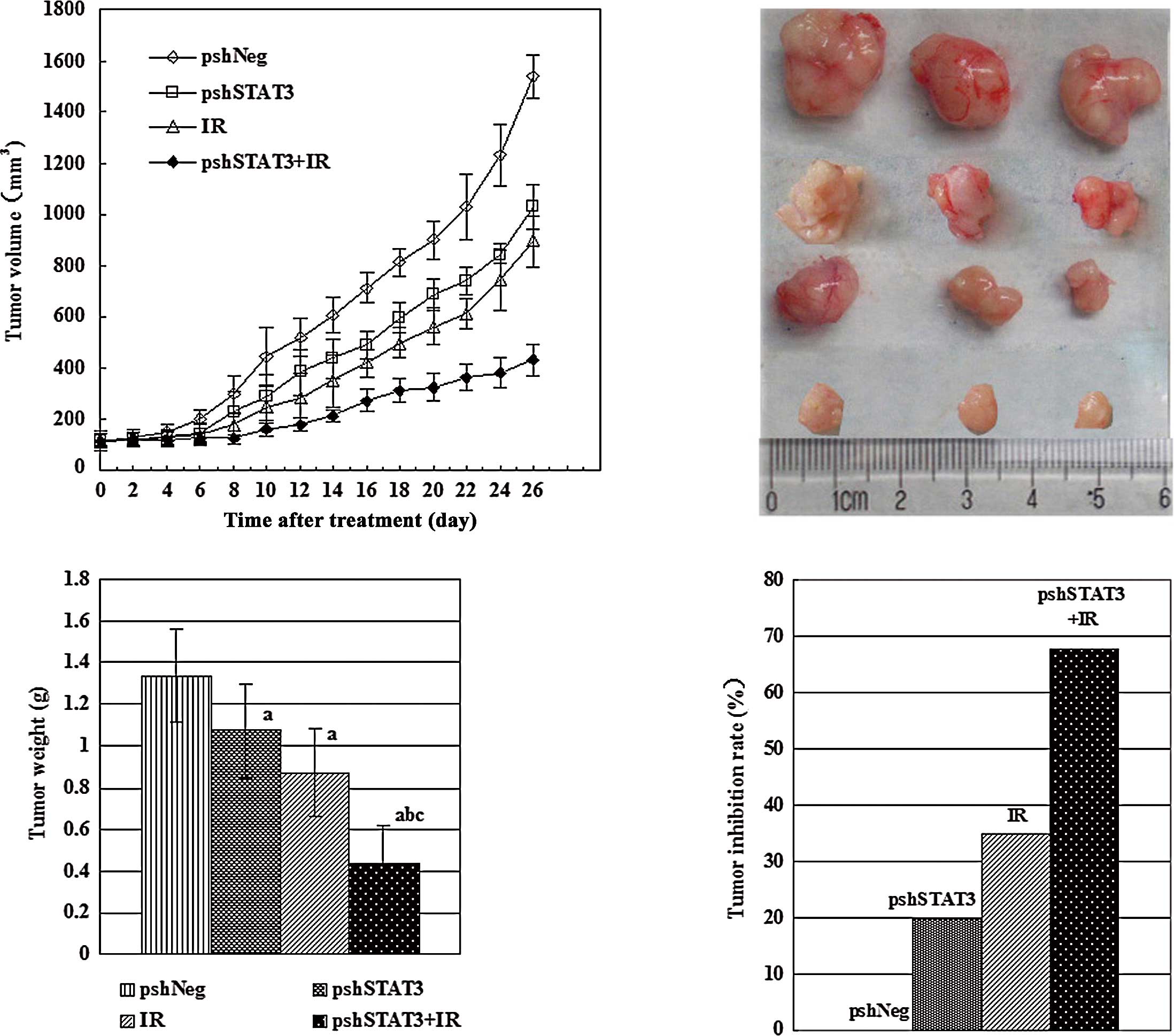
Suppression of xenograft tumor
growth
After confirming the in vivo transfection
efficiency, we analyzed the therapeutic potential of pshSTAT3 plus
irradiation. Upon termination of the experiment, tumor volumes
(mean ± SD) were 1536.83±83.27, 1030.67±89.03, 894.67±99.19 and
433.83±60.89 mm3 for the pshNeg, pshSTAT3, IR, and
pshSTAT3 plus IR groups, respectively (Fig. 2A). Differences between the groups
were statistically significant (F=174.07, P=0.000). The outcomes of
various treatments on tumor volume and appearance are shown in
Fig. 2B. There was a significant
difference in tumor weight among the different groups (F=23.10,
P=0.000), with the pshSTAT3 plus IR group having the lightest tumor
weight (Fig. 2C). The strongest
tumor growth inhibitory effect was observed in the pshSTAT3 plus IR
group, which had a tumor inhibition rate of 67.7% (Fig. 2D).
Protein expression of STAT3, p-STAT3,
p53, VEGF and Bcl-2
Immunostaining for STAT3 and VEGF was mainly
detectable in the cytoplasmic membrane and cytoplasm. Positive
expression of p-STAT3, p53 and Bcl-2 proteins was mainly found in
the nuclei of the tumor cells. Strong staining for STAT3, p53,
Bcl-2 and VEGF was observed in the negative control group. Most
tumor cells had weak or undetectable staining for the above
proteins in the pshSTAT3 plus IR group (Fig. 3A–D). Computerized image analysis
revealed that the mean density of p-STAT3 decreased in a stepwise
pattern in the pshNeg (115.6±14.2), IR (92.7±16.4), pshSTAT3
(89.7±1.0) and pshSTAT3 plus IR (65.2±8.9) groups (F=17.779,
P<0.05). For each individual protein, including p53, Bcl-2 and
VEGF, the level of protein expression among the different groups
was statistically different (F=22.969, 34.285 and 13.306, all
P<0.05). Compared to the other three groups, p53, Bcl-2 and VEGF
protein expression was significantly reduced in the pshSAT3 plus IR
group (P=0.000, 0.000 and 0.018) (Fig.
3E).
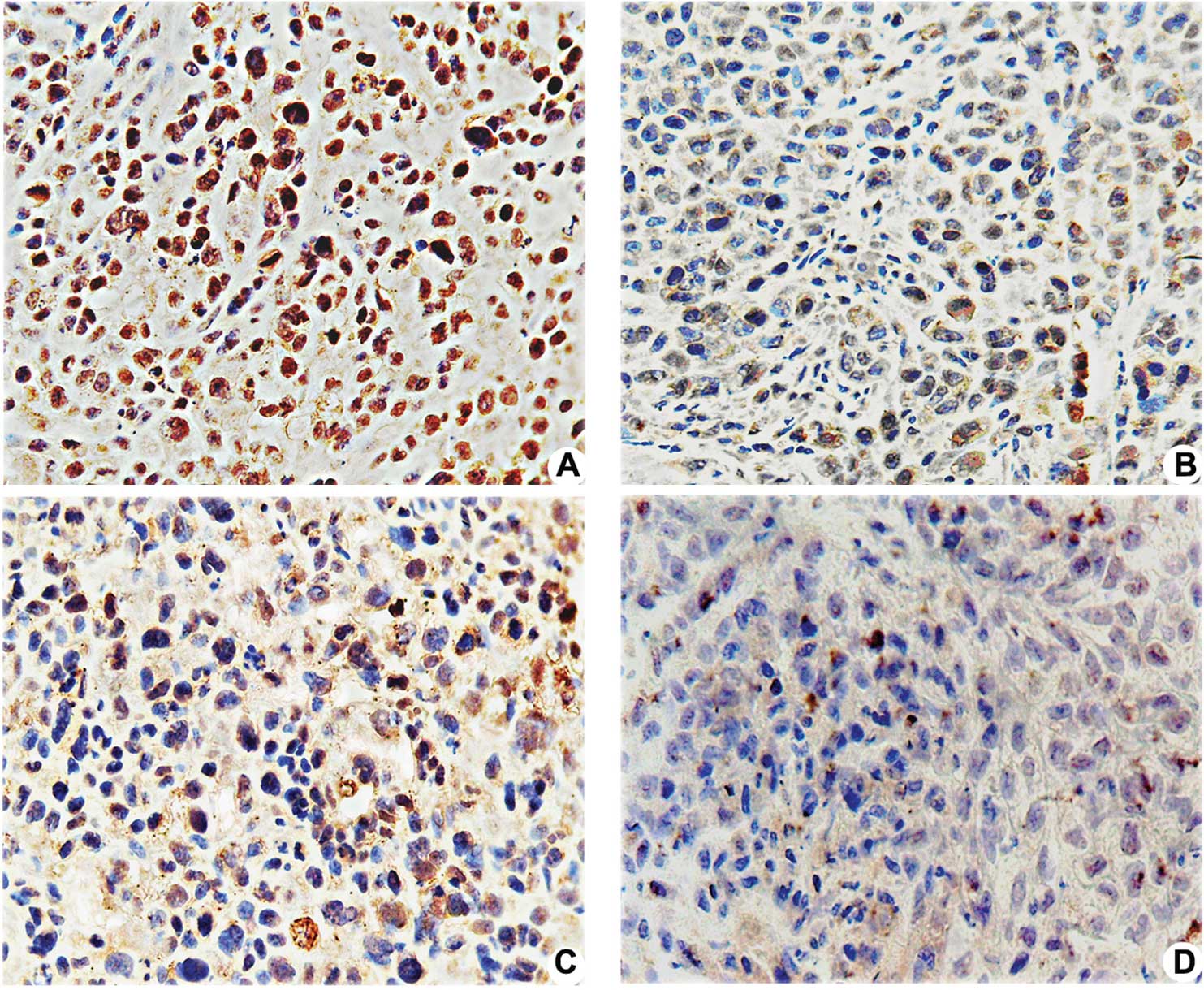 | Figure 3.Effects of pshSTAT3 on p-STAT3
expression in vivo. The expression of p-STAT3 protein in the
xenografts was evaluated by immunohistochemistry. Image analysis
was carried out in four randomly selected fields per section, from
seven consecutive sections per group (magnification, x400). (A)
pshNeg; (B) pshSTAT3; (C) IR; (D) pshSTAT3 plus IR. (E)
Computerized image analysis of the expression levels of the related
proteins, including STAT3, p-STAT3, p53, Bcl-2 and VEGF, showing
notable features of protein expression. STAT3, p-STAT3, p53, Bcl-2
and VEGF protein expression were significantly reduced in the
pshSAT3 plus IR group (aP<0.05 vs. negative control
group; #P<0.05 vs. irradiation group;
*P<0.05 vs. pshSTAT3 group, n=7). |
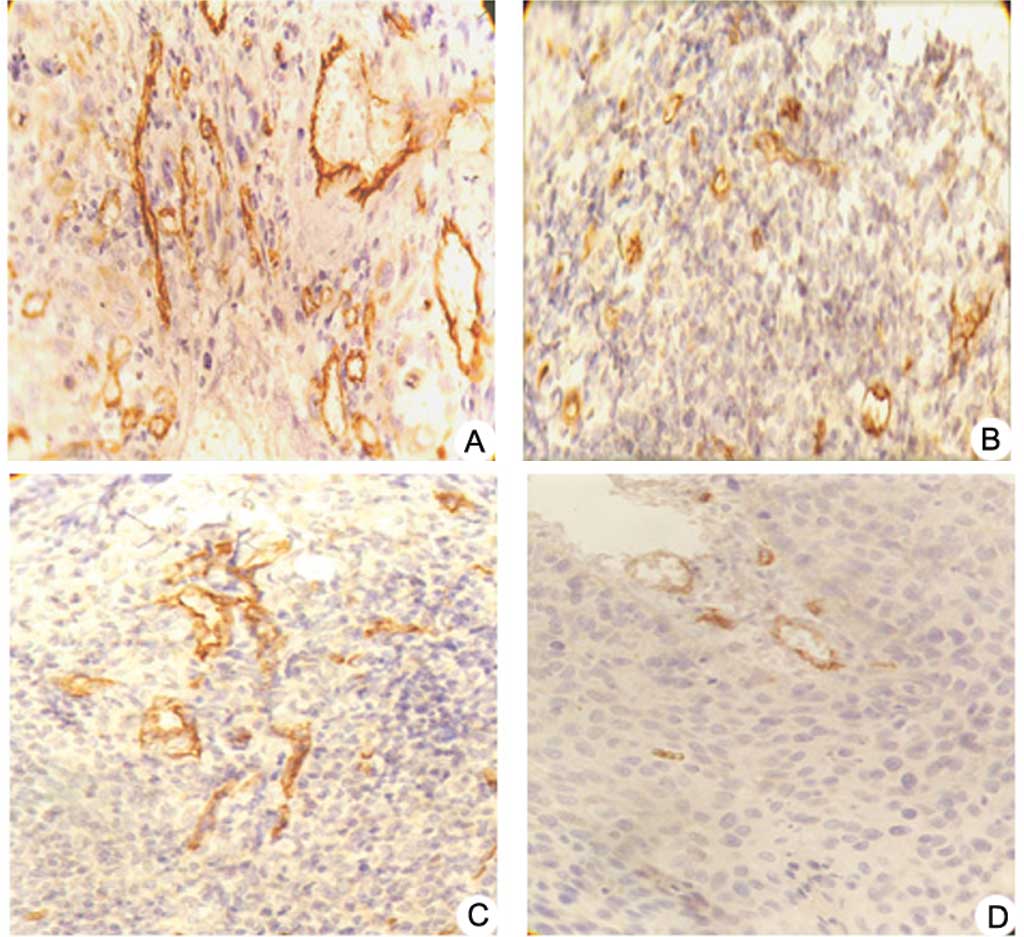
CD34 expression and MVD counting
Expression of CD34 in the pshSTAT3 plus IR group was
weak or even negative, whereas it was strong in the negative
control group. The newborn vascular endothelial cells were stained
brown or yellow. Irregular lumens and immature vessels were often
present (Fig. 4A–D). Results from
MVD counting revealed that the MVD values varied widely and showed
significant difference among the four groups (F=19.196, P<0.05).
MVD values were 31.43±4.76 and 11.67±3.41 in the negative control
group and the pshSTAT3 plus IR group, respectively (Fig. 4E).
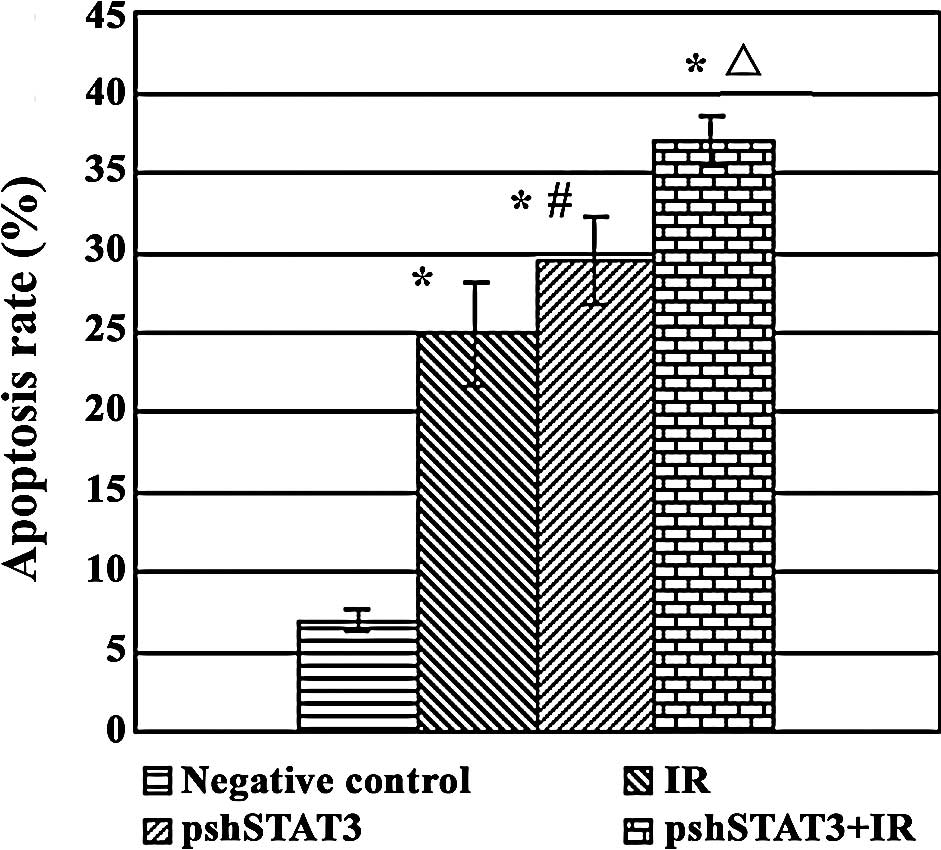
Apoptosis assay
The results of FCM revealed statistical differences
among the different treatment groups in the apoptotic rate
(F=122.40, P<0.05). As shown in Fig. 5, apoptotic rates were 37.04±1.59,
24.87±3.26, 29.49±2.69 and 6.89±0.67% in the pshSTAT3 plus IR, IR,
pshSTAT3 and negative control groups, respectively, with the
highest rate in the pshSTAT3 plus IR group and the lowest rate in
the negative control group.
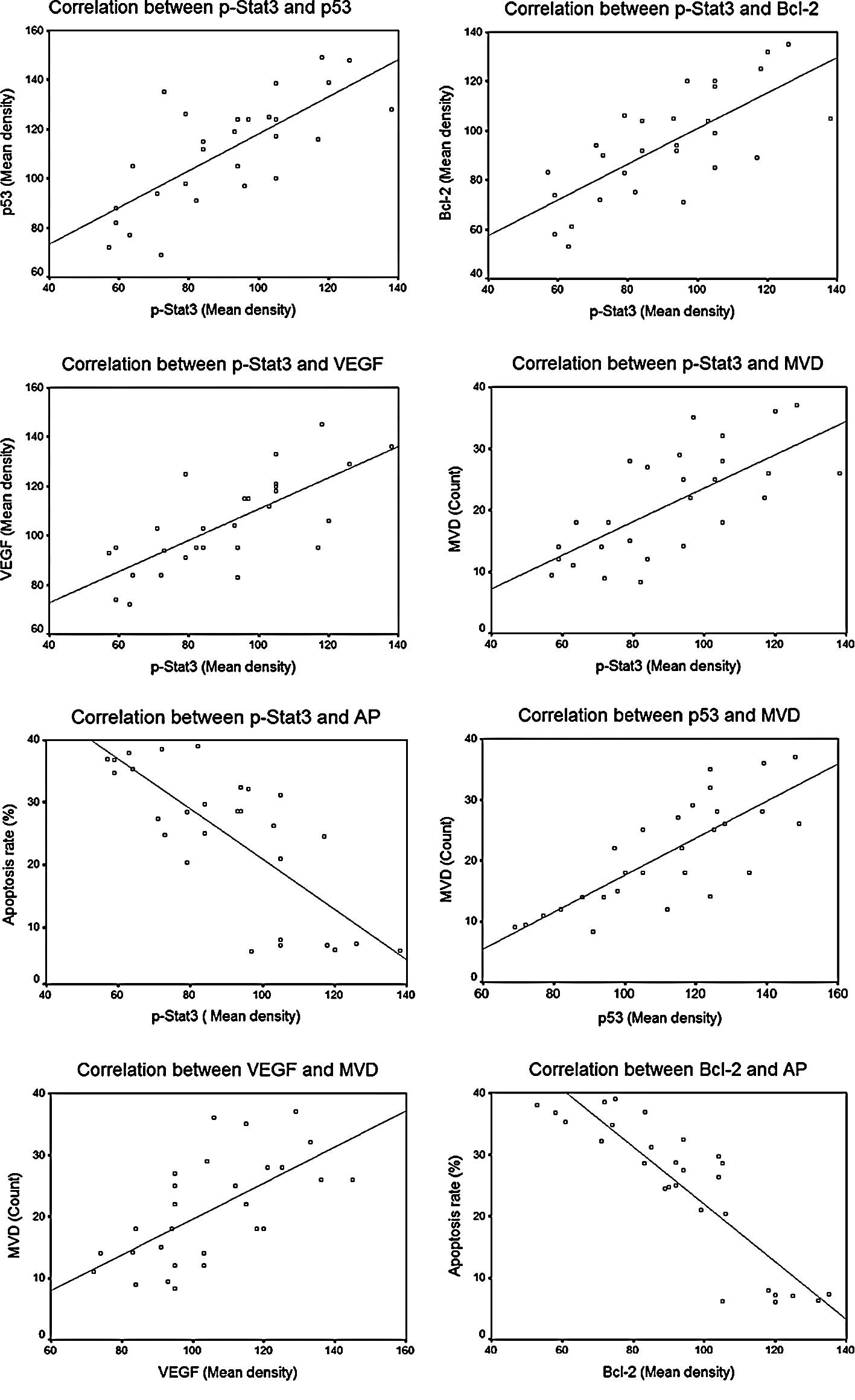
Correlation analysis
Statistically significant correlations between
different factors are summarized in Table I and Fig. 6. As shown, significant correlations
were noted between p-STAT3 protein expression and expression of the
other three downstream regulating proteins, including p-STAT3 vs.
p53 (r=0.738, P=0.000), p-STAT3 vs. VEGF (r=0.735, P=0.000) and
p-STAT3 vs. Bcl-2 (r=0.727, P=0.000), and also between the protein
expression of p53 and VEGF and MVD counts, including p53 vs. MVD
(r=0.784, P=0.000) and VEGF vs. MVD (r=0.641, P=0.000). An inverse
correlation was observed between the protein expression of Bcl-2
and the cell apoptosis rate (r=−0.883, P=0.000).
 | Table I.Analysis of correlation between
factors. |
Table I.
Analysis of correlation between
factors.
| r* | P-value |
|---|
| p-STAT3 vs. p53 | 0.738 | 0.000<0.01 |
| p-STAT3 vs.
Bcl-2 | 0.727 | 0.000<0.01 |
| p-STAT3 vs. VEGF | 0.735 | 0.000<0.01 |
| p53 vs. MVD | 0.784 | 0.000<0.01 |
| VEGF vs. MVD | 0.641 | 0.000<0.01 |
| Bcl-2 vs. AP | −0.883 | 0.000<0.01 |
Discussion
STATs are a family of proteins that act as signal
messengers and transcription factors and participate in normal
cellular responses to cytokines and growth factors. STAT3 is an
important member of the STAT family, and is often associated with a
wide variety of human malignancies, including head and neck cancer
(21). STAT3 may mediate
resistance to ionizing radiation or chemotherapeutic agents in
certain malignant tumors, as evidenced by previous studies
(22–24). For example, one study indicated
that when the STAT3 gene is knocked out, B-1 cells become more
susceptible to irradiation (25).
It has also been reported that STAT3 inhibition with a STAT3
antisense oligonucleotide enhances radiation-induced apoptosis in
prostate cancer cells (26).
Furthermore, our previous study demonstrated that blocking STAT3
expression by siRNA potentiates radiation-induced cell death in
Hep-2 human laryngeal carcinoma cells (17). However, it is unclear whether the
inhibition of STAT3 expression by means of RNAi promotes
radiosensitivity in human laryngeal carcinoma in vivo.
Essentially, RNAi specifically degrades target mRNA
without affecting the stability of non-homologous mRNA. With the
properties of high stability and a reliable inhibitory efficacy on
the targeted mRNA, it is easier for cells to uptake siRNA than
antisense oligonucleotides (27).
In the present study, we successfully transfected a plasmid
carrying STAT3 shRNA into xenograft human laryngeal squamous
carcinoma cells using liposome as a delivery carrier. The effects
of STAT3 shRNA following a planned experimental scheme on
radiosensitization were investigated in the xenograft tumors. Upon
termination of the experiment, tumor volume and weight in the
pshSTAT3 plus radiation group were found to be dramatically reduced
compared to the other groups. This indicates that a specific tumor
inhibitory effect is achieved by pshSTAT3 plus radiation, superior
to that achieved by radiotherapy or RNAi alone.
To explore the mechanism of radiosensitization by
STAT3 shRNA in laryngeal carcinoma xenografts, the expression of
STAT3 and its downstream regulating proteins as well as the
associated cell apoptosis rates were evaluated. The results of
protein expression from immunohistochemistry achieved by
computerized image analysis demonstrated that the level of p-STAT3
decreased notably in animals treated by pshSTAT3 plus radiation,
with simultaneous down-regulation of Bcl-2, p53 and VEGF protein
expression. Tumor angiogenesis was also significantly suppressed,
as evidenced by the MVD count. Furthermore, the results of FCM
demonstrated that the apoptosis rate of tumor cells in the pshSTAT3
plus radiation group was the highest among the different groups.
These changes may be attributed to the regulation of downstream
signaling proteins and tumor cell apoptosis by p-STAT3, an
activated form of STAT3.
It is known that numerous important signaling
proteins are present downstream of the STAT3 pathway. These include
cell cycle regulators (c-Myc and CyclinD1/D2), anti-apoptotic
proteins (Mcl-1 and Bcl-2) and tumor suppressor factors (p53 and
VEGF). These are regulated to promote cell proliferation, inhibit
cell apoptosis and potentiate tumor angiogenesis, and participate
in the oncogenesis and development of tumors (4,28,29).
In the present study, the protein expression of p-STAT3, Bcl-2, p53
and VEGF was investigated. Correlation analysis of protein
expression in the different groups demonstrated that the
down-regulation of Bcl-2, p53 and VEGF by p-Stat3 is responsible
for an increase in apoptosis and suppression of angiogenesis,
resulting in radiosensitization effects in laryngeal carcinoma
xenografts. In conclusion, STAT3 shRNA potentiate the
radiosensitivity of laryngeal carcinoma xeno-grafts in vivo
by regulating downstream signaling proteins in the STAT3 pathway,
which exerts potent effects on the induction of apoptosis and
inhibition of angiogenesis.
References
|
1.
|
Rhee JG, Li D, O’Malley BW Jr and
Suntharalingam M: Combination radiation and adenovirus-mediated
P16(INK4A) gene therapy in a murine model for head and neck cancer.
ORL J Otorhinolaryngol Relat Spec. 65:144–154. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Rhee JG, Li D, Suntharalingam M, Guo C,
O’Malley BW Jr and Carney JP: Radiosensitization of head/neck
squamous cell carcinoma by adenovirus-mediated expression of the
Nbs1 protein. Int J Radiat Oncol Biol Phys. 67:273–278. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Takeda K, Noguchi K, Shi W, et al:
Targeted disruption of the mouse Stat3 gene leads to early
embryonic lethality. Proc Natl Acad Sci USA. 94:3801–3804. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Fukada T, Ohtani T, Yoshida Y, et al:
STAT3 orchestrates contradictory signals in cytokine-induced G1 to
S cell-cycle transition. EMBO J. 17:6670–6677. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Catlett-Falcone R, Landowski TH, Oshiro
MM, et al: Constitutive activation of Stat3 signaling confers
resistance to apoptosis in human U266 myeloma cells. Immunity.
10:105–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bromberg JF: Activation of STAT proteins
and growth control. Bioessays. 23:161–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Niu G, Wright KL, Ma Y, et al: Role of
Stat3 in regulating p53 expression and function. Mol Cell Biol.
25:7432–7440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Odajima J, Matsumura I, Sonoyama J, et al:
Full oncogenic activities of v-Src are mediated by multiple
signaling pathways. Ras as an essential mediator for cell survival.
J Biol Chem. 275:24096–24105. 2000.PubMed/NCBI
|
|
9.
|
Ning ZQ, Li J, McGuinness M and Arceci RJ:
STAT3 activation is required for Asp(816) mutant c-Kit induced
tumorigenicity. Oncogene. 20:4528–4536. 2001. View Article : Google Scholar
|
|
10.
|
Bowman T, Broome MA, Sinibaldi D, et al:
Stat3-mediated Myc expression is required for Src transformation
and PDGF-induced mitogenesis. Proc Natl Acad Sci USA. 98:7319–7324.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sinibaldi D, Wharton W, Turkson J, Bowman
T, Pledger WJ and Jove R: Induction of p21WAF1/CIP1 and cyclin D1
expression by the Src oncoprotein in mouse fibroblasts: role of
activated STAT3 signaling. Oncogene. 19:5419–5427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Karni R, Jove R and Levitzki A: Inhibition
of pp60c-Src reduces Bcl-XL expression and reverses the transformed
phenotype of cells overexpressing EGF and HER-2 receptors.
Oncogene. 18:4654–4662. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Aoki Y, Feldman GM and Tosato G:
Inhibition of STAT3 signaling induces apoptosis and decreases
survivin expression in primary effusion lymphoma. Blood.
101:1535–1542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Gritsko T, Williams A, Turkson J, et al:
Persistent activation of stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Epling-Burnette PK, Liu JH,
Catlett-Falcone R, et al: Inhibition of STAT3 signaling leads to
apoptosis of leukemic large granular lymphocytes and decreased
Mcl-1 expression. J Clin Invest. 107:351–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wei D, Le X, Zheng L, et al: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Li X, Wang H, Lu X and Di B: STAT3
blockade with shRNA enhances radiosensitivity in Hep-2 human
laryngeal squamous carcinoma cells. Oncol Rep. 23:345–353.
2010.PubMed/NCBI
|
|
18.
|
Bissery MC, Guenard D, Gueritte-Voegelein
F and Lavelle F: Experimental antitumor activity of taxotere (RP
56976, NSC 628503), a taxol analogue. Cancer Res. 51:4845–4852.
1991.PubMed/NCBI
|
|
19.
|
Crisby M, Nordin-Fredriksson G, Shah PK,
Yano J, Zhu J and Nilsson J: Pravastatin treatment increases
collagen content and decreases lipid content, inflammation,
metalloproteinases, and cell death in human carotid plaques:
implications for plaque stabilization. Circulation. 103:926–933.
2001. View Article : Google Scholar
|
|
20.
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angio-genesis and metastasis – correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991.
|
|
21.
|
Turkson J and Jove R: STAT proteins: novel
molecular targets for cancer drug discovery. Oncogene.
19:6613–6626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Bharti AC, Shishodia S, Reuben JM, et al:
Nuclear factor-kappaB and STAT3 are constitutively active in CD138+
cells derived from multiple myeloma patients, and suppression of
these transcription factors leads to apoptosis. Blood.
103:3175–3184. 2004.
|
|
23.
|
Greten FR, Weber CK, Greten TF, et al:
Stat3 and NF-kappaB activation prevents apoptosis in pancreatic
carcinogenesis. Gastroenterology. 123:2052–2063. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Real PJ, Sierra A, De Juan A, Segovia JC,
Lopez-Vega JM and Fernandez-Luna JL: Resistance to chemotherapy via
Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer
cells. Oncogene. 21:7611–7618. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Otero DC, Poli V, David M and Rickert RC:
Cutting edge: inherent and acquired resistance to radiation-induced
apoptosis in B cells: a pivotal role for STAT3. J Immunol.
177:6593–6597. 2006. View Article : Google Scholar
|
|
26.
|
Calvin DP, Nam S, Buettner R, Sekharam M,
Torres-Roca J and Jove R: Inhibition of STAT3 activity with STAT3
antisense oligonucleotide (STAT3-ASO) enhances radiation induced
apoptosis in DU145 prostate cancer cells. Int J Radiat Oncol Biol
Phys. 57:S2972003. View Article : Google Scholar
|
|
27.
|
Zhang YC, Taylor MM, Samson WK and
Phillips MI: Antisense inhibition: oligonucleotides, ribozymes, and
siRNAs. Methods Mol Med. 106:11–34. 2005.PubMed/NCBI
|
|
28.
|
Amin HM, McDonnell TJ, Ma Y, et al:
Selective inhibition of STAT3 induces apoptosis and G(1) cell cycle
arrest in ALK-positive anaplastic large cell lymphoma. Oncogene.
23:5426–5434. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Niu G, Wright KL, Huang M, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|