Introduction
Colorectal cancer is the most common malignant
disease worldwide. Despite potentially curative surgery,
approximately 30% of patients develop metastases, even when
adjuvant therapies, inclusing chemotherapy and radiochemotherapy,
are administered (1). Although
adjuvant chemotherapy provides a significant survival benefit in
stage III patients, whether this treatment has any effect on
patients with stage II colon cancer remains controversial, since
20–30% of these patients eventually experience tumor relapses
(2). Adjuvant chemotherapy was
shown to increase the survival of certain populations of stage II
patients (3). Furthermore, it was
found that 60% of stage III patients did not relapse, even when
adjuvant chemotherapy was not used (4). Therefore, identifying high-risk
patients among those with stage II or III colorectal cancer will
aid in the selection of candidates for standard or intensive
adjuvant therapy.
C-reactive protein (CRP) is an acute phase reactant
that acts as a surveillance molecule for the activation of the
adaptive immune system. It is synthesized in hepatocytes and is
up-regulated by cytokines such as interleukin (IL)-6 and tumor
necrosis factor-α (33). McMillan
et al, among others, showed that elevated CRP levels are
associated with an increased risk for early recurrence and a poor
outcome following colorectal cancer surgery (5–11).
We previously reported that CRP levels reflect IL-6
production in colorectal cancer tissues and predict poor prognosis
in colorectal cancer patients, particularly in stage I or II
patients who are not usually candidates for postoperative adjuvant
chemotherapy (12,13). It is conceivable that the
pre-operative presence of an acute phase reactant adversely affects
prognosis, since IL-6, for example, acts as a potent tumor growth
factor (14).
By contrast, it has long been recognized that low
circulating albumin concentrations prior to surgery are associated
with a poor outcome in patients with colorectal cancer (15–17).
For example, Heys et al (16) showed that low concentrations of
circulating albumin before surgery and the magnitude of the
decrease were associated with poor overall survival.
Therefore, it is of interest that the combination of
hypoalbuminemia and elevated CRP levels, as in the Glasgow
prognostic score (GPS), have been shown to provide additional
prognostic information for patients with advanced cancer in various
organs, including colorectal cancer (18–23).
Furthermore, the GPS was recently validated as a prognostic score
for patients undergoing potentially curative resection for stage II
or stage III colon cancer (24).
However, associations between the GPS and adjuvant chemotherapy in
stage II or III colorectal cancer patients have yet to be
investigated.
Therefore, in this study we first examined, using
the GPS, whether the combination of an elevated CRP and
hypoalbuminemia could identify a subset of stage II or III
colorectal cancer patients with a poor prognosis who require
postoperative adjuvant therapy. Second, we evaluated whether the
use of adjuvant chemotherapy improves the survival of patients with
a poor prognosis as predicted by the GPS.
Materials and methods
Overall, 219 patients with stage II or III
colorectal cancer who received potentially curative surgery at our
institution between January 1995 and January 2005 were enrolled in
this retrospective study. Curative resection was defined as the
absence of any gross residual tumor from the surgical bed and a
surgical resection margin that was pathologically negative for
tumor invasion. Data were retrieved from operative and pathological
reports. Follow-up data were obtained from the outpatient clinical
database.
The study group comprised 136 men and 83 women aged
29–91 years (median 66; interquartile range 58–73). Staging was
principally based on the UICC/TNM classification of colorectal
cancer. Overall, 125 patients had stage II and 94 had stage III
disease. Experienced pathologists from our institution participated
in this study and verified the accuracy of the original diagnosis.
Of the 219 registered patients, 110 had tumors located in the colon
and 109 had tumors located in the rectum. The pathologic tumor
diameter indicated the maximum microscopic length of the tumor,
irrespective of the depth. Differentiated tumors were
histologically observed in 200 patients and undifferentiated tumors
in 19 patients. Lymphatic invasion was observed in 191 patients and
vascular invasion in 96 patients. After 1997, 96 patients with a
favorable performance status who gave informed consent received
adjuvant chemotherapies. Starting 4 weeks after curative surgery,
pyrimidine-fluoride-based regimens were administered for 0.5–1
years to patients classified as mainly stage III [stage II, 45/125
(36%); stage III, 51/94 (54.3%)].
The patients were followed up every 12–16 weeks for
at least 5 years according to our standard protocol, which included
tumor-marker studies, computed tomography, colorectal fiber
examinations, ultrasonography and chest radiography. Bone scans
were performed when bone metastasis was indicated. The median
follow-up time was 52.7 months (mean 56.9±63.8). The
clinicopathologic parameters studied for prognostic value were
tumor size, T classification, vessel involvement, lymphatic
invasion, lymph node metastasis and serum carcinoembryonic antigen
(CEA) concentration.
Blood samples were taken for routine laboratory
measurements of albumin and CRP before surgery. This is the
standard practice for all patients with cancer in our institution.
The coefficient of variation for these methods, over the range of
measurement, was <5%, as established by routine quality control
procedures. Patients who underwent non-elective surgery or
pre-operative radiotherapy, succumbed within 30 days of surgery or
showed clinical evidence of infection or other inflammatory
conditions, were excluded from the study.
The GPS was determined as previously described
(18). Briefly, patients with
elevated CRP levels (>1 mg/dl) plus hypoalbuminemia (<3.5
g/dl) were allocated a score of 2 (positive). Patients with only
one of these factors were allocated a score of 1 (positive).
Patients with neither of these factors were allocated a score of 0
(negative). In the present study, elevated serum CRP and lower
albumin levels were defined according to the best predictive values
calculated by receiver operating characteristic (ROC) analyses,
which found the best pair of values for highest sensitivity and
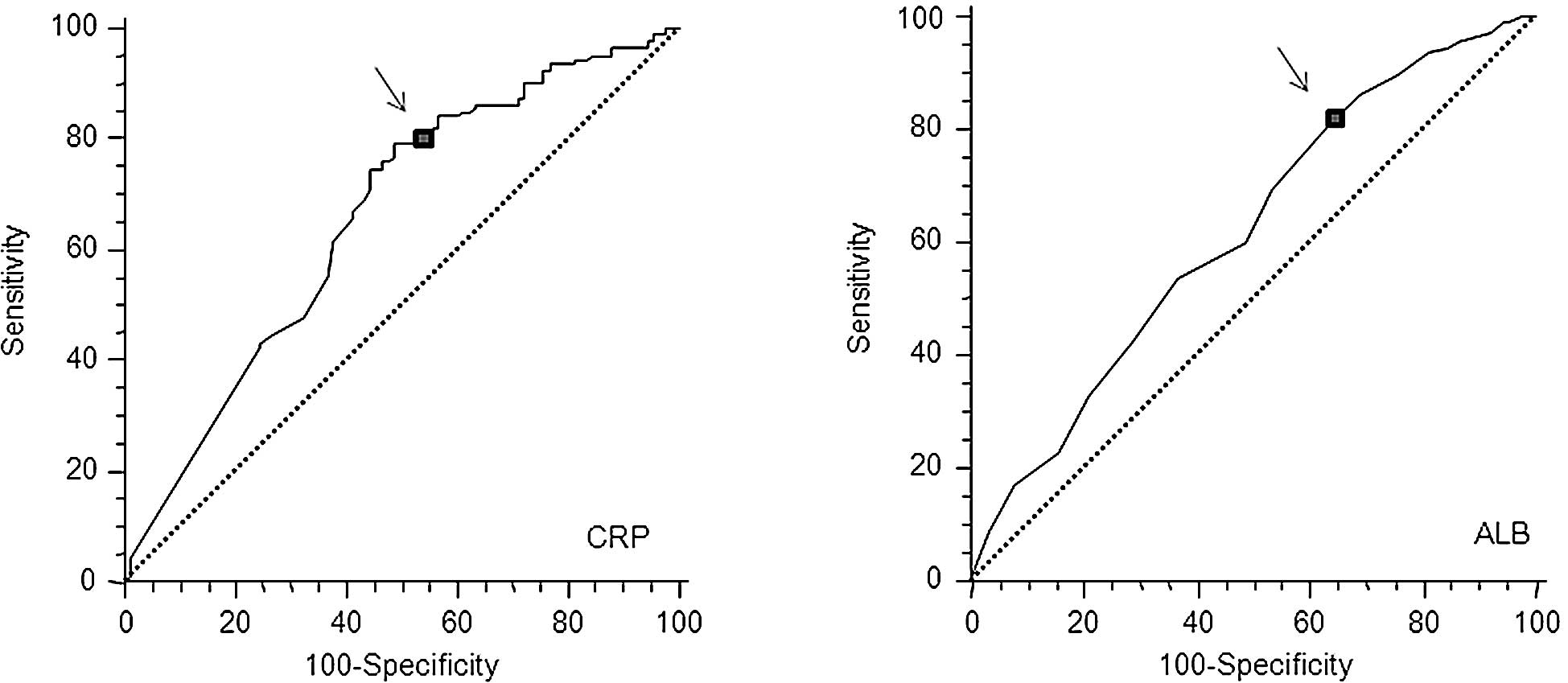
highest specificity based on the peak and cut-off points (Fig. 1A and B). Based on this analysis,
the cut-off for CRP was calculated to be 0.5 mg/dl and that for
hypoalbuminemia was unchanged (3.5 mg/dl). This modified GPS (mGPS)
was also used, and patients with elevated CRP levels (>0.5
mg/dl) and hypoalbuminemia (<3.5 g/dl) were allocated a score of
2 (mGPS 2). Patients with only one factor were allocated a score of
1 (mGPS 1) and patients with neither factor were allocated a score
of 0 (mGPS 0).
Statistical methods
Data are presented as the means ± standard deviation
(SD). Comparisons were made using the non-parametric Mann-Whitney U
test for continuous variables and the Chi-square test for
categorical data. The correlations were analyzed by Spearman’s
coefficient analysis. ROC analyses were performed to calculate the
cut-off values according to the most accurate value obtained using
Medcalc 7.2 for Windows (Mariakerke, Belgium). The survival
probabilities were calculated using the product limit method of the
Kaplan-Meier methods, considering treatment- and colorectal
cancer-related mortality. The differences between two groups were
determined using the log-rank test. The influence of each
significant predictor identified by log-rank tests was assessed by
multivariate analysis using Cox’s proportional hazards model. The
statistical analyses were carried out using StatView 5.0 (SAS
Institute Inc., Cary, NC, USA) for Windows. Two-sided p-values of
<0.5 were considered statistically significant.
Results
Association between the mGPS and the
clinicopathological characteristics of patients undergoing
potentially curative resection for colorectal cancer
During the observation period, 37 patients succumbed
to colorectal cancer. Overall, 57 patients had elevated CRP levels
(>0.5 mg/dl) and 46 had hypoalbuminemia (<3.5 mg/dl). Of the
57 patients with elevated CRP levels, 26 (45.6%) also had
hypoalbuminemia.
Table I shows the
relationship between clinicopathological characteristics and mGPS
in patients with stage II and III colorectal cancer. Gender,
vascular or lymphatic invasion, lymph node metastasis, pathological
differentiation and postoperative adjuvant chemotherapy were not
significantly associated with the mGPS classification. However,
age, serosal invasion, CEA level and TNM classification were
significantly associated with the mGPS classification.
 | Table I.Association between GPS and
clinicopathological characteristics of stage II and III patients
undergoing potentially curative resection for colon cancer. |
Table I.
Association between GPS and
clinicopathological characteristics of stage II and III patients
undergoing potentially curative resection for colon cancer.
| Patients | GPS 0 | GPS 1 | GPS 2 | p-value |
|---|
| Age (years) | | | | | 0.0080 |
| ≤64 | 95 | 72 | 17 | 6 | |
| >64 | 124 | 70 | 34 | 20 | |
| Gender | | | | | 0.9700 |
| Female | 83 | 53 | 20 | 10 | |
| Male | 136 | 89 | 31 | 16 | |
| Vascular
invasion | | | | | 0.6320 |
| No | 123 | 83 | 27 | 13 | |
| Yes | 96 | 59 | 24 | 13 | |
| Lymphatic
invasion | | | | | 0.4670 |
| No | 28 | 21 | 5 | 2 | |
| Yes | 191 | 121 | 46 | 24 | |
| Serosal invasion | | | | | 0.0040 |
| No | 139 | 100 | 29 | 10 | |
| Yes | 80 | 42 | 22 | 16 | |
| Pathology | | | | | 0.9430 |
| Diff. | 200 | 129 | 47 | 24 | |
| Non diff. | 19 | 13 | 4 | 2 | |
| TNM stage | | | | | 0.0130 |
| II | 125 | 89 | 20 | 16 | |
| III | 94 | 53 | 31 | 10 | |
| Chemotherapy | | | | | 0.5700 |
| No | 123 | 77 | 29 | 17 | |
| Yes | 96 | 65 | 22 | 9 | |
| CEA | | | | | 0.0002 |
| ≤6 | 134 | 100 | 26 | 8 | |
| >6 | 85 | 42 | 25 | 18 | |
Univariate and multivariate analyses in
relation to mortality in patients with stage II or III colorectal
cancer
The results of the univariate analysis of
postoperative mortality, using the same factors as those in
Table I, are presented in Table II. In our patients with stage II
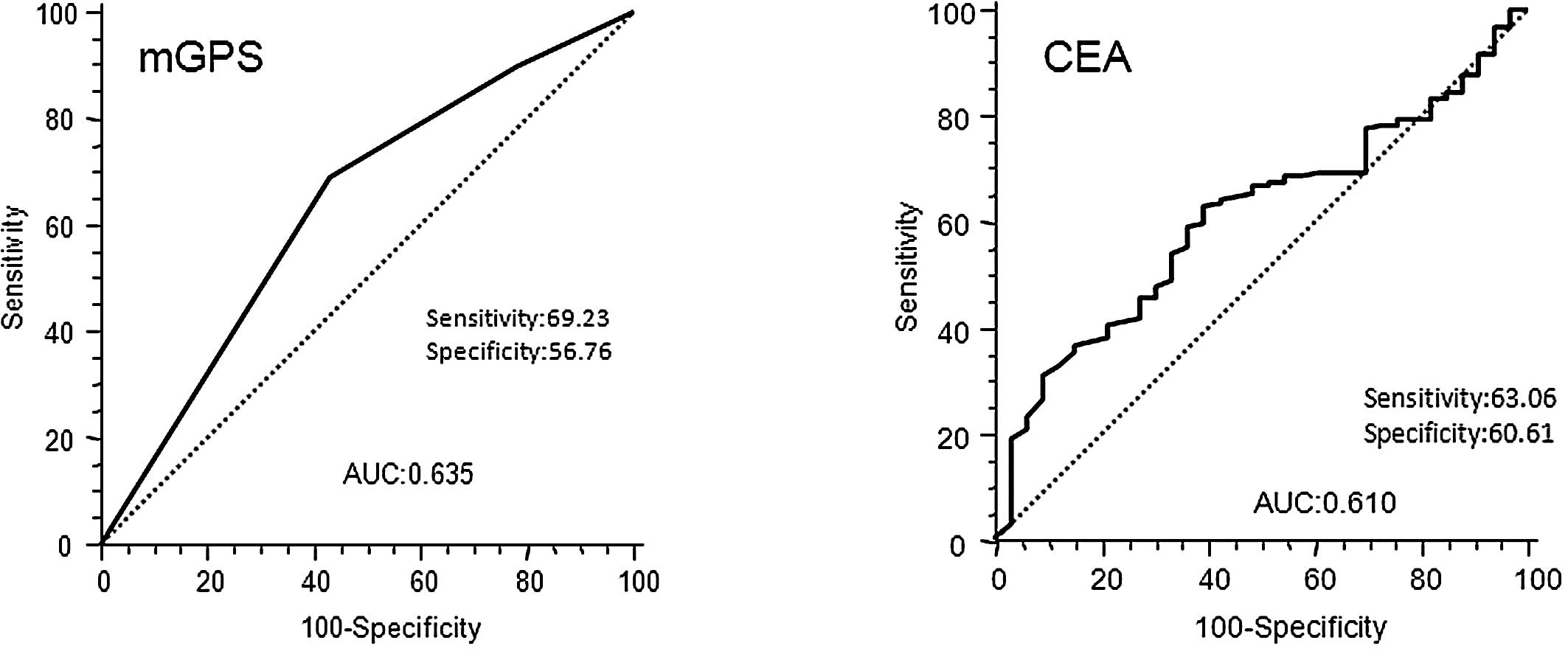
and III colorectal cancer, we defined patients with elevated mGPS
as those with mGPS 1 and mGPS 2, according to the best predictive
values calculated by ROC analyses, which found the best pair of
values for highest sensitivity (69.23%) and highest specificity
(56.76%) using a peak cut-off point (Fig. 3A). By contrast, the ROC curve in
Fig. 3B for CEA in stage II and
III colorectal cancer, showed that the best cut-off value to
predict prognosis and area under curve (AUC) of CEA was inferior to
that of mGPS (CEA 0.610; mGPS 0.635).
 | Table II.Clinicopathological characteristics
of stage II and III patients undergoing potentially curative
resection for colorectal cancer: univariate survival analysis. |
Table II.
Clinicopathological characteristics
of stage II and III patients undergoing potentially curative
resection for colorectal cancer: univariate survival analysis.
| HR | 95% CI | p-value |
|---|
| Age ( ≤64 vs.
>64) | 1.02 | 0.530-1.95 | 0.9500 |
| Gender (male vs.
female) | 1.21 | 0.610-2.44 | 0.5600 |
| Chemotherapy (yes
vs. no) | 1.04 | 0.540-2.01 | 0.9000 |
| Venous invasion
(yes vs. no) | 1.17 | 0.600-2.32 | 0.6300 |
| Lymphatic invasion
(yes vs. no) | 1.15 | 0.430-3.05 | 0.8000 |
| Lymph-node
metastasis (yes vs. no) | 1.45 | 0.760-2.86 | 0.2500 |
| Serosal invasion
(yes vs. no) | 1.99 | 1.060-4.12 | 0.0300 |
| Pathology (well vs.
poor diff.) | 0.33 | 0.046-0.58 | 0.0050 |
| CEA (≤6 vs.
>6) | 2.66 | 1.460–5.63 | 0.0020 |
| mGPS (0 vs.
1.2) | 0.33 | 0.140-0.58 | 0.0005 |
Fig. 2A and B shows
the survival curves of patients subdivided on the basis of their
mGPS (0, 1 and 2) and CEA (≤6 vs. >6 ng/ml) levels. Patients
with elevated mGPS and CEA levels had a significantly worse
prognosis than patients whose levels were below the cut-off value
(log-rank test, mGPS: p=0.0012; CEA: p=0.0023). Based on Cox’s
univariate proportional hazards analysis, serosal invasion
(p=0.03), undifferentiated tumors (poorly differentiated and
mucinous adenocarcinoma) (p=0.005), elevated serum CEA levels
(p=0.002) and elevated mGPS (mGPS1 and 2) (p=0.0005) were
significant prognostic factors for poor overall survival in
patients with stage II and III colorectal cancer (Table II). Multivariate analysis revealed
that undifferentiated tumors (p=0.013) and elevated mGPS (p=0.003)
were the only independent risk factors for predicting poor
prognosis (Table III).
 | Table III.Clinicopathological characteristics
of stage II and III patients undergoing potentially curative
resection for colorectal cancer: multivariate survival
analysis. |
Table III.
Clinicopathological characteristics
of stage II and III patients undergoing potentially curative
resection for colorectal cancer: multivariate survival
analysis.
| HR | 95% CI | p-value |
|---|
| Serosal invasion
(no vs. yes) | 0.75 | 0.38-1.47 | 0.400 |
| Pathology (poor vs.
well diff.) | 2.89 | 1.25-6.69 | 0.013 |
| CEA (≤6 vs.
>6) | 0.54 | 0.27-1.08 | 0.080 |
| mGPS (1,2 vs.
0) | 2.80 | 1.43-5.49 | 0.003 |
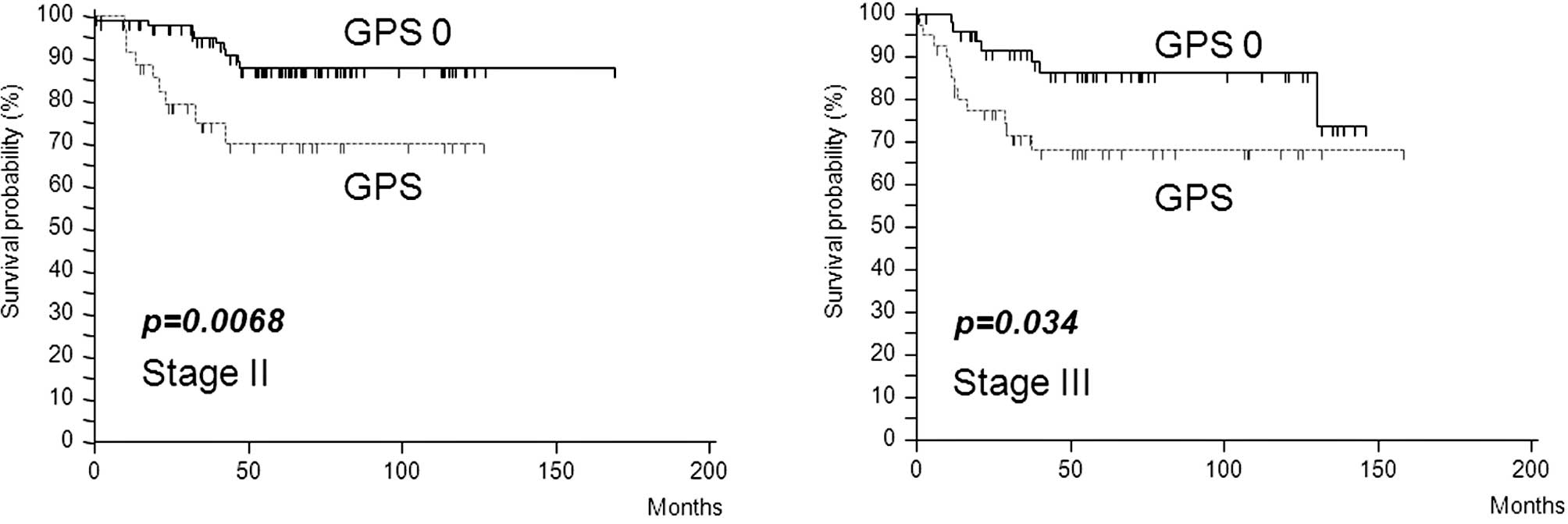
Furthermore, in stage II or III colorectal cancer,
elevated mGPS was associated with poor survival (stage II,
p=0.0068; stage III, p=0.034) (Fig. 4A
and B) and was the only independent prognostic factor in stage
II patients (p=0.005) (Table
IV).
 | Table IV.Clinicopathological characteristics
and cancer-specific survival in patients undergoing potentially
curative resection for stage II colorectal cancer: multivariate
survival analysis. |
Table IV.
Clinicopathological characteristics
and cancer-specific survival in patients undergoing potentially
curative resection for stage II colorectal cancer: multivariate
survival analysis.
| HR | 95% CI | p-value |
|---|
| Age (≤64 vs.
>64) | 0.56 | 0.19-1.70 | 0.310 |
| Gender (male vs.
female) | 0.56 | 0.17-1.81 | 0.330 |
| Chemotherapy (yes
vs. no) | 1.52 | 0.48-4.82 | 0.480 |
| Venous invasion
(yes vs. no) | 0.64 | 0.19-2.11 | 0.470 |
| Lymphatic invasion
(yes vs. no) | 2.02 | 0.45-8.18 | 0.330 |
| Serosal invasion
(yes vs. no) | 0.93 | 0.33-2.63 | 0.890 |
| Pathology (well vs.
poor diff.) | 7.61 | 1.79-32.44 | 0.006 |
| CEA (≤6 vs.
>6) | 0.40 | 0.14-1.17 | 0.090 |
| mGPS (0 vs.
1,2) | 5.01 | 1.60-15.69 | 0.005 |
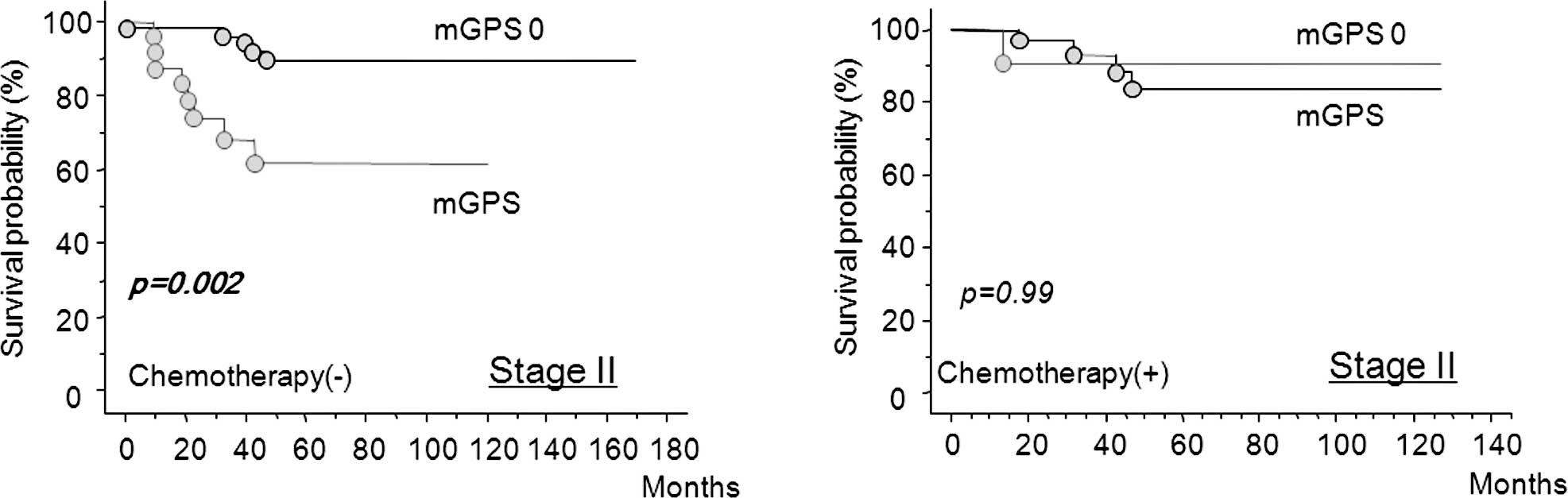
Evaluation of whether adjuvant
chemotherapy improves the survival of patients with poor prognosis
with stage II colorectal cancer as predicted by mGPS
Fig. 5 shows that
in stage II colorectal cancer, mGPS-positive patients had a
significantly worse prognosis compared to mGPS-negative patients
when adjuvant chemotherapy was not used (Fig. 5A). By contrast, chemotherapy
improved cancer-specific survival, even in the mGPS-positive
patients (Fig. 5B).
Discussion
The TNM staging system provides the most reliable
information on prognosis and aids in the discrimination of patients
with early stage disease from those with advanced stage disease.
However, it is less accurate for predicting the prognosis of
patients with an intermediate extent of tumor invasion. CEA is a
complex glycoprotein that is up-regulated in approximately 90% of
advanced colorectal cancers and contributes to the malignant
characteristics of tumors (25).
However, it is not useful in detecting asymptomatic cancer, as the
sensitivity of CEA determination for early colorectal cancer is as
low as 30–40% (26). Moreover, CEA
is not significantly associated with survival in patients with
stage I and II lesions, and CEA testing is relatively insensitive
to tumors with local or peritoneal involvement (27). Therefore, the identification of
sensitive prognostic markers in this subgroup will allow for the
use of postoperative adjuvant therapy in a subset of patients with
poor prognosis and improve survival.
Recently, the combination of hypoalbuminemia and
elevated CRP levels (>1.0 mg/dl) (original GPS) has been shown
to provide additional prognostic information for patients with
curative or advanced colorectal cancer. In the present study, we
defined that the cut-off value of CRP was 0.5 mg/dl according to
the best predictive values calculated by ROC analyses (Fig. 1A). Furthermore, the classification
of CRP using a cut-off value of 0.5 mg/dl discriminates high-risk
patients more clearly than using 1.0 mg/dl. CRP serum levels were
measured by turbidimetric immunoassays using an N-Assay TIA CRP-S
kit (Nittobo Medical, Tokyo, Japan). Since the limit of detection
of this CRP assay was lower than that in other studies (0.2 vs.
>0.5 mg/dl) (7,8), the cut-off value for abnormal
elevation of serum CRP was set at 0.5 mg/dl.
This study aimed to determine whether the mGPS
provides more accurate prognostic information than that offered by
existing staging systems or tumor markers, such as CEA, in stage
II/III colorectal cancer patients. In fact, we showed that mGPS was
significantly associated with serosal invasion, pre-operative CEA
level and TNM classification, which are established conventional
prognostic factors. Furthermore, mGPS-positive was found to have
independent prognostic value, whereas the prognostic values of CEA
or TNM classification were influenced by other clinical
factors.
Recent studies on various types of malignancies have
emphasized the importance of examining multiple lymph nodes in
determining prognosis. In colon and rectal cancer, staging accuracy
and survival are improved by increasing the number of nodes
examined and analyzed (28–30).
In addition, the failure to examine a sufficient number of lymph
nodes may result in the inability to identify patients in whom
lymph nodes are affected by cancer, thus resulting in understaging
(31). However, the number of
lymph nodes reported with colectomy varies widely and may be due to
variations in surgical technique, the thoroughness of the
pathologist in finding nodes in the specimen, or the actual number
of regional lymph nodes. Our study showed that high mGPS was the
strongest prognostic factor for stage II and III colorectal cancer,
rather than lymph node metastasis, which is routinely addressed for
postoperative adjuvant chemotherapy. Our results suggest that an
objective evaluation method using the mGPS will identify candidates
for postoperative chemotherapy to improve poor prognosis revealed
by pathological staging.
Surgical resection is highly effective for stage II
colorectal cancer, but a significant proportion of these patients
(25–30%) develop recurrence and succumb to the disease. Therefore,
identifying sensitive prognostic markers in this subgroup of
patients would prompt the use of postoperative adjuvant in these
patients with poor prognosis and thus improve survival. In the
present study, we demonstrated that pre-operative mGPS was an
independent prognostic factor for patients with stage II colorectal
cancer. This ability to identify patients with stage II colorectal
cancer with a poor prognosis and who would benefit from adjuvant
therapy to prevent recurrence could improve cancer survival. In
fact, mGPS-positive patients showed a significantly worse prognosis
compared to mGPS-negative patients when adjuvant chemotherapy was
not used, while cancer-specific survival was improved in the
mGPS-positive patients with the worst prognosis when adjuvant
chemotherapy was used.
The mechanism of CRP up-regulation is controlled by
cytokines, including IL-8, IL-6 and tumor necrosis factor-α
(32). We previously investigated
host-tumor interactions in patients with colorectal cancer focusing
on the defective immunoinflammatory adaptation system against
intrinsic IL-6. We reported that an increased serum level of CRP in
patients with colorectal cancer reflected, not only enhanced tumor
expression of IL-6, but also activation of the interaction between
IL-6 and the IL-6 receptor in tumor cells, which maintained an
active proliferative state (12,33,34).
In addition, the systemic antagonistic response against circulating
IL-6 derived from the tumor component was found to be suppressed in
malnourished cancer patients, which in turn up-regulated
IL-6-related systemic induction of CRP (35). IL-6 exerts its action on target
cells by acting through a receptor complex consisting of the IL-6
soluble receptor (sR) and a signal-transducing subunit (gp130).
IL-6sR/IL-6 complexes play a positive role in local inflammatory
reactions by activating cells through membrane-bound gp130. In our
previous study, surgery-induced stress increased the formation of
IL-6sR/ IL-6 complexes in the operative field, which systemically
enhanced IL-6 activity in malnourished colorectal cancer patients
(36). Since CRP is known to
promote a loss of the membrane-bound IL-6 receptor by proteolytic
shedding resulting in a 3-fold increase in IL-6sR production
(37), CRP may then up-regulate
IL-6-mediated inflammatory events by enabling the formation of the
IL-6sR/IL-6 complex in patients with colorectal cancer.
Taken together, the results of the previous and
present studies suggest that CRP is more than just an indicator of
tumor burden in certain patients with colorectal cancer, in whom
tumor burden and a deteriorated host-tumor interaction may
synergistically elevate systemic induction of CRP. Elevated CRP
levels may then enable the systemic formation of the IL-6sR/IL-6
complex and up-regulate IL-6-mediated tumor growth factors. The
subsequent autocrine/paracrine stimulation of residual tumor cells
protects cells from apoptosis and regrowth.
In conclusion, pre-operative mGPS levels may provide
valuable prognostic information in patients with stage II and III
colorectal cancer, and is independent of the CEA test or
pathological N classification system. Accordingly, the mGPS may
provide valuable information concerning specific subgroups of
patients who might benefit from adjuvant chemotherapy for stage II
colorectal cancer. However, further studies are required to confirm
our results.
References
|
1.
|
Haller DG: An overview of adjuvant therapy
for colorectal cancer. Eur J Cancer. 31A:1255–1263. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wein A, Hahn EG, Merkel S and Hohenberger
W: Adjuvant chemotherapy for stage II colon cancer. Eur J Surg
Oncol. 26:730–732. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Figueredo A, Charette ML, Maroun J,
Brouwers MC and Zuraw L: Adjuvant therapy for stage II colon
cancer: a systematic review from the Cancer Care Ontario Program in
evidence-based care’s gastrointestinal cancer disease site group. J
Clin Oncol. 22:3395–3407. 2004.
|
|
4.
|
Gill S, Loprinzi CL, Sargent DJ, et al:
Pooled analysis of fluorouracil-based adjuvant therapy for stage II
and III colon cancer: Who benefits and by how much? J Clin Oncol.
22:1797–1806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Canna K, McMillan DC, McKee RF, McNicol
AM, Horgan PG and McArdle CS: Evaluation of a cumulative prognostic
score based on the systemic inflammatory response in patients
undergoing potentially curative surgery for colorectal cancer. Br J
Cancer. 90:1707–1709. 2004.
|
|
6.
|
Nozoe T, Matsumata T, Kitamura M and
Sugimachi K: Significance of preoperative elevation in serum
C-reactive protein as an indicator for prognosis in colorectal
cancer. Am J Surg. 176:335–338. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
McMillan DC, Canna K and McArdle CS:
Systemic inflammatory response predicts survival following curative
resection for colorectal cancer. Br J Surg. 90:215–219. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Crozier JE, McKee RF, McArdle CS, et al:
The presence of a systemic inflammatory response predicts poorer
survival in patients receiving adjuvant 5-FU chemotherapy following
potentially curative resection for colorectal cancer. Br J Cancer.
94:1833–1836. 2006. View Article : Google Scholar
|
|
9.
|
McMillan DC, Wotherspoon HA, Fearon KC,
Sturgeon C, Cooke TG and McArdle CS: A prospective study of tumor
recurrence and the acute-phase response after apparently curative
colorectal cancer surgery. Am J Surg. 170:319–322. 1995. View Article : Google Scholar
|
|
10.
|
Nozoe T, Matsumata T and Sugimachi K:
Preoperative elevation of serum C-reactive protein is related to
impaired immunity in patients with colorectal cancer. Am J Clin
Oncol. 23:263–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gunter MJ, Stolzenberg-Solomon R, Cross
AJ, et al: A prospective study of serum C-reactive protein and
colorectal cancer risk in men. Cancer Res. 66:2483–2487. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Miki C, Konishi N, Ojima E, Hatada T,
Inoue Y and Kusunoki M: C-reactive protein as a prognostic variable
that reflects uncontrolled up-regulation of the IL-1-IL-6 network
system in colorectal carcinoma. Dig Dis Sci. 49:970–976. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Koike Y, Miki C, Okugawa Y, et al:
Preoperative C-reactive protein as a prognostic and therapeutic
marker for colorectal cancer. J Surg Oncol. 98:540–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kawano M, Hirano T, Matsuda T, et al:
Autocrine generation and requirement of BSF-2/IL-6 for human
multiple myelomas. Nature. 332:83–85. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Longo WE, Virgo KS, Johnson FE, et al:
Outcome after proctectomy for rectal cancer in Department of
Veterans Affairs Hospitals: a report from the National Surgical
Quality Improvement Program. Ann Surg. 228:64–70. 1998. View Article : Google Scholar
|
|
16.
|
Heys SD, Walker LG, Deehan DJ and Eremin
OE: Serum albumin: a prognostic indicator in patients with
colorectal cancer. J R Coll Surg Edinb. 43:163–168. 1998.PubMed/NCBI
|
|
17.
|
Longo WE, Virgo KS, Johnson FE, et al:
Risk factors for morbidity and mortality after colectomy for colon
cancer. Dis Colon Rectum. 43:83–91. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ, Dagg K and Scott HR: A prospective longitudinal study
of performance status, an inflammation-based score (GPS) and
survival in patients with inoperable non-small-cell lung cancer. Br
J Cancer. 92:1834–1836. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Al Murri AM, Bartlett JM, Canney PA,
Doughty JC, Wilson C and McMillan DC: Evaluation of an
inflammation-based prognostic score (GPS) in patients with
metastatic breast cancer. Br J Cancer. 94:227–230. 2006.PubMed/NCBI
|
|
21.
|
Crumley AB, McMillan DC, McKernan M,
McDonald AC and Stuart RC: Evaluation of an inflammation-based
prognostic score in patients with inoperable gastro-oesophageal
cancer. Br J Cancer. 94:637–641. 2006.PubMed/NCBI
|
|
22.
|
Glen P, Jamieson NB, McMillan DC, Carter
R, Imrie CW and McKay CJ: Evaluation of an inflammation-based
prognostic score in patients with inoperable pancreatic cancer.
Pancreatology. 6:450–453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ishizuka M, Nagata H, Takagi K, Horie T
and Kubota K: Inflammation-based prognostic score is a novel
predictor of postoperative outcome in patients with colorectal
cancer. Ann Surg. 246:1047–1051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Roxburgh CS, Crozier JE, Maxwell F, et al:
Comparison of tumour-based (Petersen Index) and inflammation-based
(Glasgow Prognostic Score) scoring systems in patients undergoing
curative resection for colon cancer. Br J Cancer. 100:701–706.
2009. View Article : Google Scholar
|
|
25.
|
Wiggers T, Arends JW, Schutte B, Volovics
L and Bosman FT: A multivariate analysis of pathologic prognostic
indicators in large bowel cancer. Cancer. 61:386–395. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Fletcher RH: Carcinoembryonic antigen. Ann
Int Med. 104:66–73. 1986. View Article : Google Scholar
|
|
27.
|
Moertel CG, Fleming TR, Macdonald JS,
Haller DG, Laurie JA and Tangen C: An evaluation of the
carcinoembryonic antigen (CEA) test for monitoring patients with
resected colon cancer. JAMA. 270:943–947. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tepper JE, O’Connell MJ, Niedzwiecki D, et
al: Impact of number of nodes retrieved on outcome in patients with
rectal cancer. J Clin Oncol. 19:157–163. 2001.PubMed/NCBI
|
|
29.
|
Joseph NE, Sigurdson ER, Hanlon AL, et al:
Accuracy of determining nodal negativity in colorectal cancer on
the basis of the number of nodes retrieved on resection. Ann Surg
Oncol. 10:213–218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Joseph NE, Sigurdson ER, Hanlon AL, et al:
Colon cancer survival is associated with increasing number of lymph
nodes analyzed: A secondary survey of intergroup trial INT-0089. J
Clin Oncol. 21:2912–2919. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Swanson RS, Compton CC, Stewart AK and
Bland KI: The prognosis of T3N0 colon cancer is dependent on the
number of lymph nodes examined. Ann Surg Oncol. 10:65–71. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Nakazaki H: Preoperative and postoperative
cytokines in patients with cancer. Cancer. 70:709–713. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Wakuda R, Miki C and Kusunoki M:
Autoreactivity against interleukin 6 as a risk factor in elderly
patients with colorectal carcinoma. Arch Surg. 136:1274–1279. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Kinoshita T, Ito H and Miki C: Serum
interleukin-6 level reflects the tumor proliferative activity in
patients with colorectal carcinoma. Cancer. 85:2526–2531. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Miki C, Inoue Y, Toiyama Y, et al:
Deficiency in systemic interleukin-1 receptor antagonist production
as an operative risk factor in malnourished elderly patients with
colorectal carcinoma. Crit Care Med. 33:177–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Hatada T and Miki C: Nutritional status
and postoperative cytokine reponse in colorectal cancer patients.
Cytokine. 12:1331–1336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Jones SA, Novick D, Horiuchi S, Yamamoto
N, Szalai AJ and Fuller GM: C-reactive protein: a physiological
activator of interleukin 6 receptor shedding. J Exp Med.
189:599–604. 1999. View Article : Google Scholar : PubMed/NCBI
|