Introduction
The extracellular matrix (ECM) is considered to play
an crucial role in the stability of tissues and in regulating the
growth and differentiation of cells (1,2).
Synthesis, accumulation and catabolism of the ECM occur during
wound healing and during the initiation and progression of numerous
diseases (3).
Moreover, it is generally acknowledged that the ECM
does not function as a passive scaffold for connective tissue
within the organ architecture, but also plays an ‘informational’
role through a network of interactions between cells and signal
molecules. This role is crucial in the control of cellular
proliferation and motility during histogenesis for maintenance of
tissue homeostasis and in cancer development.
Uterine leiomyomata, or fibroids, are the most
common pelvic tumors in women of reproductive age. Despite their
prevalence and impact on normal reproductive and menstrual
function, little is understood regarding their basic biology and
growth. Uterine leiomyoma contains abundant quantities of ECM
(4–7). However, the proteins comprising the
ECM and the regulation of their expression have yet to be
characterized. The study of ECM of uterine leiomyomata and normal
myometrium is key in the elucidation of the growth of these
neoplasms. Leiomyomata exhibit a low mitotic index, yet undergo
rapid growth and, conversely, a rapid decrease in size upon GnRH
agonist treatment (8). A
significant component of this growth and/or regression may be
mediated by changes in the composition and content of the ECM.
Therefore, the precise control of ECM metabolism in leiomyomata and
normal myometrium is critical for the pathology and development of
uterine leiomyomata.
In the present study, the expression of various
types of collagen, a major component of ECM, was investigated in
human uterine leiomyoma and normal myometrium tissues by
immunofluorescent staining. The results were compared to normal
myometrium obtained throughout the menstrual cycle.
Materials and methods
This study was approved by the Committee on
Investigations Involving Human Subjects of Wakayama Medical
College. Informed consent was obtained from each subject after the
purpose and nature of the study had been fully explained.
Tissues
Leiomyomata and matched myometrium were processed
for immunohistochemistry and SDS-PAGE. The tissues were obtained
from 40 pre-menopausal women (29–53 years of age) who were
undergoing abdominal hysterectomy for symptomatic uterine
leiomyomata at various stages of the menstrual cycle. None of the
patients received any hormone therapy prior to surgery. The stage
of the menstrual cycle was determined by histological dating of the
endometrium for all secretory phase samples. Proliferative phase
samples were dated by either dating the endometrium or determining
the date of the last menstrual period. The leiomyoma and
corresponding myometrium specimens from the proliferative (n=20)
and secretory (n=20) phase were studied. No submucosal leiomyomata
were collected so as to avoid possible contamination with
endometrium. The leiomyomata and myometrium tissues were
immediately frozen in liquid nitrogen.
Primary antibodies
Monoclonal antibodies (mAbs) against each α1 chain
of human type I, III and IV collagen were used. Preparation of the
antibodies has been previously described (9). In brief, BALB/C mice were immunized
with each type of collagen, after it had been extracted from human
placentas. The spleen cells of the mice were then hybridized with
myeloma cells. Following hypoxanthine-aminopterinethymidine (HAT)
selection, positive hybrids were identified using an enzyme-linked
immunosorbent assay. The specificity of each antibody was
determined using immunoblotting or inhibition in an enzyme-linked
immunosorbent assay. No cross-reaction was observed among the
antibodies.
Immunohistochemistry
Immunohistochemical analysis was performed using the
standard indirect immunofluorescence method. In brief, 3-μm
frozen sections were rehydrated in phosphate-buffered saline (PBS)
at room temperature and then incubated with the primary antibody
(diluted 1:100 in PBS) for 12 h at 4°C in a humidified chamber.
Following incubation, the sections were washed twice in PBS for 3
min. Each section was then incubated for 1 h at room temperature
with human plasma-preabsorbed, fluorescein
isothiocyanate-conjugated goat antibodies against mouse
immunoglobulins diluted 1:100 in PBS (Organon Teknik, Co., West
Chester, PA, USA). Subsequently, the sections were washed again in
PBS, mounted in buffered glycerol and examined under a fluorescence
microscope (Olympus Co., Tokyo, Japan).
Results
The control sections were stained with goat
antibodies against mouse immunoglobulin G without prior application
of the appropriate primary antibody (Figs. 1A, 2A and 3A). When the mAbs were first allowed to
react with an excess of each specific type of collagen, no
immunostaining was observed.
The intensity and distribution of
immunohistochemistry for type I, III and IV collagen in the
myometrium and leiomyoma samples showed no significant difference
between the proliferative and secretory phases, respectively.
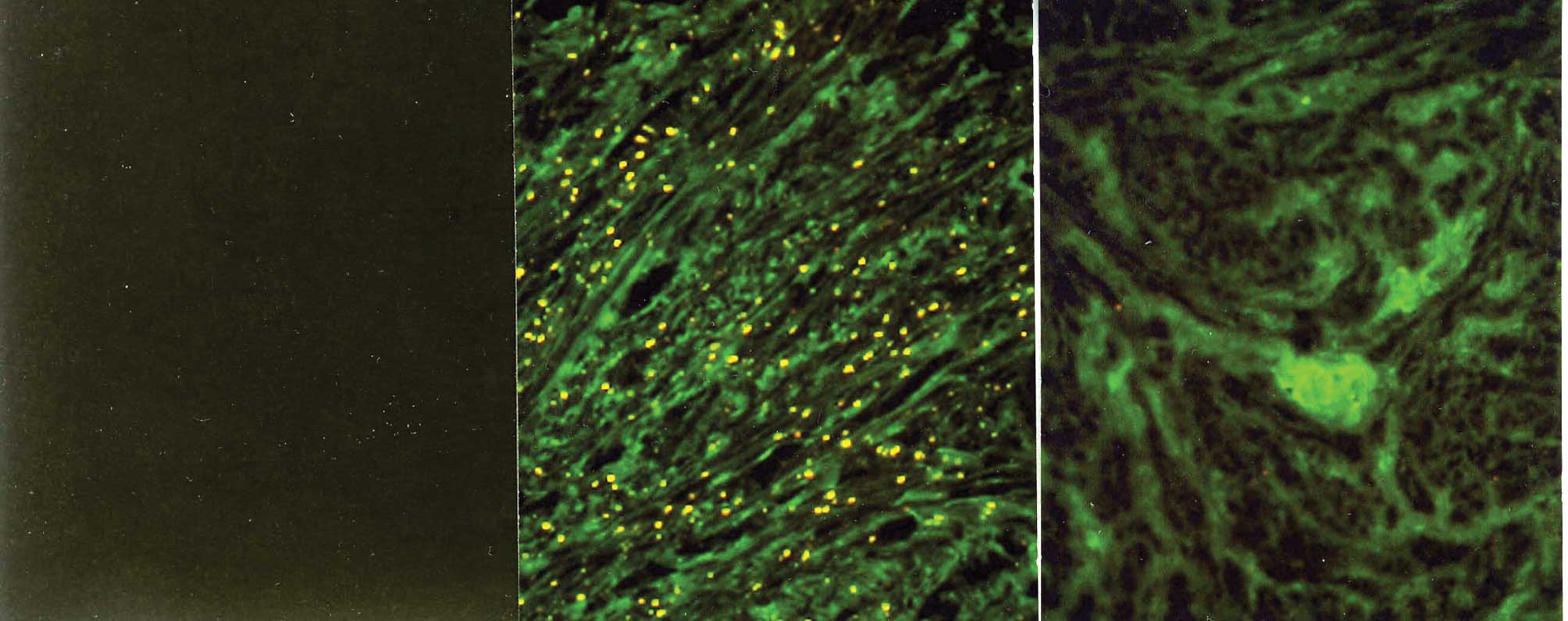
Immunostaining with the mAbs against type I and III
collagen showed a fibrillary pattern in the ECM of the myometrium
and the leiomyomata (Figs. 1B and
C and 2B and C).
Immunostaining for type I collagen showed an intense and
accumulated pattern in much larger areas of the ECM in the
leiomyomata as compared to the normal myometrium (Fig. 1B and C). However, immunostaining
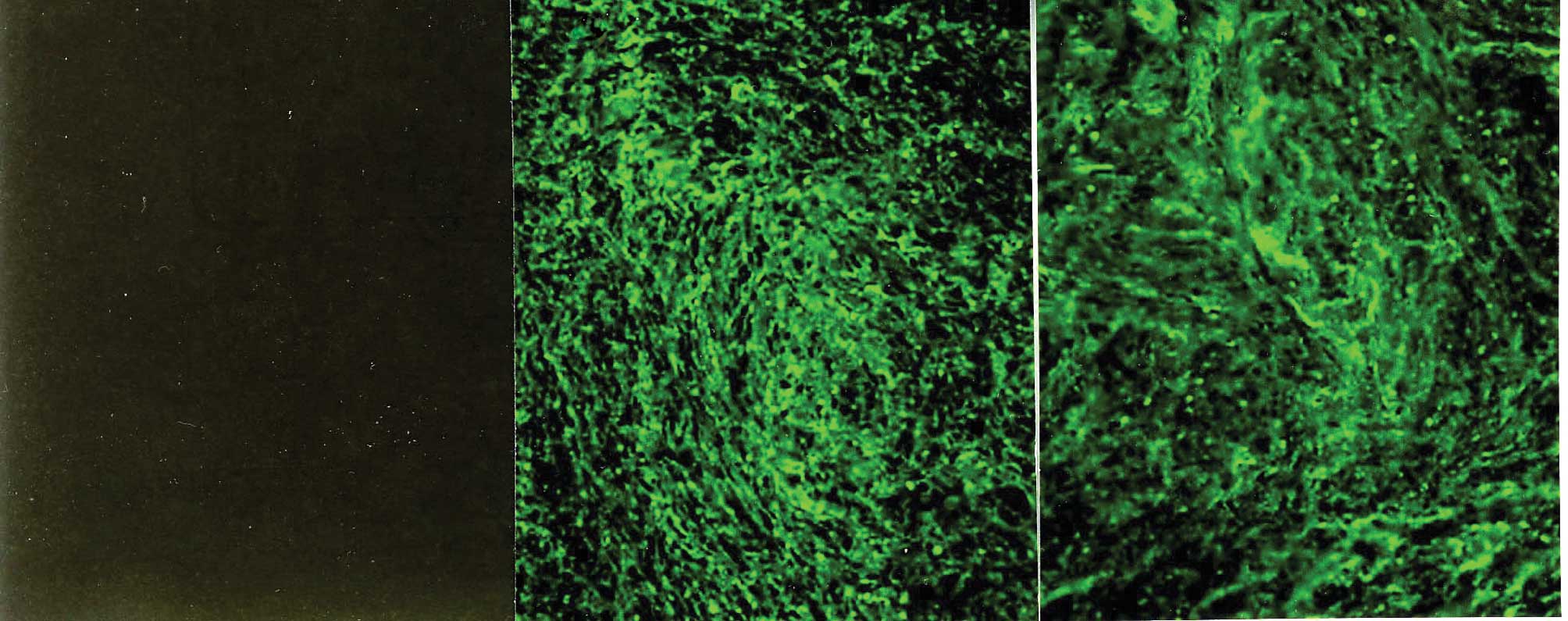
for type III collagen showed a similarly intense and
microfibrillary pattern in the ECM of the leiomyomata and the
normal myometrium (Fig. 2B and
C).
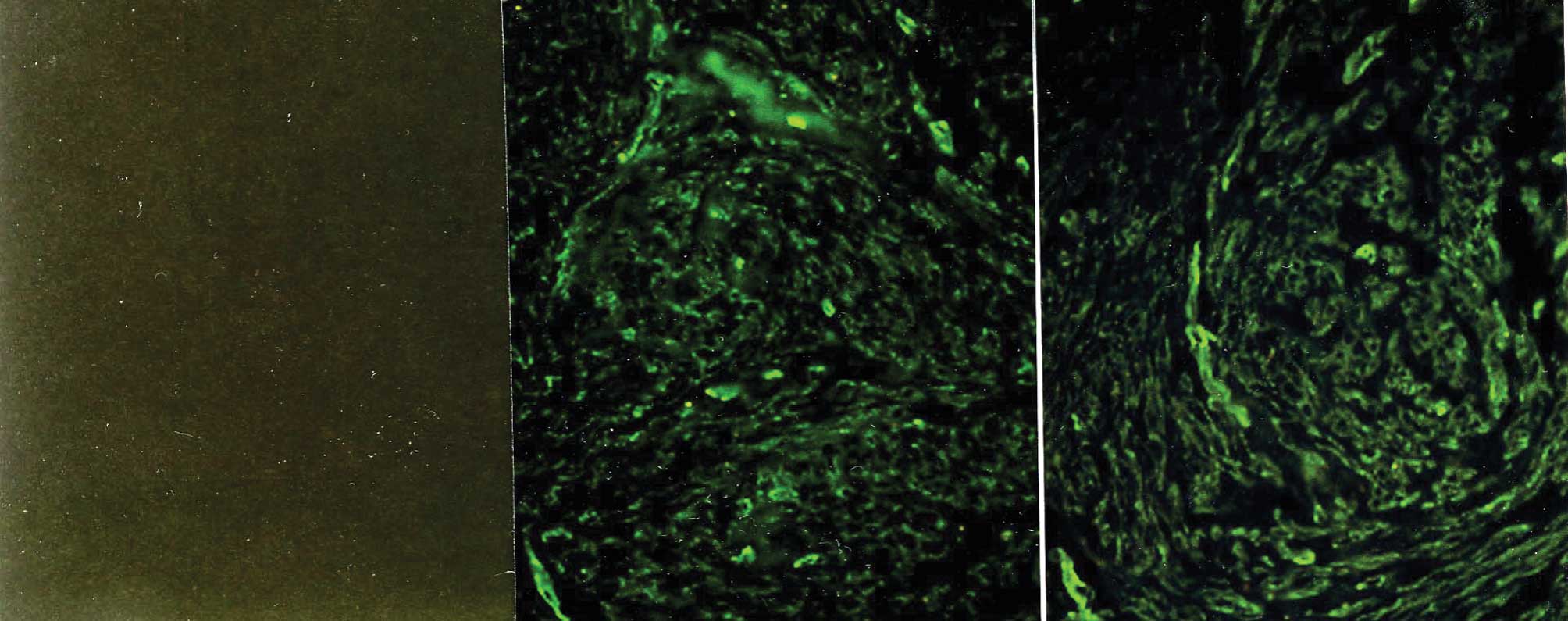
Immunostaining with the mAbs against type IV
collagen showed positive staining of a similar intensity in the
myometrium and the leiomyomata (Fig.
3) throughout the menstrual cycle. In the leiomyomata, type IV
collagen was localized in the ECM-embedded bundles of smooth muscle
cells, whereas areas of ECM accumulation were negative for this
collagen (Fig. 3C). Similarly, in
the myometrial samples, type IV collagen surrounded smooth muscle
cell bundles, but did not contribute to the formation of larger
septa separating the muscle fibers. The intensity of staining of
the myometrium and leiomyomata by each of the antibodies against
various types of collagen was subjectively graded from 1+ to 3+,
and the results are summarized in Table I. Although the immunostaining did
not allow an accurate quantitative comparison of ECM proteins
between the leiomyomata and myometrium, our results suggest that
the leiomyomata contained more type I collagen than the normal
myometrium due to the larger areas of ECM. There appeared to be no
variation in the staining of these collagen types in tissues
obtained at different times throughout the menstrual cycle.
 | Table I.Immunostaining of human uterine
myometrium and myoma with antibodies for type I, III and IV
collagens. |
Table I.
Immunostaining of human uterine
myometrium and myoma with antibodies for type I, III and IV
collagens.
| No. of patients | Collagen type
|
|---|
| I | III | IV |
|---|
| Myometrium | 20 | ++ | ++ | + |
| Myoma uteri | 20 | +++ | ++ | + |
Discussion
In the present study, we investigated changes in the
composition of the ECM, including type I, III and IV collagens, in
human leiomyomata and normal myometrium tissues obtained throughout
the menstrual cycle.
Although type I and III collagens are commonly found
in combination, our present study showed that the ratios of type I
collagen in the tissues of leiomyomata were significantly higher
than those in the normal myometrium throughout the menstrual cycle.
However, immunostaining for type III and IV collagen demonstrated a
similar pattern in the leiomyomata and the normal myometrium
throughout the menstrual cycle. Thus, type I collagen deposition
may result in the fibrotic nature of leiomyomata, so called
‘fibroids’. Type I collagen has been demonstrated in the human skin
(10) and in human atherosclerosis
(11,12). One possible cause is an increase in
the density of cells in the leiomyoma tissues.
Cell density-dependent effects have been previously
reported in various types of cell, including mesangial (13,14),
endothelial (15,16), vascular smooth muscle (17,18),
fibroblasts (19,20) and primitive mesenchymal cells
(21). It has been suggested that
cell density may modulate biological behavior, with changes in
signal-transduction responses to hormonal stimulation in growth, in
the synthesis and composition of the ECM, and in the synthesis of
specific proteins (13,14). Therefore, our findings suggest that
leiomyomata exhibit an alteration in cell density or proliferation
of their cells and that accumulation of ECM, particularly increased
type I collagen in leiomyoma tissues, may play a key role in
biological behavior in terms of the reaction to ovarian hormones,
such as estrogen and progesterone, as well as cytokines and growth
factors, such as TGF-β (22–24).
Moreover, type I collagen has been shown to mediate
cell behavior, including attachment, spread, proliferation and
morphogenesis (25–28). Therefore, it is suggested that
increased relative levels of type I collagen in leiomyomata provide
a biochemical basis for the functional regulation of leiomyoma
cells as compared to normal myometrium.
In conclusion, an increased level of the protein
expression of type I collagen was found in the uterine leiomyomata
as compared to that in the normal myometrium throughout the
menstrual cycle. Therefore, changes in the composition of
collagens, which modulate the expression of various cytokines and
growth factors, result in the morphologic and functional
characteristics of uterine leiomyomata. This study enhanced the
understanding of the pathophysiology of uterine leiomyomata in
terms of ECM metabolism. Further research is required to elucidate
the mechanisms which regulate the expression of genes of other
types of collagen in uterine leiomyomata throughout the menstrual
cycle and during hormone therapy.
References
|
1.
|
Lin CQ and Bissell MJ: Multi-faceted
regulation of cell differentiation by extracellular matrix. FASEB
J. 7:737–743. 1993.PubMed/NCBI
|
|
2.
|
Madri JA and Basson MD: Extracellular
matrix-cell interactions: dynamic modulators of cell, tissue and
organism structure and function. Lab Invest. 66:519–521.
1992.PubMed/NCBI
|
|
3.
|
Haralson MA: Extracellular matrix and
growth factors: an integrated interplay controlling tissue repair
and progression to disease. Lab Invest. 69:369–372. 1993.PubMed/NCBI
|
|
4.
|
Ferency A, Richart RM and Okazaki T: A
comparative ultra-structural study of leiomyosarcoma, cellular
leiomyoma, and leiomyoma of the uterus. Cancer. 28:1004–1018. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Srewart AE, Friedman AJ, Peck K and Nowak
RA: Relative overexpression of collagen type I and collagen III
messenger ribonucleic acids by uterine leiomyoma during the
proliferative phase of the menstrual cycle. J Clin Endocrinol
Metab. 79:900–906. 1994.
|
|
6.
|
Catherino WH, Leppert PC, Stenmark MH, et
al: Reduced dermatopontin expression is a molecular link between
uterine leiomyomas and keloids. Genes Chromosomes Cancer.
40:204–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Leppert PC, Baginski T, Prupas C,
Catherino WH, Pletcher S and Segars JH: Comparative ultrastructure
of collagen fibrils in uterine leiomyomas and normal myometrium.
Fertil Steril. 82(Suppl 3): 1182–1187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chavez NF and Stewart EA: Medical
treatment of uterine fibroids. Clin Obstet Gynecol. 44:372–384.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ooshima A and Muragaki Y: Collagen
metabolism in atherogenesis. Ann NY Acad Sci. 598:582–584. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
McCullagh KA and Balian G: Collagen
characterisation and cell transformation in human atherosclerosis.
Nature. 258:73–75. 1975. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ooshima A: Collagen α B chain: increased
proportion in human atherosclerosis. Science. 213:666–668.
1981.
|
|
13.
|
Ishimura E, Sterzel RB, Budde K and
Kashgarian M: Formation of extracellular matrix by cultured rat
mesangial cells. Am J Pathol. 134:843–855. 1989.PubMed/NCBI
|
|
14.
|
Worthuis A, Boes A and Grond J: Cell
density modulates growth, extracellular matrix, and protein
synthesis of cultured rat mesangial cells. Am J Pathol.
143:1209–1219. 1993.PubMed/NCBI
|
|
15.
|
Patton WF, Yoon MU, Alexander JS, et al:
Expression of simple epithelial cytokeratins in bovine pulmonary
microvascular endothelial cells. J Cell Physiol. 143:140–149. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Orpana A, Ranta V, Mikkola T, Viinikka L
and Ylikorkala O: Inducible nitric oxide and prostacyclin
productions are differently controlled by extracellular matrix and
cell density in human vascular endothelial cells. J Cell Biochem.
64:538–546. 1997. View Article : Google Scholar
|
|
17.
|
Goodman LV and Majack RA: Vascular smooth
muscle cells express distinct transforming growth factor-β receptor
phenotypes as a function of cell density in culture. J Biol Chem.
264:5241–5244. 1989.
|
|
18.
|
Kato S, Shanley JR and Fox JC: Serum
stimulation, cell-cell interactions, and extracellular matrix
independently influence smooth muscle cell phenotype in vitro. Am J
Pathol. 149:687–697. 1996.PubMed/NCBI
|
|
19.
|
Ellis IR and Schor SL: Differential
effects of TGF-beta 1 on hyaluronan synthesis by fetal and adult
skin fibroblasts: implications for cell migration and wound
healing. Exp Cell Res. 228:326–333. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Brenn T, Aoyama T, Francke U and Furthmayr
H: Dermal fibroblast culture as a model system for studies of
fibrillin assembly and pathogenetic mechanisms: defects in distinct
groups of individuals with Marfan's syndrome. Lab Invest.
75:389–402. 1996.PubMed/NCBI
|
|
21.
|
Tsonis PA and Goetinck PK: Cell density
effects of a tumor promotor on proliferation and chondrogenesis of
limb bud mesenchymal cells. Exp Cell Res. 190:247–253. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tang XM, Dou Q, Zhao Y, McLean F, Davis J
and Chegini N: The expression of transforming growth factor-beta
and TGF-beta receptor mRNA and protein and the effect of TGF-beta
on human myometrial smooth muscle cells in vitro. Mol Hum Reprod.
3:233–240. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lee BS and Nowak RA: Human leiomyoma
smooth muscle cells show increased expression of transforming
growth factor-beta 3 (TGF beta 3) and altered responses to the
antiproliferative effects of TGF beta. J Clin Endocrinol Metab.
86:913–920. 2001.PubMed/NCBI
|
|
24.
|
Sozen I and Arici A: Interactions of
cytokines, growth factors, and extracellular matrix in the cellular
biology of uterine leiomyoma. Fertil Steril. 78:1–12. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Stein CA, Wu S, Voskresenskiy AM, et al:
G3139, an anti-BCL-2 antisense oligomer that binds heparin-binding
growth factors and collagen I, alters in vitro endothelial cell
growth and tubular morphogenesis. Clin Cancer Res. 15:2797–2807.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Hubchak SC, Sparks EE, Hayashida T and
Schnaper W: Rac1 promotes TGF-β-stimulated mesangial cell type I
collagen expression through a PI3K/Akt-dependent mechanism. Am J
Physiol Renal Physiol. 297:F1316–F1323. 2009.
|
|
27.
|
Barkan D, El Touny LH, Michalowski AM, et
al: Metastatic growth from dormant cells induced by a
Col-I-enriched fibrotic environment. Cancer Res. 70:5706–5716.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Moore AB, Yu L, Swartz CD, et al: Human
uterine leiomyoma-derived fibroblasts stimulate uterine leiomyoma
cell proliferation and collagen type I production, and activate
RTKs and TGF beta receptor signaling in coculture. Cell Commun
Signal. 8:102010. View Article : Google Scholar : PubMed/NCBI
|