Introduction
Ovarian cancer is the most lethal gynecological
malignancy in developed countries and is the 9th most common cancer
in Japanese females. An estimated 8,304 new cases and 4,467
mortalities occurred in 2005 (Center for Cancer Control and
Information Services, National Cancer Center, Japan). Although
ovarian cancer patients respond to cytoreductive surgery and
combination chemotherapy satisfactorily, advanced cases exhibit a
high level of recurrence, and the overall survival (OS) rate has
not significantly changed for decades. However, clinical trials
have been undertaken to improve prognosis. Since there are
different clinical behavior patterns for certain histopathological
subgroups, separate trials have been developed for clear cell
(1) and mucinous carcinomas
(2). The alteration of
dose/schedule and the use of intraperitoneal therapy have been
shown to be superior in at least one trial (3,4).
A number of clinicopathological factors, including
the volume of residual tumor after primary surgery, FIGO stage and
tumor grade, are reported to be the key prognostic factors
(5,6). Cytoreduction to a non-macroscopic
residual tumor is the ultimate goal, and it improves prognosis
(7–9). However, in order to improve the
prognosis of advanced epithelial ovarian cancer (EOC) cases, other
predictive biomarkers should also be elucidated.
We previously described that cyclin E (CCNE1)
amplification was strongly associated with resistance to treatment
in serous ovarian cancer by high-resolution oligonucleotide copy
number analysis (10). Therefore,
the amplification status of cyclin E has potential for therapeutic
exploitation, whereby patients exhibiting cyclin E amplification
may benefit from novel, cyclin-related targeted treatments.
Dysregulation of cell cycle control has been implicated as the key
event in human oncogenesis, and aberrant expression of G1-S
phase-related genes in particular has been reported in a number of
human cancers, including EOC (5,11).
Aberrant expression of the p16-cyclin D1-CDK4/6-pRb and
p21-p27-cyclin E-CDK2 pathways have been reported to correlate with
prognosis (5,11–14).
Barbieri et al reported in their series of 70
EOC cases that overexpression of cyclin D1 was associated with a
shorter OS. In particular, among patients with stage III/IV tumors
and residual disease greater than 2 cm, cyclin D1 expression
significantly influenced clinical outcome (5). Bali et al reported on 134
serous ovarian cancers for which molecular markers predicted
reduced OS in univariate analysis, which included overexpression of
cyclin D1 and p53, and reduced expression of p27Kip1 and
p21Waf1/Cip1 (11). In
contrast, it was reported that low nuclear p27 expression was
associated with improved 3-year OS and progression-free survival
(PFS) in 150 advanced stage (FIGO stages II, III and IV) EOC
patients (12). Kommoss et
al carried out immunohistochemical analysis of
p16Ink4a and pRb expression levels and found that they
correlated with survival in a series of 300 patients with FIGO
stage II–IV ovarian carcinoma. They reported that
p16Ink4a-negative tumors had a significantly worse
prognosis in both univariate and multivariate analyses. High
expression levels of pRb were associated with an incremental
deterioration of prognosis, which was also the case in the subgroup
of optimally debulked patients (15). Meanwhile, Khouja et al,
using immunohistochemistry, evaluated 171 primary stage III ovarian
carcinoma tumors for expression of Ki-67, p16, p14 and p57. High
expression of p16 was correlated with poor differentiation and
survival in univariate analysis. However, in multivariate analysis,
p16 expression was not significantly associated with shorter
survival (13). Some of the
results are contradictory, probably due to the variety of
histotypes and stages of EOC as well as disease heterogeneity,
different research methodologies or the sample sizes of the
studies. As serous ovarian cancer is the most common histological
type of EOC and the prognosis of advanced cases remains poor, we
limited our analysis to the serous histotype and advanced cases to
eliminate such bias.
We focused on advanced serous EOC (stage III/IV)
cases in particular and investigated the association between the
expression of G1-S phase-regulatory proteins and the
clinicopathological parameters. We aimed to identify the utility of
these proteins as prognostic factors and to evaluate whether these
targets reflect chemoresistance of advanced serous EOC.
Patients and methods
Patients and tumor specimens
The Jikei University School of Medicine Ethics
Review Committee approved the study protocol, and informed consent
was obtained from the patients. The tumor specimens were surgically
obtained from a group of 66 patients with advanced primary ovarian
serous adenocarcinoma who were treated at the Department of
Obstetrics and Gynecology, The Jikei University School of Medicine,
and Jikei University Kashiwa Hospital. The tumors were staged in
accordance with the International Federation of Gynecology and
Obstetrics (FIGO) system (1988). The clinical and pathological
characteristics of the patient cohort are shown in Table I. The age at diagnosis, volume of
postoperative residual disease, FIGO stage, presence of
intraoperative ascites and patient outcome were obtained
retrospectively from patient records as shown. The median follow-up
time for the cohort was 15.5 months (range 3–72). The 66 patients
received first-line platinum-based chemotherapy. Among them, 62
cases received taxane simultaneously as T-C chemotherapy following
primary surgery (93.9%).
 | Table I.Clinical and pathological
characteristics of the serous epithelial ovarian cancer patient
cohort (n=66). |
Table I.
Clinical and pathological
characteristics of the serous epithelial ovarian cancer patient
cohort (n=66).
| Clinicopathological
parameters | No. of patients
(%) |
|---|
| Age | |
| ≤65 years | 55 (83.3) |
| >65 years | 11 (16.7) |
| FIGO stage | |
| III | 52 (78.8) |
| IV | 14 (21.2) |
| Residual disease | |
| ≤2 cm | 28 (42.4) |
| >2 cm | 38 (57.6) |
| Ascites | |
| ≤500 ml | 25 (37.9) |
| >500 ml | 41 (62.1) |
| Disease
progression | |
| No | 9 (13.6) |
| Yes | 57 (86.4) |
Immunohistochemistry
Immunostaining was performed on buffered
formalin-fixed, paraffin-embedded tissue sections. The sections
were deparaffinized, and standard immunohistochemical techniques
were performed using Ventana XT system (BenchMark® XT;
Ventana Medical Systems, Inc., Tuscon, AZ, USA) in accordance with
the manufacturer’s instructions. Antigen epitopes were retrieved
using Ventana Benchmark CC1 standard program. The primary
antibodies used in this study were: anti-cyclin D1 (rabbit
monoclonal clone SP4; Ventana Medical Systems, Inc.), anti-pRb
(mouse monoclonal clone 13A10; Novocastra Laboratories Ltd., UK;
1:100), anti-p16 (mouse monoclonal clone 16P04; Ventana Medical
Systems, Inc.), anti-p53 (mouse monoclonal clone DO-7; Ventana
Medical Systems, Inc.), anti-p21Waf1 (mouse monoclonal
clone EA10; Calbiochem, Darmstadt, Germany; 1:50),
anti-p27Kip1 (mouse monoclonal clone SX53G8; Dako,
Glostrup, Denmark; 1:20) and anti-cyclin E (mouse monoclonal clone
HE12; Medical and Biological Laboratories Co., Ltd., Japan; 1:500).
Antibodies from Ventana Medical Systems, Inc. were pre-diluted. The
antibodies were incubated at 37°C for 32 min (60 min for p21, Rb
and cyclin E). The slides were counterstained with hematoxylin and
mounted for microscopic examination. Positive and negative controls
were tested in parallel for each staining.
Immunostaining evaluation
At least 500 tumor cells were evaluated for
immunostaining, and the percentage of stained cells was calculated.
The evaluation of immunostaining was conducted in a blinded manner
by two independent screeners, without knowledge of the clinical and
pathological characteristics of the cases. Standardization of
scoring was achieved by comparison of scores between screeners, and
discrepancies were resolved by consensus. The percentage of
positive nuclear immunostaining in cells of the tumor sections was
calculated. Scores are expressed as a percentage of positive
nuclear staining within representative areas of the tumor sample.
The percentage score above, whose staining is considered
representative of overexpression, is based on published reports
(11). The range and the median
percentage of immunostaining, percentage values regarded as
overexpression (cutoff value) and the numbers of specimens
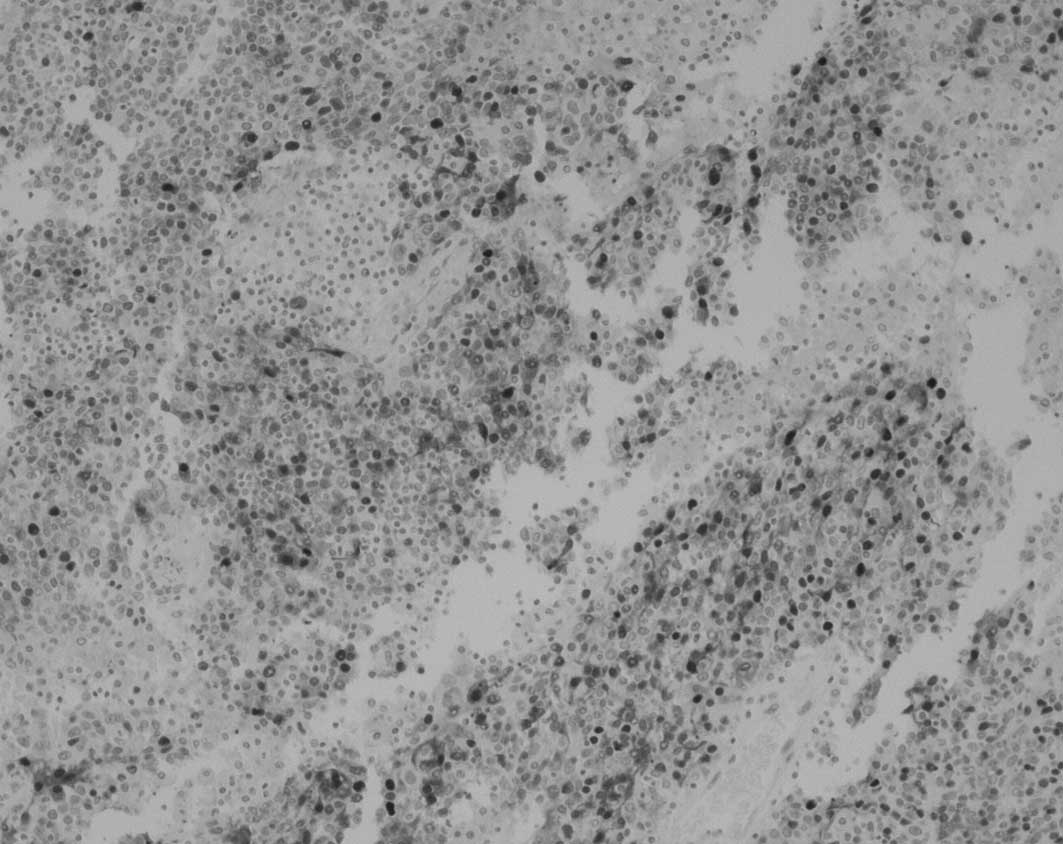
displaying positive staining are shown in Table II. Representative photomicrographs
of tumor tissue showing positive and negative staining for the
specific antigens are presented in Fig. 1.
 | Table II.Immunohistochemical analysis of cell
cycle gene expression in advanced serous epithelial ovarian
cancer. |
Table II.
Immunohistochemical analysis of cell
cycle gene expression in advanced serous epithelial ovarian
cancer.
| Cyclin D1 | pRb |
p16Ink4a | p53 |
p27Kip1 |
p21Waf1/Cip1 | Cyclin E |
|---|
| Range of staining
(% positive) | 0–50 | 0–90 | 0–90 | 0–90 | 0–80 | 0–70 | 0–90 |
| Median staining
(%) | 10 | 50 | 80 | 60 | 50 | 5 | 50 |
| Cutoff value (%)
(positive staining) | >20 | >50 | >50 | >40 | >40 | >5 | >70 |
| Positive tumors
(%) | 11 (16.7) | 26 (39.4) | 42 (63.6) | 37 (56.1) | 37 (56.1) | 20 (30.3) | 11 (16.7) |
Statistical analysis
The associations between clinicopathological
parameters and the immunostaining scores were analyzed. The
correlations between the expression of each gene and the
clinicopathological parameters were analyzed using the Chi-square
test. p≤0.05 was considered to be statistically significant. For
survival analysis, event time distributions were evaluated using
the Kaplan-Meier method, and differences in survival rates were
compared using the log-rank test for univariate analysis and Cox
proportional hazards regression model for multivariate analysis.
PFS was calculated from the date of primary surgery to the date of
disease progression. The duration of OS was defined from the date
of primary surgery to the date the patient succumbed to the disease
or to the date of last follow-up. The treatment-free interval (TFI)
was defined as being from the last date of first-line chemotherapy
to the date of recurrence or last follow-up without recurrence.
Results
Expression of G1-S phase-regulatory
proteins and the association with clinicopathological
parameters
The expression of G1-S phase-regulatory proteins was
analyzed by immunohistochemistry in advanced serous EOC.
Overexpression of cyclin D1, pRb, p16, p53, p27Kip1,
p21Waf1/Cip1 and cyclin E was detected with incidences
of 16.7, 39.4, 63.6, 56.1, 56.1, 30.3 and 16.7%, respectively.
Associations of the expression of each protein and
clinicopathological parameters are shown in Table III. The volume of postoperative
residual disease and the presence of ascites were not correlated
with the expression pattern of any of the studied proteins. The
expression of p53 appeared to be positively correlated with that of
p16Ink4a (Table III).
No other significant association among the gene expressions was
observed.
 | Table III.Association of gene expression and
clinicopathological parameters in serous epithelial ovarian
cancer. |
Table III.
Association of gene expression and
clinicopathological parameters in serous epithelial ovarian
cancer.
| Cyclin D1 | pRb |
p16Ink4a | p53 |
p27Kip1 |
p21Waf/Cip1 | Cyclin E |
|---|
| Residual
disease | 0.15 | 0.99 | 0.14 | 0.7300 | 0.097 | 0.17 | 0.91 |
| Ascites | 0.82 | 0.10 | 0.12 | 0.3000 | 0.310 | 0.81 | 0.82 |
| Cyclin D1 | | 0.43 | 0.30 | 0.6600 | 0.820 | 0.19 | 0.77 |
| pRb | | | 0.45 | 0.0820 | 0.420 | 0.95 | 0.14 |
|
p16Ink4a | | | | 0.0049 | 0.190 | 0.34 | 0.73 |
| p53 | | | | | 0.380 | 0.51 | 0.37 |
|
p27Kip1 | | | | | | 0.67 | 0.82 |
|
p21Waf/Cip1 | | | | | | | 0.90 |
Correlation between G1-S phase-regulatory
protein expression and patient outcome in advanced serous
epithelial ovarian cancer
The relationship between gene expression and patient
prognosis was assessed. Upon univariate analysis, the
clinicopathological determinants of reduced OS included age and
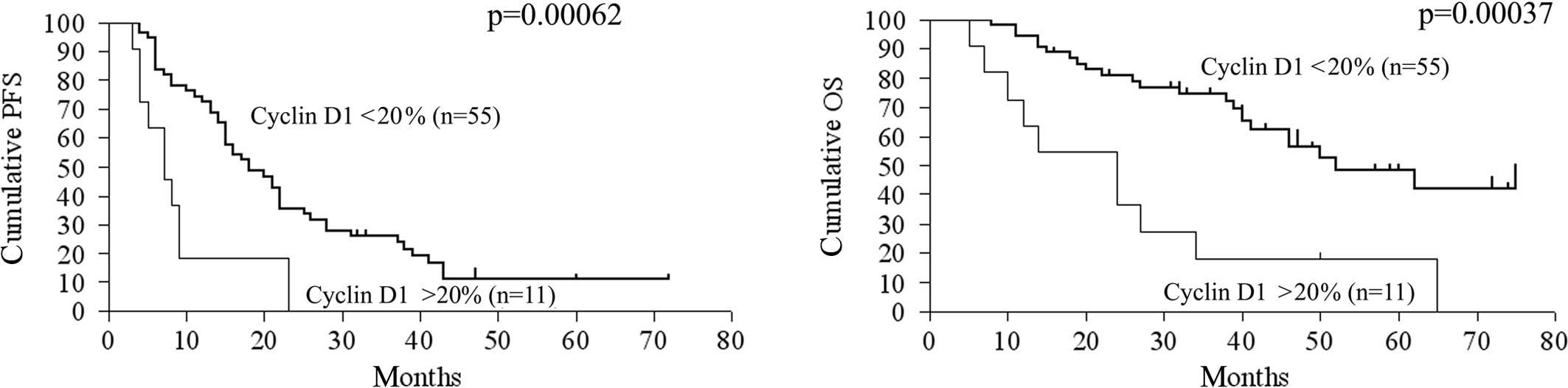
volume of residual disease >2 cm (Table IV). A molecular marker predictive
of reduced OS upon univariate analysis was overexpression of cyclin
D1 (p=0.00037, RR=0.28, 95% CI 0.044-0.40). Reduced expression of
p27Kip1 had a trend of association with a shorter OS
(p=0.064, RR=1.88, 95% CI 0.97–4.21). Regarding PFS, overexpression
of cyclin D1 (p=0.00063, RR=0.34, 95% CI 0.054-0.43) was
significantly correlated with reduced PFS, but reduced expression
of p27Kip1 had no statistically significant correlation
with PFS. The CA125 level, volume of intraoperative ascites, pRb,
p16, p53, p21Waf1/Cip1 and cyclin E expression exhibited
no statistically significant correlation with either OS or PFS.
Kaplan-Meier curves and log-rank p-values according to cyclin D1
expression, p27Kip1 expression and residual tumor volume
are shown in Fig. 2.
 | Table IV.Univariate analysis for the
association of clinicopathological parameters and gene expression
with clinical outcome in serous epithelial ovarian cancer. |
Table IV.
Univariate analysis for the
association of clinicopathological parameters and gene expression
with clinical outcome in serous epithelial ovarian cancer.
| Parameters | PFS
| OS
|
|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value |
|---|
| Age (≤65 vs. >65
years) | 0.71 | 0.300-1.46 | 0.32000 | 0.42 | 0.100-0.87 | 0.02900 |
| Residual disease
(≤2 vs. >2 cm) | 0.62 | 0.360-1.04 | 0.07800 | 0.27 | 0.150-0.60 | 0.00087 |
| CA125 (≤500 vs.
>500) | 0.83 | 0.440-1.54 | 0.56000 | 1.10 | 0.470-2.65 | 0.81000 |
| Cyclin D1 (≤20 vs.
>20%) | 0.34 | 0.054-0.43 | 0.00063 | 0.28 | 0.044-0.40 | 0.00037 |
| pRb (≤50 vs.
>50%) | 1.00 | 0.580-1.73 | 0.99000 | 0.90 | 0.440-1.81 | 0.76000 |
| p16Ink4a
(≤50 vs. >50%) | 1.13 | 0.650-2.00 | 0.65000 | 0.97 | 0.480-1.96 | 0.93000 |
| p53 (≤40 vs.
>40%) | 1.43 | 0.850-2.59 | 0.17000 | 1.38 | 0.690-2.83 | 0.35000 |
| p27Kip1
(≤40 vs. >40%) | 1.08 | 0.630-1.87 | 0.78000 | 1.88 | 0.970-4.21 | 0.06400 |
|
p21Waf1/Cip1 (≤5 vs.
>5%) | 0.82 | 0.440-1.45 | 0.48000 | 1.48 | 0.710-3.00 | 0.31000 |
| Cyclin E (≤70 vs.
>70%) | 0.97 | 0.460-2.04 | 0.94000 | 0.86 | 0.330-2.19 | 0.75000 |
| Ascites (≤500 vs.
>500 ml) | 0.81 | 0.470-1.37 | 0.43000 | 0.63 | 0.320-1.28 | 0.21000 |
In the multivariate analysis using the Cox
proportional hazards model, overexpression of cyclin D1 was
identified as the key determinant of OS (p=0.0019, RR=3.61, 95% CI
1.61–8.12) and PFS (p=0.0052, RR=2.70, 95% CI 1.35–5.41) (Table V). The volume of residual disease
and reduced expression of p27Kip1 were found to be
independent predictors of OS (p=0.0092 and p=0.042, respectively),
but not of PFS when incorporated into a multivariate model
(Table V).
 | Table V.Multivariate Cox regression analysis
of PFS and OS of patients with serous EOC. |
Table V.
Multivariate Cox regression analysis
of PFS and OS of patients with serous EOC.
| Clinical
parameter | PFS
| OS
|
|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value |
|---|
| Residual disease
(≤2 vs. >2 cm) | 1.53 | 0.89–2.64 | 0.1200 | 3.06 | 1.32–7.12 | 0.0093 |
| Cyclin D1 (≤20 vs.
>20%) | 2.70 | 1.35–5.41 | 0.0052 | 3.61 | 1.61–8.12 | 0.0019 |
| p27Kip1
(≤40 vs. >40%) | 1.01 | 0.59–1.72 | 0.9700 | 2.15 | 1.03–4.51 | 0.0420 |
Association between chemosensitivity and
G1-S phase-regulatory protein expression
In order to assess whether the clinicopathological
parameters reflect the chemosensitivity, the cohort was divided
into two groups: patients who relapsed within 6 months after the
last date of first-line chemotherapy; and patients who had no
disease progression within 6 months after the last date of
first-line chemotherapy. Using the Chi-square test, overexpression
of cyclin D1 (p=0.011) as well as residual tumor volume >2 cm
(p=0.006) were found to be significantly associated with TFI,
suggesting that these parameters are correlated with first-line
chemosensitivity (Table VI). In
contrast, expression of pRb, p16, p53, p27Kip1,
p21Waf1/Cip1 and cyclin E had no statistical correlation
with chemosensitivity.
 | Table VI.Association of the TFI and
clinicopathological parameters in serous EOC. |
Table VI.
Association of the TFI and
clinicopathological parameters in serous EOC.
| TFI <6 months, n
(%) | TFI ≥6 months, n
(%) | p-value |
|---|
| Residual
disease | | | |
| ≤2 cm (n=28) | 4 (6.0) | 24 (36.4) | 0.006 |
| >2 cm
(n=38) | 19 (28.8) | 19 (28.8) | |
| Cyclin D1 | | | |
| ≤20% (n=55) | 15 (22.7) | 40 (60.6) | 0.011 |
| >20%
(n=11) | 8 (12.1) | 3 (4.6) | |
| pRB | | | |
| ≤50% (n=40) | 12 (18.2) | 28 (42.4) | 0.305 |
| >50%
(n=26) | 11 (16.7) | 15 (22.7) | |
|
p16Ink4a | | | |
| ≤50% (n=24) | 7 (10.6) | 17 (25.8) | 0.464 |
| >50%
(n=42) | 16 (24.3) | 26 (39.4) | |
| p53 | | | |
| ≤40% (n=29) | 11 (16.7) | 18 (27.3) | 0.642 |
| >40%
(n=37) | 12 (18.2) | 25 (37.9) | |
|
p27Kip1 | | | |
| ≤40% (n=29) | 13 (19.7) | 16 (24.3) | 0.132 |
| >40%
(n=37) | 10 (15.2) | 27 (40.9) | |
|
p21Waf/Cip1 | | | |
| ≤5% (n=46) | 19 (28.8) | 27 (40.9) | 0.165 |
| >5%
(n=20) | 4 (6.0) | 16 (24.3) | |
| Cyclin E | | | |
| ≤70% (n=55) | 18 (27.3) | 37 (56.1) | 0.644 |
| >70%
(n=11) | 5 (7.6) | 6 (9.1) | |
Discussion
Various studies exist concerning the association
between G1-S phase-related genes and EOC prognosis, however, the
results are conflicting. Amplification of cyclin E in
high-resolution oligonucleotide microarrays was previously found to
be associated with poor response to primary treatment in serous
ovarian cancer (10), but in the
present study, cyclin E expression in immunohistochemical analysis
revealed no significant correlation with patient outcome of
advanced serous EOC. It is considered that the variety of
histological types of EOC, different tumor stages, tumor
heterogeneity, racial backgrounds of patients, research
methodologies and sample sizes may contribute to inconsistent
results. In this study, we focused on advanced serous cases
(limited to stage III/IV cases) at a single institution with
similar surgical and chemotherapeutic procedures administered in
order to eliminate such bias.
Cyclin D1, a regulatory kinase subunit that is
selectively associated with cyclin-dependent kinase 4 (CDK4), is a
crucial modulator of G1 progression in the cell cycle (16). In our analysis, overexpression of
cyclin D1 was detected in 11% of the cases (Table II). The overexpression of cyclin D1
was previously observed in 14–89% of EOC cases (11,17–19),
but the underlying mechanism has yet to be elucidated.
Amplification of cyclin D1 in ovarian tumors occurs infrequently
(20). Furthermore, the mostly
small cyclin D1 copy gains are not associated with an increase in
detectable cyclin D1 protein by immunohistochemistry (21). These findings suggest that the
post-transcriptional regulation of cyclin D1 protein production is
complex. Recently, Jiang et al performed a systematic
validation of the predicted microRNAs for cyclin D1 and revealed
that microRNAs suppressed the endogenous cyclin D1 protein and mRNA
levels in vitro (22).
microRNAs may aid in determining the mechanism of cyclin D1
expression.
Barbieri et al reported that cyclin D1
overexpression significantly influenced the clinical outcome in
advanced EOC cases with residual disease greater than 2 cm. They
identified cyclin D1 overexpression as an independent prognostic
factor in multivariate analysis (5). Similarly, Bali et al
identified cyclin D1 overexpression as an independent prognostic
factor in the multivariate analysis of 134 serous EOC cases
(11). In our study,
overexpression of cyclin D1 was significantly correlated with
reduced OS and PFS in both univariate and multivariate analyses,
suggesting that overexpression of cyclin D1 actually contributes to
the prognosis of advanced serous EOCs; therefore, its application
to clinical practice is expected.
We found that both overexpression of cyclin D1 and
residual tumor volume were significantly associated with TFI,
suggesting that these parameters are correlated with first-line
chemosensitivity (Table VI). Zhou
et al showed that inhibition of cyclin D1 expression by
siRNA in oral squamous cell carcinoma cells resulted in a decrease
in cisplatin IC50 level. In vivo transplantation
models also confirmed a cisplatin-sensitizing effect of cyclin D1
knockdown in these cell lines (23). In addition, it was reported that
overexpression of cyclin D1 was associated with reduced
chemosensitivity and a higher survival rate upon cisplatin
administration in a pancreatic cancer model (24). Moreover, inhibition of cyclin D1
expression rendered cells more susceptible to cisplatin-mediated
apoptosis in the same model (24).
Taken together, these findings indicate that cyclin D1 expression
may contribute to chemoresistance in a number of cancers, although
further investigation is required. Therefore, we speculate that
overexpression of cyclin D1 contributes to poor prognosis, which
may in part be mediated by chemoresistance in ovarian cancer.
p27Kip1 is a cyclin-dependent kinase
(cdk) inhibitor that regulates cell cycle progression from the G1
to S-phase. In non-cycling cells, p27Kip1 binds to
cyclin E-cdk2 complexes and inhibits their activation. In contrast,
p27Kip1 binding to catalytically active cyclin D-cdk4/6
complexes results in p27Kip1 degradation and the
subsequent release of cdk2 from inhibition in proliferating cells
(12,25). Therefore, p27Kip1 helps
to coordinate a balance between proliferation and arrest (12,25).
In the present study, reduced expression of p27Kip1 was
detected in 43.9% of the cases. In previous immunohistochemical
studies of ovarian tumors, 36.2-100% exibited low expression of
p27Kip1 (26). We found
that reduced expression of p27Kip1 was associated with
shorter OS (p=0.064), but had no statistically significant
correlation with PFS (p=0.78). When incorporated into a
multivariate model, reduced expression of p27Kip1 was
found to be an independent predictor of OS (p=0.042) (Table V). The relationship between
p27Kip1 expression levels and prognosis is
controversial. Conflicting data regarding the possible prognostic
role of p27Kip1 status also exist for ovarian cancer.
Psyrri et al evaluated subcellular localization and protein
levels of p27Kip1 in 150 advanced EOCs and found that
low nuclear p27Kip1 expression was associated with
improved prognosis, suggesting its potential as a strong predictor
of outcome in advanced EOCs (12).
On the other hand, Shigemasa et al reported that negative
p27Kip1 expression was significantly correlated with
poor survival in serous EOC patients, suggesting that the
underexpression of p27Kip1 caused by a
post-translational mechanism may contribute to development and
progression and may result in poor prognosis of serous EOCs
(27). It is hypothesized that
different methodologies of immunohistochemical grading may account
for these discrepancies. Our result suggests that
p27Kip1 is associated with the prognosis of this
disease, as previously reported.
In conclusion, overexpression of cyclin D1
contributed markedly to poor prognosis in advanced serous EOC; this
may in part be mediated by chemoresistance. Cyclin D1 may be a
target for overcoming the refractory nature of advanced serous
EOC.
Acknowledgements
We thank Dr Miho Takao and Dr Hideo
Shinozaki for the assistance in this research. This study was
supported in part by Grants-in-Aid for Scientific Research from the
Japanese Ministry of Education, Culture, Sports, Science and
Technology to N.Y., A.O. and S.T., and by the Japanese Ministry of
Health, Labour and Welfare to K.O.
References
|
1.
|
Takakura S, Takano M, Takahashi F, Saito
T, Aoki D, Inaba N, Noda K, Sugiyama T and Ochiai K; Japanese
Gynecologic Oncology Group: Randomized phase II trial of paclitaxel
plus carboplatin therapy versus irinotecan plus cisplatin therapy
as first-line chemotherapy for clear cell adenocarcinoma of the
ovary: a JGOG study. Int J Gynecol Cancer. 20:240–247. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Shimada M, Kigawa J, Ohishi Y, Yasuda M,
Suzuki M, Hiura M, Nishimura R, Tabata T, Sugiyama T and Kaku T:
Clinicopathological characteristics of mucinous adenocarcinoma of
the ovary. Gynecol Oncol. 113:331–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Katsumata N, Yasuda M, Takahashi F,
Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura
E, Ochiai K and Noda K; Japanese Gynecologic Oncology Group:
Dose-dense paclitaxel once a week in combination with carboplatin
every 3 weeks for advanced ovarian cancer: a phase 3, open-label,
randomised controlled trial. Lancet. 374:1331–1338. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA:
Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl
J Med. 354:34–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Barbieri F, Lorenzi P, Ragni N, Schettini
G, Bruzzo C, Pedullà F and Alama A: Overexpression of cyclin D1 is
associated with poor survival in epithelial ovarian cancer.
Oncology. 66:310–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
NIH Consensus Conference: Ovarian cancer.
Screening, treatment, and follow-up. JAMA. 273:491–497. 1995.
View Article : Google Scholar
|
|
7.
|
Terauchi F, Nishi H, Moritake T, et al:
Prognostic factor on optimal debulking surgery by maximum effort
for stage IIIC epithelial ovarian cancer. J Obstet Gynaecol Res.
35:315–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Du Bois A, Reuss A, Pujade-Lauraine E,
Harter P, Ray-Coquard I and Pfisterer J: Role of surgical outcome
as prognostic factor in advanced epithelial ovarian cancer: a
combined exploratory analysis of 3 prospectively randomized phase 3
multicenter trials: by the Arbeitsgemeinschaft Gynaekologische
Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe
d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire
(GINECO). Cancer. 115:1234–1244. 2009.
|
|
9.
|
Chi DS, Eisenhauer EL, Zivanovic O, et al:
Improved progression-free and overall survival in advanced ovarian
cancer as a result of a change in surgical paradigm. Gynecol Oncol.
114:26–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Etemadmoghadam D, deFazio A, Beroukhim R,
Mermel C, George J, Getz G, Tothill R, Okamoto A, Raeder MB,
Harnett P, Lade S, Akslen LA, Tinker AV, Locandro B, Alsop K, Chiew
YE, Traficante N, Fereday S, Johnson D, Fox S, Sellers W, Urashima
M, Salvesen HB, Meyerson M and Bowtell D; AOCS Study Group:
Integrated copy number and expression analysis of chemoresistant
ovarian carcinomas. Clin Cancer Res. 15:1417–1427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bali A, O’Brien PM, Edwards LS, Sutherland
RL, Hacker NF and Henshall SM: Cyclin D1, p53, and
p21Waf1/Cip1 expression is predictive of poor clinical
outcome in serous epithelial ovarian cancer. Clin Cancer Res.
10:5168–5177. 2004.
|
|
12.
|
Psyrri A, Bamias A, Yu Z, et al:
Subcellular localization and protein levels of cyclin-dependent
kinase inhibitor p27 independently predict for survival in
epithelial ovarian cancer. Clin Cancer Res. 11:8384–8390. 2005.
View Article : Google Scholar
|
|
13.
|
Khouja MH, Baekelandt M, Nesland JM and
Holm R: The clinical importance of Ki-67, p16, p14, and p57
expression in patients with advanced ovarian carcinoma. Int J
Gynecol Pathol. 26:418–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Milde-Langosch K and Riethdorf S: Role of
cell-cycle regulatory proteins in gynecological cancer. J Cell
Physiol. 196:224–244. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kommoss S, du Bois A, Ridder R, Trunk MJ,
Schmidt D, Pfisterer J and Kommoss F; AGO-OVAR: Independent
prognostic significance of cell cycle regulator proteins p16(INK4a)
and pRb in advanced-stage ovarian carcinoma including optimally
debulked patients: a translational research subprotocol of a
randomised study of the Arbeitsgemeinschaft Gynaekologische
Onkologie Ovarian Cancer Study Group. Br J Cancer. 96:306–313.
2007.
|
|
16.
|
Sherr CJ: G1 phase progression: cycling on
cue. Cell. 79:551–555. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Dhar KK, Branigan K, Parkes J, et al:
Expression and subcellular localization of cyclin D1 protein in
epithelial ovarian tumour cells. Br J Cancer. 81:1174–1181. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Worsley SD, Ponder BA and Davies BR:
Overexpression of cyclin D1 in epithelial ovarian cancers. Gynecol
Oncol. 64:189–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Barbieri F, Cagnoli M, Ragni N, Foglia G,
Bruzzo C, Pedullà F and Alama A: Increased cyclin D1 expression is
associated with features of malignancy and disease recurrence in
ovarian tumors. Clin Cancer Res. 5:1837–1842. 1999.PubMed/NCBI
|
|
20.
|
Masciullo V, Scambia G, Marone M, et al:
Altered expression of cyclin D1 and CDK4 genes in ovarian
carcinomas. Int J Cancer. 74:390–395. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Diebold J, Mösinger K, Peiro G, et al:
20q13 and cyclin D1 in ovarian carcinomas. Analysis by fluorescence
in situ hybridization. J Pathol. 190:564–571. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Jiang Q, Feng MG and Mo YY: Systematic
validation of predicted microRNAs for cyclin D1. BMC Cancer.
9:1942009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhou X, Zhang Z, Yang X, Chen W and Zhang
P: Inhibition of cyclin D1 expression by cyclin D1 shRNAs in human
oral squamous cell carcinoma cells is associated with increased
cisplatin chemosensitivity. Int J Cancer. 124:483–489. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Biliran H Jr, Wang Y, Banerjee S, et al:
Overexpression of cyclin D1 promotes tumor cell growth and confers
resistance to cisplatin-mediated apoptosis in an elastase-myc
transgene-expressing pancreatic tumor cell line. Clin Cancer Res.
11:6075–6086. 2005. View Article : Google Scholar
|
|
25.
|
Blain SW, Scher HI, Cordon-Cardo C and
Koff A: p27 as a target for cancer therapeutics. Cancer Cell.
3:111–115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Nam EJ and Kim YT: Alteration of
cell-cycle regulation in epithelial ovarian cancer. Int J Gynecol
Cancer. 18:1169–1182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Shigemasa K, Shiroyama Y, Sawasaki T, et
al: Underexpression of cyclin-dependent kinase inhibitor p27 is
associated with poor prognosis in serous ovarian carcinomas. Int J
Oncol. 18:953–958. 2001.PubMed/NCBI
|