Introduction
Obesity, diabetes and hyperlipidemia are
lifestyle-related diseases that are receiving attention throughout
the world. It is known that the development and progression of
lifestyle-related diseases are closely associated with overeating,
lack of exercise and heredity (1,2). The
lack of exercise not only increases fat that stores surplus energy,
but also decreases the function of bones and muscles.
Adiponectin is a protein hormone, that is produced
and secreted exclusively by adipocytes, regulates the metabolism of
lipids and glucose, and exhibits anti-inflammatory properties. It
has been reported to play a role in the inhibition of the
development of cardiovascular diseases, type 2 diabetes and
atherosclerosis, and its levels have been shown to be suppressed in
insulin-resistant and obese animals and humans (3–5).
Adiponectin levels have been reported to rise in response to weight
loss and glitazone therapy (6–8).
Nesfatin-1 is a recently discovered anorexigenic
peptide encoded as the precursor protein nucleobindin-2 (9). Nesfatin-1 is distributed in
peripheral tissues, such as adipose tissue, and also exists in the
blood (9–11). A recent study reported that
nesfatin-1 immunoreactivity is also detected in the rat gastric
oxyntic mucosa (12). The
intraperitoneal administration of nesfatin-1 inhibits food intake
and thereby reduces body weight (13). Notably, nesfatin-1 also inhibits
food intake in Zucker fatty rats whose leptin receptor is mutated,
indicating that the anorexigenic effect of nesfatin-1 is
leptin-independent (9). Nesfatin-1
is relatively stable in the circulation and it has a moderate
influx from the blood to the brain compared to other peptides of
similar size (8,9,14).
Nesfatin-1 is also present in the hypothalamus, including the
paraventricular nucleus, supraoptic nucleus, arcuate nucleus, the
lateral hypothalamic area and the nucleus tractus solitarius
in the brain stem (9,10,15,16).
Nesfatin-1 suppresses eating behavior not only by peripheral
administration, but also by central administration (13), and is thus expected to improve
glucose metabolism in conditions such as impaired glucose
tolerance, through the suppression of food intake and body weight
gain. In this study, the effect of exercise and high-fat diet on
the levels of adiponectin and nesfatin-1 was investigated.
Materials and methods
Laboratory animals and breeding
environment
Male C57BL/6J mice (21.06±0.60 g, 7-week-old; CLEA
Japan, Inc., Tokyo, Japan) were used in this study. The mice were
maintained in breeding rooms at a temperature of 22±1°C and a
humidity of 55±11% under 12-h light/dark cycles, wherein the light
cycle began at 7:00 am daily. The mice were individually housed.
All experiments were approved by the animal experimental ethics
committee of our university.
Experimental procedures
Twenty-four male C57BL/6J mice were divided into 4
groups and housed for 4 weeks. Six mice were included in each of
the 4 groups: the non-exercise and normal diet (SN), exercise and
normal diet (EN), non-exercise and high-fat diet (SF) and the
exercise and high-fat diet (EF) group. All cages were equipped with
a running wheel. The running wheels were locked for the
non-exercise groups, while the exercise groups were subjected to
voluntary wheel running. The C57BL/6J mice of the normal diet group
were fed daily with a normal diet (CE-2 diet containing 11% kcal
%fat, 59% kcal %carbohydrate and 30% kcal %protein; CLEA Japan,
Inc.). The C57BL/6J mice of the high-fat diet group were fed with a
high-fat diet (D12492 diet containing 60% kcal %fat, 20% kcal
%carbohydrate and 20% kcal %protein; Research Diets, Inc., NJ,
USA). Water was available ad libitum. Motor activity, food
intake and body weight were measured at the same time each day.
Running distances were calculated as the number of rotations of the
running wheel x the circumference of the wheel (0.7 m). Four weeks
later, the mice were deprived of food for 6 h for tissue sampling.
Rectal temperature was determined using a digital thermometer
(Technol Seven Co. Ltd., Yokohama, Japan) in a room maintained at
22±0.5°C. A lubricated thermocouple was inserted 1.5 cm into the
rectum of conscious mice. Blood samples were obtained from the
orbital sinus under diethyl ether anesthesia. The mice were
sacrificed by cervical dislocation. Visceral fat was removed and
weighed. Plasma glucose was measured by the glucose oxidase method.
Plasma insulin was measured by an enzyme-linked immunosorbent assay
(ELISA) kit (Morinaga, Co., Tokyo, Japan). Plasma cholesterol and
triglyceride (TG) concentrations were measured using the
Cholesterol E-test Wako and Triglyceride E-test Wako kits (Wako
Pure Chemical, Ind., Osaka, Japan), respectively. Mice plasma
adiponectin (Otsuka Pharmaceutical, Co., Tokyo, Japan) and
nesfatin-1 levels (Phoenix Pharmaceuticals, Inc., CA, USA) were
measured by their corresponding ELISA-based kits.
Statistics
Analysis of variance followed by Bonferroni's test
were used to assess the differences among the groups. A value of
P<0.05 was considered to be statistically significant.
Results
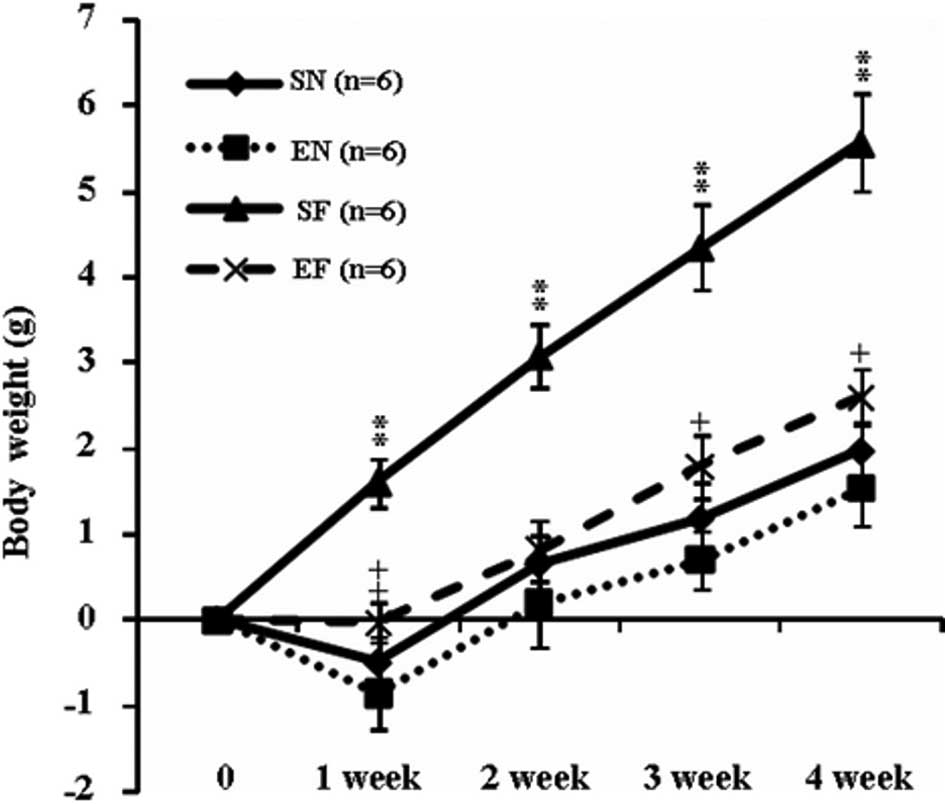
The weekly body weight in the SF group significantly
increased compared to that in the other groups (P<0.01). The
body weight in the EF group significantly increased at week 1
(P<0.01) and at weeks 3 and 4 (P<0.05) compared to that in
the EN group (Fig. 1A). The motor
activity did not differ significantly between the EN and EF groups,
but an increasing trend was observed in the motor activity in the
EF group compared to that in the EN group (Fig. 1B). The mean daily food intake
(Kcal) in the high-fat diet groups significantly increased compared
to that in the normal diet groups (P<0.01). The mean daily food
intake (Kcal) in the exercise groups significantly increased
compared to that in the non-exercise groups (P<0.01) (Fig. 1C). The temperature of the mice in
the EF group significantly increased compared to that of the mice
in the SN group (P<0.05) (Fig.
1D).
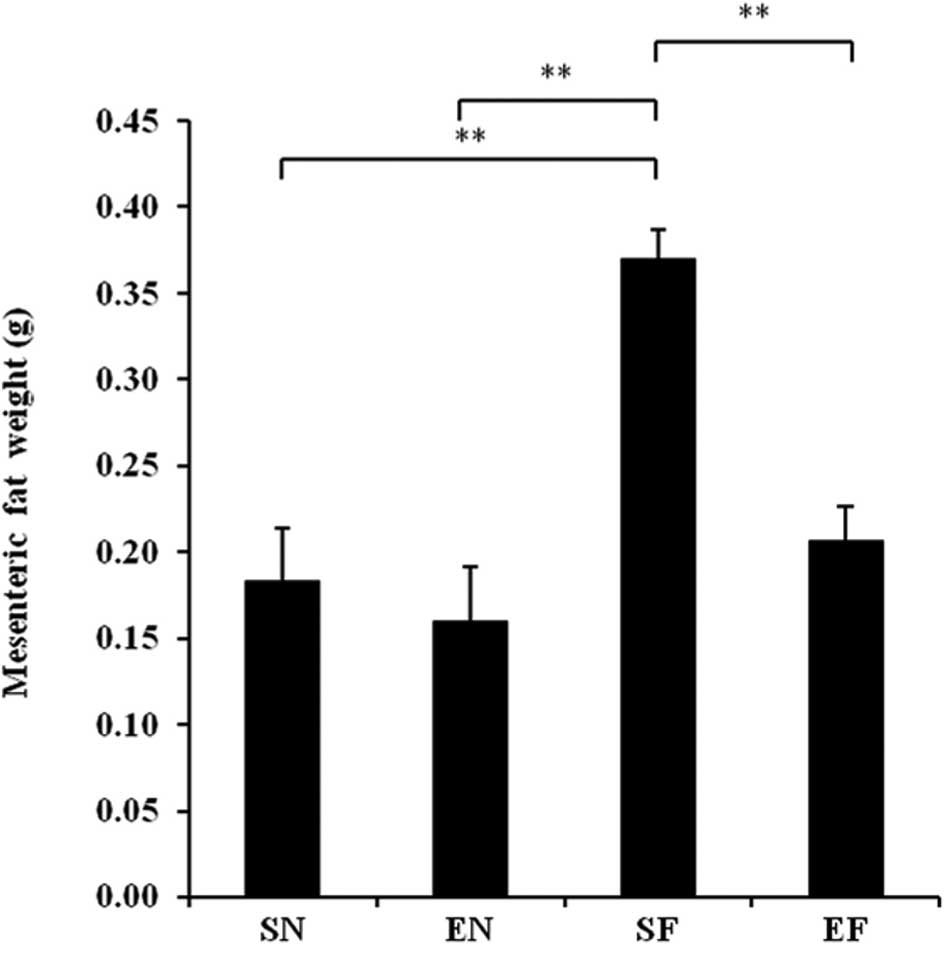
The mesenteric fat in the SF group significantly
increased compared to that in the EF and the other groups
(P<0.01), while the mesenteric fat in the EF group showed no
significant difference compared to that in the normal diet groups
(Fig. 2A). The epididymal fat in
the SF group significantly increased compared to that in the other
groups (P<0.01), while the epididymal fat in the EF group did
not significantly differ from that in the SN group (Fig. 2B).
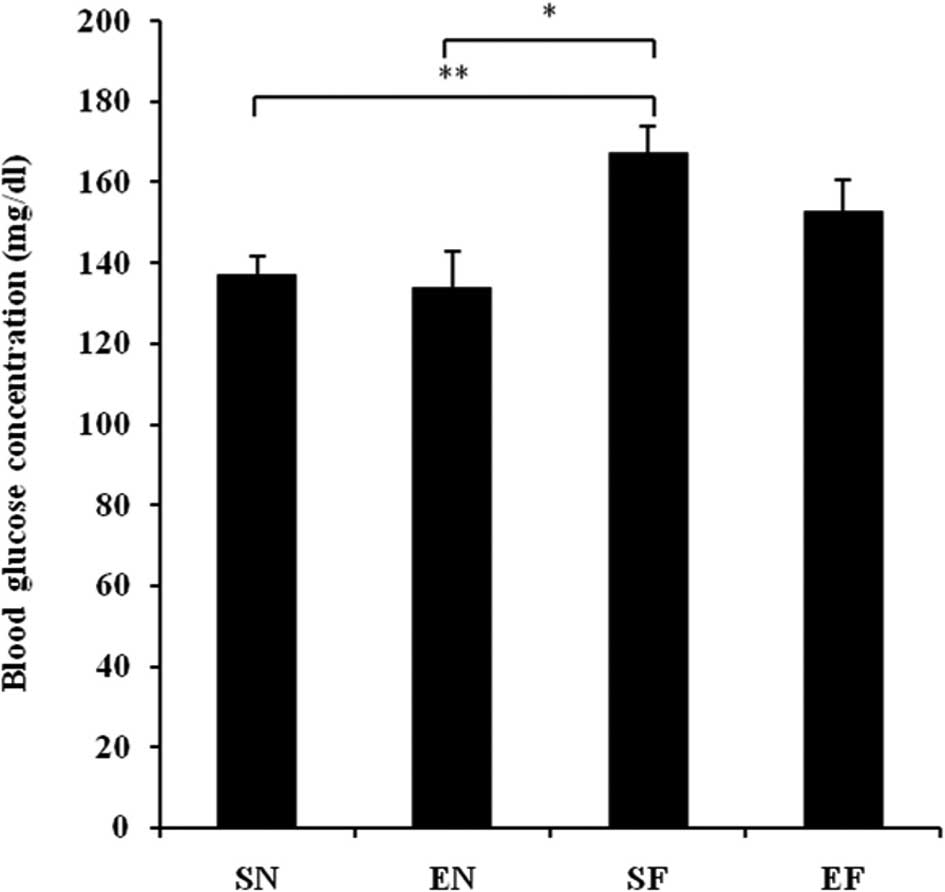
The glucose concentration in the SF group
significantly increased compared to that in the normal diet groups
(P<0.01), while the blood glucose level in the EF group did not
differ significantly from that in the normal diet groups (Fig. 3A). The plasma insulin concentration
in the SF group significantly increased compared to that in the
other groups (P<0.01), while the plasma insulin concentration in
the EF group did not differ significantly from that in the normal
diet groups (Fig. 3B). The plasma
TG concentration in the SF group significantly increased compared
to that in the normal diet groups (P<0.01), while the plasma TG
concentration in the EF group did not differ significantly from
that in the normal diet groups (Fig.
3C). The plasma cholesterol concentration in the high-fat diet
groups significantly increased compared to that in the normal diet
groups (P<0.01) (Fig. 3D). With
the high-fat diet, the plasma cholesterol concentration in the
exercise group significantly decreased compared to that in the
non-exercise group (P<0.05). The plasma adiponectin level in the
SF group significantly decreased compared to that in the normal
diet groups (P<0.05 and P<0.01). The plasma adiponectin level
in the EF group significantly decreased compared to that in the SN
group (P<0.05) (Fig. 3E). The
plasma adiponectin concentration did not differ significantly
between the EF and EN groups. The plasma nesfatin-1 level in the SF
group significantly decreased compared to that in the SN group
(P<0.05), while the plasma nesfatin-1 level in the EF group did
not differ significantly from that in the normal diet groups
(Fig. 3F).
Discussion
To date, it has been reported that exercise is
closely related to body weight, especially the accumulation of fat
(17). Physical exercise is a
strategy used to counteract obesity, as it lowers the energetic
balance by increasing energy expenditure (18). This study reveals that exercise
suppresses body weight gain caused by a high-fat diet. Although the
high-fat diet increased visceral fat, visceral fat did not increase
in the exercise groups. The food intake (Kcal) of the mice in the
exercise groups significantly increased compared to that in the
non-exercise groups. It is thought that exercise increases the need
for energy intake, resulting in an increased appetite.
It is known that the energy generated after the
intake of a high-fat diet is relatively higher than that generated
after a normal diet (19). In this
study, the temperature of the mice in the EF group significantly
increased compared to that of the mice in the SN group. In
addition, an increasing trend was observed in the temperature of
the mice in the EF group compared to that of the mice in the EN
group. In the EF group, the glucose and insulin concentrations that
increased with the high-fat diet, decreased. Hence, it is
considered that exercise increases insulin sensitivity. In
addition, in the EF group, TG and total cholesterol concentrations
that increased with the high-fat diet, decreased.
It has previously been shown that adiponectin has
certain effects on insulin sensitivity, the regulation of blood
glucose, the repair of vascular lesions and the suppression of
arteriosclerosis (20). Hattori
et al have shown that adiponectin activates the
AMP-activated protein kinase in the skeletal muscle and liver, and
thus promotes the combustion of fatty acids and the uptake of
glucose to improve insulin resistance (21). In this study, the plasma
adiponectin level in the high-fat diet groups significantly
decreased compared to that in the SN group. However, in the
exercise groups, the plasma adiponectin level did not differ
significantly between the high-fat diet and normal diet groups.
No reports are as yet available on the effects of
exercise and high-fat diet on nesfatin-1. In this study, the plasma
nesfatin-1 level in the SF group significantly decreased compared
to that in the SN group, while exercise under the high-fat diet
antagonized this significant decrease. It has recently been
reported that the fasting levels of nesfatin-1 are significantly
lower in type 2 diabetes mellitus patients than in healthy subjects
(22). In addition, Su et
al have reported that the intravenous administration of
nesfatin-1 reduces the blood glucose level in hyperglycemic db/db
mice (23). Moreover,
nesfatin-1-like immunoreactivity has been detected in human and rat
islet β cells (24). Therefore, it
is suggested that plasma nesfatin is associated with
lifestyle-related diseases, such as diabetes and obesity.
In conclusion, a high-fat diet decreases plasma
adiponectin and nesfatin levels, but this decrease is suppressed by
exercise. Accordingly, it has been revealed that not only diet, but
also exercise, are important in the development and progression of
lifestyle-related diseases.
References
|
1.
|
Yamauchi T, Oike Y, Kamon J, et al:
Increased insulin sensitivity despite lipodystrophy in Crebbp
heterozygous mice. Nat Genet. 30:221–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hara K, Boutin P, Mori Y, et al: Genetic
variation in the gene encoding adiponectin is associated with an
increased risk of type 2 diabetes in the Japanese population.
Diabetes. 51:536–540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Okamoto Y, Arita Y, Nishida M, et al: An
adipocyte-derived plasma protein, adiponectin, adheres to injured
vascular walls. Horm Metab Res. 32:47–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Arita Y, Kihara S, Ouchi N, et al:
Paradoxical decrease of an adipose-specific protein, adiponectin,
in obesity. Biochem Biophys Res Commun. 257:79–83. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hotta K, Funahashi T, Arita Y, et al:
Plasma concentrations of a novel, adipose-specific protein,
adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc
Biol. 20:1595–1599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hulver MW, Zheng D, Tanner CJ, et al:
Adiponectin is not altered with exercise training despite enhanced
insulin action. Am J Physiol Endocrinol Metab. 283:E861–E865. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Boudou P, Sobngwi E, Mauvais-Jarvis F,
Vexiau P and Gautier JF: Absence of exercise-induced variations in
adiponectin levels despite decreased abdominal adiposity and
improved insulin sensitivity in type 2 diabetic men. Eur J
Endocrinol. 149:421–424. 2003. View Article : Google Scholar
|
|
8.
|
Yatagai T, Nishida Y, Nagasaka S, et al:
Relationship between exercise training-induced increase in insulin
sensitivity and adiponectinemia in healthy men. Endocr J.
50:233–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Oh-I S, Shimizu H, Satoh T, et al:
Identification of nesfatin-1 as a satiety molecule in the
hypothalamus. Nature. 443:709–712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Brailoiu GC, Dun SL, Brailoiu E, Inan S,
Yang J, Chang JK and Dun NJ: Nesfatin-1: distribution and
interaction with a G protein-coupled receptor in the rat brain.
Endocrinology. 148:5088–5094. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kohno D, Nakata M, Maejima Y, et al:
Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the
rat hypothalamus coexpress oxytocin and vasopressin and are
activated by refeeding. Endocrinology. 149:1295–1301. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Stengel A, Goebel M, Yakubov I, et al:
Identification and characterization of nesfatin-1 immunoreactivity
in endocrine cell types of the rat gastric oxyntic mucosa.
Endocrinology. 150:232–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shimizu H, Oh-I S, Hashimoto K, et al:
Peripheral administration of nesfatin-1 reduces food intake in
mice: the leptin-independent mechanism. Endocrinology. 150:662–671.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Price TO, Samson WK, Niehoff ML and Banks
WA: Permeability of the blood-brain barrier to a novel satiety
molecule nesfatin-1. Peptides. 28:2372–2381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Cowley MA and Grove KL: To be or NUCB2, is
nesfatin the answer? Cell Metab. 4:421–422. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Schwartz MW, Woods SC, Porte D Jr, Seeley
RJ and Baskin DG: Central nervous system control of food intake.
Nature. 404:661–671. 2000.PubMed/NCBI
|
|
17.
|
Cox KL, Burke V, Beilin LJ and Puddey IB:
A comparison of the effects of swimming and walking on body weight,
fat distribution, lipids, glucose, and insulin in older women-the
Sedentary Women Exercise Adherence Trial 2. Metabolism.
59:1562–1573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
MacLean PS, Higgins JA, Wyatt HR, et al:
Regular exercise attenuates the metabolic drive to regain weight
after long-term weight loss. Am J Physiol Regul Integr Comp
Physiol. 297:793–802. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Michailidou Z, Carter RN, Marshall E, et
al: Glucocorticoid receptor haploinsufficiency causes hypertension
and attenuates hypothalamic-pituitaryadrenal axis and blood
pressure adaptions to high-fat diet. FASEB J. 22:3896–3907. 2008.
View Article : Google Scholar
|
|
20.
|
Qi Y, Takahashi N, Hileman SM, et al:
Adiponectin acts in the brain to decrease body weight. Nat Med.
10:524–529. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hattori Y, Nakano Y, Hattori S, Tomizawa
A, Inukai K and Kasai K: High molecular weight adiponectin
activates AMPK and suppresses cytokine-induced NF-kappaB activation
in vascular endothelial cells. FEBS Lett. 582:1719–1724. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Li QC, Wang HY, Chen X, Guan HZ and Jiang
ZY: Fasting plasma levels of nesfatin-1 in patients with type 1 and
type 2 diabetes mellitus and the nutrient-related fluctuation of
nesfatin-1 level in normal humans. Regul Pept. 159:72–77. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Su Y, Zhang J, Tang Y, Bi F and Liu JN:
The novel function of nesfatin-1: anti-hyperglycemia. Biochem
Biophys Res Commun. 391:1039–1042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Foo KS, Brauner H, Ostenson CG and
Broberger C: Nucleobindin-2/nesfatin in the endocrine pancreas:
distribution and relationship to glycaemic state. J Endocrinol.
204:255–263. 2010. View Article : Google Scholar : PubMed/NCBI
|