Introduction
There has been evidence that inflammation is
implicated in cancer development and progression. Various
approaches have been used to clarify the relationship between
inflammation and cancer. Recent reports have demonstrated that the
gene expression profiles of cytokines related to inflammation in
cancer tissues and the surrounding non-malignant tissues may be
used as possible indexes of the association with
clinicopathological factors and prognosis in liver and lung cancer
(1,2). In gastric cancer, it has been
suggested that Helicobacter pylori is involved in the onset
of cancer development through the induction of immunocompetent
cells that evoke chronic inflammation and expression of
immune-related cytokines (3).
Although the major cause of colon cancer is considered to be an
accumulation of cancer-related gene mutations, as represented by
adenoma-carcinoma sequence and de novo types (4,5), the
involvement of cytokines in colitis-associated colon cancer
coexisting with inflammatory enteritis has been reported (6). It is essential to analyze the balance
of all cytokines related to inflammation which are intricately
linked to cancer development and progression, and it is important
to elucidate the function of inflammation-related molecules.
Annexin A1 (ANXA1), which belongs to the
calcium-dependent phospholipid-linked protein family, is associated
with anti-inflammatory effects through the inhibition of
phospholipase A2, including arachadonic acid (7). The functions of ANXA1 in tumor growth
and development have been examined in various types of cancer, but
are not clearly understood, since ANXA1 expression is
differentially expressed in different cancer types, including
esophageal cancer, pancreatic cancer, skin squamous cell carcinoma,
colon cancer, cervical cancer, oral squamous cell carcinoma,
prostate cancer, breast cancer and laryngeal squamous cell
carcinoma (8–18). In the present study, we
investigated ANXA1 expression in gastric and colon cancer, and
analyzed the relationship between ANXA1 expression and
clinicopathological factors and patient prognosis.
Materials and methods
Clinical samples of patients
For the analysis of mRNA expression, cancer tissues
and adjacent normal mucosa were collected from 25 gastric cancer
patients (19 males and 6 females) and 37 colon cancer patients (24
males and 13 females) who underwent surgery at our institute
between 2003 and 2008. The median age of the gastric and colon
cancer patients was 73 and 64 years, respectively. Tissues were
collected fresh during surgery, immediately frozen using liquid
nitrogen and stored at −80°C until total RNA was extracted.
Formalin-fixed, paraffin-embedded specimens obtained from 135
patients with gastric cancer and 210 patients with colon cancer who
underwent surgery at our institute between 1990 and 2007, including
the cases mentioned above, were examined in this study. Regarding
gastric cancer patients, the median age of the patients was 66
years. The carcinomas were staged according to UICC classification.
These cases included stage I, 66 cases; stage II, 25 cases; stage
III, 37 cases; and stage IV, 7 cases. In colon cancer, the median
age of the patients was 67 years. The carcinomas were staged
according to UICC classification. These cases included stage I, 40
cases; stage II, 82 cases; stage III, 60 cases; and stage IV, 28
cases. This study was performed in accordance with ethical
guidelines for clinical research with the approval of our
institutional ethics committee.
RNA extraction and quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from 5-mm2 blocks
of each cancer and normal tissues obtained from resected specimens
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance
with the manufacturer’s instruction manual. Complementary DNA
(cDNA) was synthesized from 5 μg of total RNA with random hexamer
using the SuperScript III First-Strand Synthesis kit (Invitrogen).
These cDNAs were used for the measurement of ANXA1 gene expression
with a 7500 Real-Time PCR system (Applied Biosystems, Foster City,
CA, USA). The level of mRNA expression for each tissue was
normalized to that of β-actin. Probes for each gene were purchased
from Applied Biosystems.
Immunohistochemical staining and
evaluation
Paraffin-embedded histological sections (4 μm) were
deparaffinized and washed with water, and then endogenous
peroxidase activity was blocked with 0.3% hydrogen peroxide in
methanol. After washing in PBS, blocking agent (Dako, Glostrup,
Denmark) was used to regulate the non-specific reactivity.
Anti-ANXA1 monoclonal antibodies (mouse IgG1, clone 29, 1:100; BD
Biosciences) were allowed to react overnight at 4°C and after
antigen-antibody reaction, diaminobenzidine (Envision; Dako) was
used for visualization. Sections were counterstained with
hematoxylin. The expression of ANXA1 protein was evaluated as
positive when the nucleus and/or cytoplasm of cancerous and normal
tissue indicated at least 5% dye-affinity when the total field of
view was observed at x40 magnification. Assessment of the staining
was evaluated by two independent pathologists without knowledge of
the clinical status of the patients.
Statistical analysis
The paired t-test was used to determine the
correlations between the amounts of ANXA1 mRNA expression in cancer
and normal tissues. The Chi-square test or the Mann-Whitney U test
was used to analyze the correlations between ANXA1 protein
expression and each clinicopathological factor upon
immunohistochemical staining. Survival analysis was performed using
the log-rank test followed by the Kaplan-Meier method. We assumed
there was a significant difference at P<0.05. These analyses
were performed using SPSS II for Windows 11.0.1J (Lead
Technologies, Inc., Charlotte, NC, USA).
Results
ANXA1 mRNA expression in gastric and
colon cancer
Levels of ANXA1 mRNA expression in the cancer and
adjacent normal tissues obtained from 25 cases of gastric cancer
and 37 cases of colon cancer were examined by quantitative RT-PCR.
ANXA1 mRNA expression was differentially altered in cancer and
normal tissues depending on the cases. As a result, there was no
statistical significancy between the ANXA1 expression level of
cancer tissue and that of normal tissues in the gastric and colon
cancer tissues (P=0.2231 and P=0.7331, respectively) (Fig. 1).
Immunohistochemical determination of
ANXA1 protein expression in gastric and colon cancer
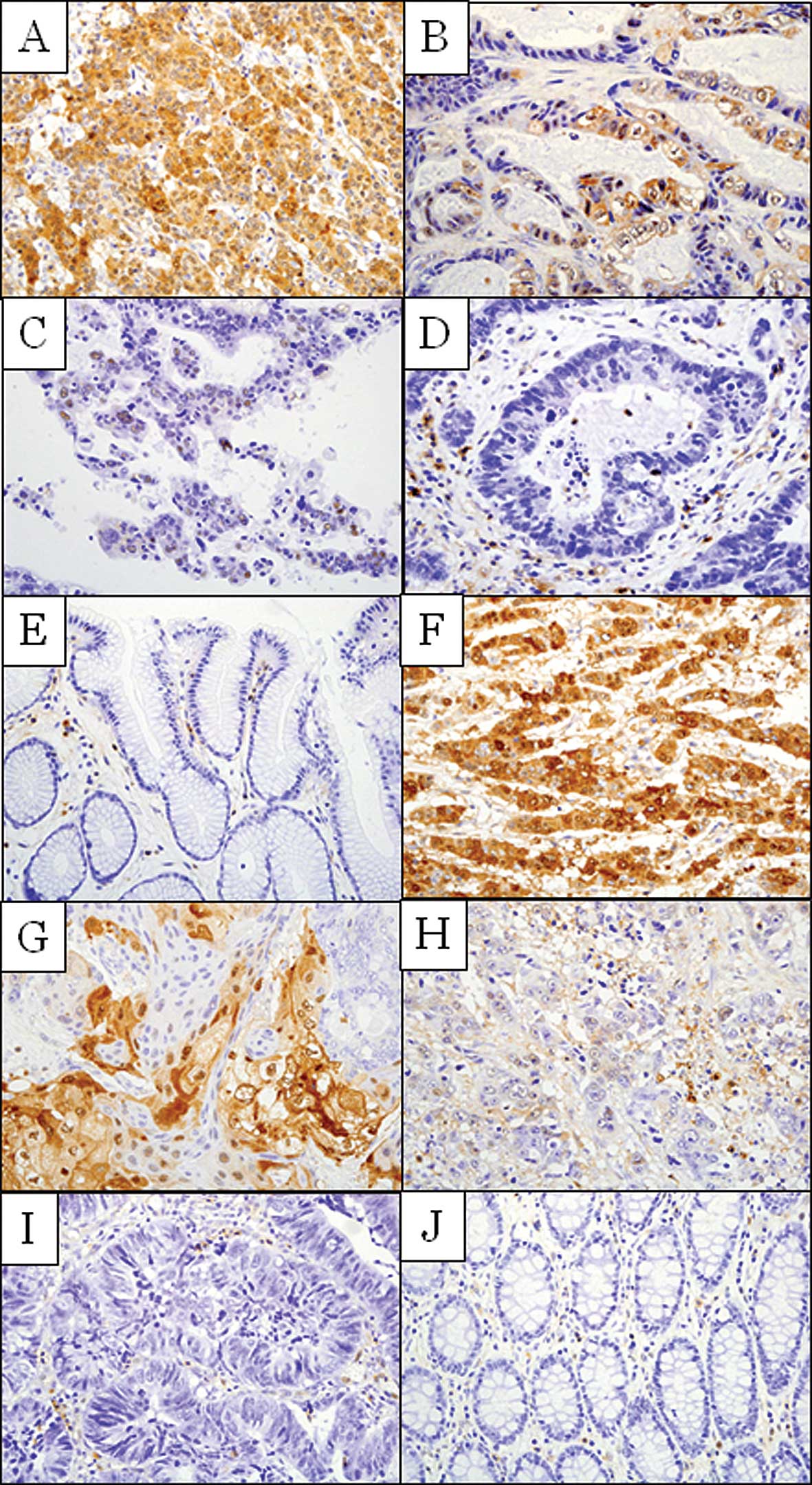
ANXA1 protein expression in 135 gastric cancer and
210 colon cancer tissues was evaluated by immunohistochemical
staining using an anti-ANXA1 antibody. ANXA1 expression was
detected in the nucleus and cytoplasm of the malignant cells. ANXA1
expression was observed in 76 of 135 cases (56.3%) in gastric
cancer and 61 of 210 cases (29.0%) in colon cancer (Fig. 2). The staining pattern in the
cancerous region showed a tendency to be localized in the invasive
lesion of the cancer tissue. ANXA1 expression was not detected in
the normal mucosa in almost all cases, although ANXA1 expression of
immunocompetent cells, such as lymphocytes in interstitial and
connective tissues, including vessels, was sometimes observed,
depending on the case.
Association of ANXA1 protein expression
and clinicopathological factors
In gastric cancer, high ANXA1 protein expression
showed significant positive correlations with depth of wall
invasion (P<0.001), lymphatic invasion (P=0.023), venous
invasion (P=0.002), lymph node metastasis (P=0.001) and UICC stage
(P<0.001) (Table I). There were
no correlations between ANXA1 protein expression and gender, age or
histological differentiation. In colon cancer, high ANXA1 protein
expression showed significant positive correlations with gender
(P=0.038), lymphatic invasion (P=0.011), venous invasion (P=0.023),
lymph node metastases (P=0.042) and UICC stage (P=0.041) (Table II). However, no significant
correlations were observed between ANXA1 expression and age, tumor
location, histological differentiation, depth of wall invasion and
the presence of hepatic metastases.
 | Table I.Relationship between ANXA1 expression
and clinico-pathological findings in gastric cancer. |
Table I.
Relationship between ANXA1 expression
and clinico-pathological findings in gastric cancer.
| Characteristics | ANXA1 expression
| P-value |
|---|
| Negative (n=59) | Positive (n=76) |
|---|
| Gender | | | 0.649 |
| Male | 41 (69%) | 50 (66%) | |
| Female | 18 (31%) | 26 (34%) | |
| Age (years) | | | 0.610 |
| <60 | 20 (34%) | 29 (38%) | |
| >60 | 39 (66%) | 47 (62%) | |
| Tumor
differentiation | | | 0.368 |
| Differentiated | 31 (53%) | 34 (45%) | |
|
Undifferentiated | 28 (47%) | 42 (55%) | |
| Wall invasion | | | <0.001 |
| T1 | 31 (53%) | 12 (16%) | |
| T2 | 20 (34%) | 34 (45%) | |
| T3 | 8 (13%) | 30 (39%) | |
| Lymphatic
invasion | | | 0.023 |
| Absent | 16 (27%) | 9 (12%) | |
| Present | 43 (73%) | 67 (88%) | |
| Venous invasion | | | 0.002 |
| Absent | 25 (42%) | 14 (18%) | |
| Present | 34 (58%) | 62 (82%) | |
| Lymph node
metastasis | | | 0.001 |
| Negative | 36 (61%) | 25 (33%) | |
| Positive | 23 (39%) | 51 (67%) | |
| UICC stage | | | <0.001 |
| I | 40 (68%) | 26 (34%) | |
| II | 8 (14%) | 17 (22%) | |
| III | 9 (15%) | 28 (37%) | |
| IV | 2 (3%) | 5 (7%) | |
 | Table II.Relationship between ANXA1 expression
and clinicopathological findings in colon cancer. |
Table II.
Relationship between ANXA1 expression
and clinicopathological findings in colon cancer.
| Characteristics | ANXA1 expression
| P-value |
|---|
| Negative (n=149) | Positive (n=61) |
|---|
| Gender | | | 0.038 |
| Male | 94 (63%) | 29 (48%) | |
| Female | 55 (37%) | 32 (52%) | |
| Age (years) | | | 0.816 |
| <60 | 44 (30%) | 19 (31%) | |
| >60 | 105 (70%) | 42 (69%) | |
| Tumor location | | | 0.180 |
| Proximal | 44 (30%) | 24 (39%) | |
| Distant | 61 (40%) | 17 (28%) | |
| Rectum | 44 (30%) | 20 (33%) | |
| Tumor
differentiation | | | 0.719 |
| Well | 67 (45%) | 28 (46%) | |
| Moderate | 68 (46%) | 24 (39%) | |
| Poor | 4 (3%) | 2 (3%) | |
| Mucinous | 10 (6%) | 7 (12%) | |
| Wall invasion | | | 0.274 |
| T1 | 18 (12%) | 4 (7%) | |
| T2 | 18 (12%) | 7 (11%) | |
| T3 | 103 (70%) | 45 (74%) | |
| T4 | 10 (6%) | 5 (8%) | |
| Lymphatic
invasion | | | 0.011 |
| Absent | 38 (26%) | 6 (10%) | |
| Present | 111 (74%) | 55 (90%) | |
| Venous
invasion | | | 0.023 |
| Absent | 35 (23%) | 6 (10%) | |
| Present | 114 (77%) | 55 (90%) | |
| Lymph node
metastasis | | | 0.042 |
| Negative | 97 (65%) | 30 (49%) | |
| Positive | 52 (35%) | 31 (51%) | |
| Hepatic
metastasis | | | 0.404 |
| Negative | 131 (88%) | 51 (84%) | |
| Positive | 18 (12%) | 10 (16%) | |
| UICC stage | | | 0.041 |
| I | 32 (21%) | 8 (13%) | |
| II | 61 (41%) | 21 (35%) | |
| III | 38 (26%) | 22 (36%) | |
| IV | 18 (12%) | 10 (16%) | |
Relationship between ANXA1 expression and
prognosis in gastric and colon cancer
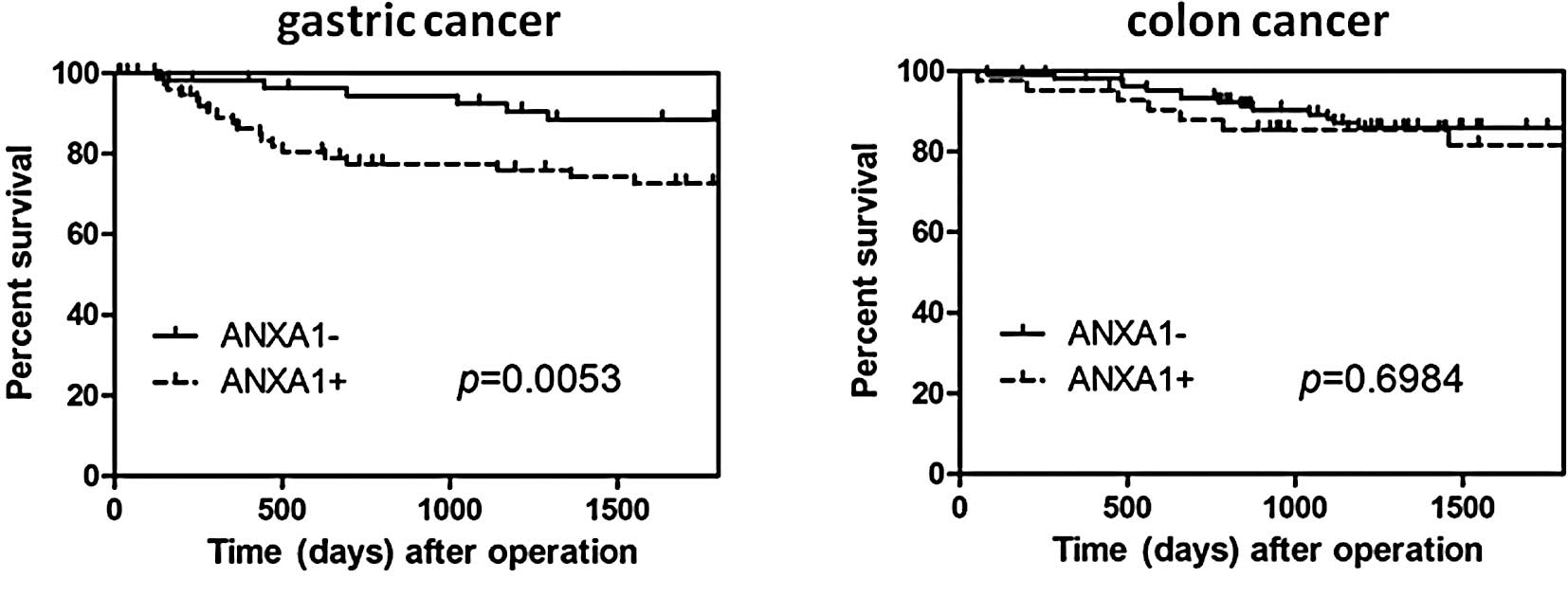
The disease-specific survival rate of the gastric
cancer patients with high ANXA1 expression was significantly lower
as compared to the patients without ANXA1 expression (P=0.0053)
(Fig. 3A). However, this
difference in expression was not an independent predictor on
multivariate analysis using the Cox regression model. In colon
cancer, the disease-specific survival rate of the patients with
high ANXA1 expression tended to be lower than that in patients
without ANXA1 expression, although this was not statistically
significant (P=0.6984) (Fig.
3B).
Discussion
ANXA1 is a calcium-dependent phospholipid-linked
protein with a molecular weight of 37 kDa that shows phospholipase
A2 inhibitory activity and is derived from glucocorticoid (19). ANXA1 is an endogenous
anti-inflammatory protein with a number of biological functions,
such as cell adhesion, inhibition of migration, inflammation,
phagocytosis, proliferation and apoptosis (20,21).
ANXA1 also has an important role in tumor development and
progression, although the exact mechanisms in cancer remain
unknown. To date, numerous reports suggest that ANXA1 is
differentially expressed depending on cancer types. In fact, ANXA1
was found to be up-regulated in esophageal cancer (8), pancreatic cancer (9), skin squamous cell carcinoma (10) and colon cancer (11), and down-regulated in cervical
cancer (12), oral squamous cell
carcinoma (13), prostate cancer
(14), breast cancer (15–17)
and laryngeal squamous cell carcinoma (18). For example, high levels of ANXA1
expression were detected in approximately 40% of cases of
esophageal and gastroesophageal junction adenocarcinoma and were
correlated with the progression of T factor and distant metastases
(8). The studies concluded that
ANXA1 is an independent prognostic factor for patient survival.
Regarding breast cancer, suppression of ANXA1 expression was found
to be related to cancer development and progression (15–17).
This discrepancy indicates that a physiological range of ANXA1
levels is important to maintain cellular homeostasis. In
particular, the evaluation of ANXA1 expression has not been
accurately carried out in gastric and colon cancer. In the present
study, we found that ANXA1 protein expression was up-regulated in
approximately 60% of gastric cancer and 30% of colon cancer
tissues, and high expression of ANXA1 was associated with advanced
stage, including deeper wall invasion, lymphatic invasion, venous
invasion and lymph node metastasis. Consequently, ANXA1 expression
was implicated in poor prognosis. Our results suggest that ANXA1
plays an essential role in tumor growth, invasion and
metastasis.
There are only a few reports on ANXA1 in gastric and
colon cancer. A previous report found that the lack of ANXA1
protein expression was correlated with the degree of T factor
progression, lymph node metastasis, advanced stage and histological
low differentiation by microarray analysis using tissue samples
obtained from gastric cancer patients, suggesting that ANXA1 is a
negative biomarker of gastric cancer growth and development
(22). Although the results
conflict with our data, various reasons explaining this discrepancy
include the experimental condition and the different antibody used
for immunohistochemical staining. Therefore, whether ANXA1
expression is up- or down-regulated in gastric cancer is difficult
to determine. In fact, similar findings were recognized in breast
cancer. Our findings provide new insight to the association between
ANXA1 expression and gastric cancer. Regarding colon cancer, one
supportive study found increased ANXA1 protein expression in
primary and metastatic lesions when compared to the normal mucosa.
However, no correlation was found between the extent of ANXA1
expression and tumor stage (11).
Recently, the result of an ANXA1-knockout mice study
suggested that tumor growth and metastasis using cancer cell lines
were significantly decreased in ANXA1-knockout mice compared to
ANXA1 wild-type mice (23).
Furthermore, ANXA1 acts directly on the EGFR to regulate the
EGFR/Ras pathway, and binds to the EGFR adaptor protein Grb2 and
increases Ras activity, resulting in the activation of the
mitogen-activated protein kinase extracellular signal-regulated
kinase (24). An in vitro
experiment of a colorectal cancer cell line, knocked down for ANXA1
expression using an siRNA method, found attenuated cellular
invasion (25). These data
indicate that ANXA1 expression may be associated with tumor growth,
tumor invasion and metastasis, and may support our current results
that ANXA1 expression is up-regulated in a subset of gastric and
colon cancer and is significantly associated with lymphatic
invasion, venous invasion, lymph node metastases and UICC stage in
both gastric and colon cancer.
When ANXA1 mRNA was measured in the cancer and
non-cancer tissues, no significant differences were observed in the
ANXA1 expression level in either type of cancer. Although the
number of cases included in the study was small, we realized that
it was difficult to evaluate the ANXA1 expression level accurately
in cancer cells as ANXA1 was expressed non-specifically in
infiltrating cells of interstitial tissues as determined by
immunohistochemical staining. Therefore, immunohistochemical
staining is a more accurate method than real-time PCR for
evaluating ANXA1 expression in clinical samples.
Our results indicate that the clinical significance
of ANXA1 expression is related to tumor invasion and metastasis,
resulting in poor prognosis. Thus, ANXA1 expression may be a useful
marker to predict malignant potential of gastric and colon cancer.
Further investigation is needed before its application as a target
therapy for cancer.
References
|
1.
|
Budhu A, Forgues M, Ye QH, et al:
Prediction of venous metastases, recurrence, and prognosis in
hepatocellular carcinoma based on a unique immune response
signature of the liver microenvironment. Cancer Cell. 10:99–111.
2006. View Article : Google Scholar
|
|
2.
|
Seike M, Yanaihara N, Bowman ED, Zanetti
KA, Budhu A, Kumamoto K, Mechanic LE, Matsumoto S, Yokota J,
Shibata T, Sugimura H, Gemma A, Kudoh S, Wang XW and Harris CC: Use
of a cytokine gene expression signature in lung adenocarcinoma and
the surrounding tissue as a prognostic classifier. J Natl Cancer
Inst. 99:1257–1269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bronte-Tinkew DM, Terebiznik M, Franco A,
Ang M, Ahn D, Mimuro H, Sasakawa C, Ropeleski MJ, Peek RM Jr and
Jones NL: Helicobacter pylori cytotoxin-associated gene A
activates the signal transducer and activator of transcription 3
pathway in vitro and in vivo. Cancer Res. 69:632–639. 2009.
View Article : Google Scholar
|
|
4.
|
Muto T, Bussey HJ and Morson BC: The
evolution of cancer of the colon and rectum. Cancer. 36:2251–2270.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Minamoto T, Sawaguchi K, Mai M, Yamashita
N, Sugimura T and Esumi H: Infrequent K-ras activation in
superficial-type (flat) colorectal adenomas and adenocarcinomas.
Cancer Res. 54:2841–2844. 1994.PubMed/NCBI
|
|
6.
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L and Karin M: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lim LH and Pervaiz S: Annexin 1: the new
face of an old molecule. FASEB J. 21:968–975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang KL, Wu TT, Resetkova E, Wang H,
Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton
SR and Albarracin CT: Expression of annexin A1 in esophageal and
esophagogastric junction adenocarcinomas: association with poor
outcome. Clin Cancer Res. 12:4598–4604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bai XF, Ni XG, Zhao P, Liu SM, Wang HX,
Guo B, Zhou LP, Liu F, Zhang JS, Wang K, Xie YQ, Shao YF and Zhao
XH: Overexpression of annexin 1 in pancreatic cancer and its
clinical significance. World J Gastroenterol. 10:1466–1470.
2004.PubMed/NCBI
|
|
10.
|
Hummerich L, Müller R, Hess J, Kokocinski
F, Hahn M, Fürstenberger G, Mauch C, Lichter P and Angel P:
Identification of novel tumour-associated genes differentially
expressed in the process of squamous cell cancer development.
Oncogene. 25:111–121. 2006.PubMed/NCBI
|
|
11.
|
Duncan R, Carpenter B, Main LC, Telfer C
and Murray GI: Characterisation and protein expression profiling of
annexins in colorectal cancer. Br J Cancer. 98:426–433. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wang LD, Yang YH, Liu Y, Song HT, Zhang LY
and Li PL: Decreased expression of annexin A1 during the
progression of cervical neoplasia. J Int Med Res. 36:665–672. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lin CY, Jeng YM, Chou HY, Hsu HC, Yuan RH,
Chiang CP and Kuo MY: Nuclear localization of annexin A1 is a
prognostic factor in oral squamous cell carcinoma. J Surg Oncol.
97:544–550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kang JS, Calvo BF, Maygarden SJ, Caskey
LS, Mohler JL and Ornstein DK: Dysregulation of annexin I protein
expression in high-grade prostatic intraepithelial neoplasia and
prostate cancer. Clin Cancer Res. 8:117–123. 2002.PubMed/NCBI
|
|
15.
|
Chuthapisith S, Bean BE, Cowley G, Eremin
JM, Samphao S, Layfield R, Kerr ID, Wiseman J, El-Sheemy M,
Sreenivasan T and Eremin O: Annexins in human breast cancer:
possible predictors of pathological response to neoadjuvant
chemotherapy. Eur J Cancer. 45:1274–1281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Cao Y, Li Y, Edelweiss M, Arun B, Rosen D,
Resetkova E, Wu Y, Liu J, Sahin A and Albarracin CT: Loss of
annexin A1 expression in breast cancer progression. Appl
Immunohistochem Mol Morphol. 16:530–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Shen D, Nooraie F, Elshimali Y, Lonsberry
V, He J, Bose S, Chia D, Seligson D, Chang HR and Goodglick L:
Decreased expression of annexin A1 is correlated with breast cancer
development and progression as determined by a tissue microarray
analysis. Hum Pathol. 37:1583–1591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
lves VA, Nonogaki S, Cury PM, Wünsch-Filho
V, de Carvalho MB, Michaluart-Júnior P, Moyses RA, Curioni OA,
Figueiredo DL, Scapulatempo-Neto C, Parra ER, Polachini GM,
Silistino-Souza R, Oliani SM, Silva-Júnior WA, Nobrega FG; Head and
Neck Genome Project/GENCAPO; Tajara EH and Zago MA: Annexin A1
subcellular expression in laryngeal squamous cell carcinoma.
Histopathology. 53:715–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kim SW, Rhee HJ, Ko J, Kim YJ, Kim HG,
Yang JM, Choi EC and Na DS: Inhibition of cytosolic phospholipase
A2 by annexin I. Specific interaction model and mapping of the
interaction site. J Biol Chem. 276:15712–15719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Gerke V and Moss SE: Annexins: from
structure to function. Physiol Rev. 82:331–371. 2002.PubMed/NCBI
|
|
21.
|
Parente L and Solito E: Annexin 1: more
than an anti-phospholipase protein. Inflamm Res. 53:125–132. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yu G, Wang J, Chen Y, Wang X, Pan J, Li Q
and Xie K: Tissue microarray analysis reveals strong clinical
evidence for a close association between loss of annexin A1
expression and nodal metastasis in gastric cancer. Clin Exp
Metastasis. 25:695–702. 2008. View Article : Google Scholar
|
|
23.
|
Yi M and Schnitzer JE: Impaired tumor
growth, metastasis, angiogenesis and wound healing in annexin
A1-null mice. Proc Natl Acad Sci USA. 106:17886–17891. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Grewal T and Enrich C: Annexins –
modulators of EGF receptor signalling and trafficking. Cell Signal.
21:847–858. 2009.
|
|
25.
|
Babbin BA, Lee WY, Parkos CA, Winfree LM,
Akyildiz A, Perretti M and Nusrat A: Annexin I regulates SKCO-15
cell invasion by signaling through formyl peptide receptors. J Biol
Chem. 281:19588–19599. 2006. View Article : Google Scholar : PubMed/NCBI
|