Introduction
Triple-negative breast cancer (TNBC) is a type of
breast cancer that lacks expression of estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor 2 (HER2). This subtype accounts for 10–25% of all breast
cancers (1–7) and is frequently observed in patients
of African or Hispanic descent, whereas it is relatively uncommon
among Asian patients (4,6,8).
TNBC is known to be associated with a poor prognosis (3–5,7).
Recently, gene expression studies using DNA
microarrays have identified several distinct breast cancer
subtypes: two ER-positive subtypes (luminal A and B) and three
ER-negative subtypes (HER2-positive, basal-like and normal
breast-like) (9,10). Among these subtypes, the basal-like
subtype is clinically relevant since it shows a clinical course
that is as aggressive as the HER2-positive subtype (9,10).
Most breast cancers of the basal-like subtype have been reported to
be immunohistochemically positive for cytokeratin (CK) 5/6 and/or
epidermal growth factor receptor (EGFR), but negative for ER and
HER2 (11). It appears that the
basal-like subtype constitutes 80% of TNBCs and is more aggressive
than the non-basal-like subtype of TNBCs (2,3).
Due to the lack of proper therapeutic targets,
patients with TNBC usually do not benefit from appropriate therapy,
such as hormone or HER2-targeted therapy. An increasing number of
patients with TNBC, which are not always at an advanced stage, have
been receiving neoadjuvant chemotherapy (NAC), and this has
resulted in a higher rate of partial resection, rather than
mastectomy. Several studies have reported that TNBC responds more
frequently to NAC than non-TNBC, and consequently 20–30% of TNBC
patients achieve a pathological complete response (pCR) (1,8,12).
Breast cancer patients who achieve a pCR to NAC generally have an
excellent prognosis, while patients with residual disease (RD),
whose invasive carcinoma cells do not disappear completely even
after NAC, usually have a poor prognosis (8,13).
It seems that TNBC patients with RD after NAC have a considerably
worse prognosis than non-TNBC patients with RD. Liedtke et
al (1) reported that 68% of
TNBC patients with RD after NAC survived for at least 3 years after
surgery, in comparison to 88% of patients with non-TNBC.
Collectively, TNBC includes NAC-sensitive and -resistant subgroups,
which show a significant difference in outcome after NAC.
Therefore, it is necessary to identify markers that distinguish
these subgroups.
In the present study, we analyzed TNBC patients who
received combination NAC with anthracycline and taxane at a single
institution. Our main aim was to identify markers that could
predict pCR to NAC by analyzing the correlation between
clinicopathological parameters and pathological response. We also
attempted to clarify prognostic factors that have a major influence
on the outcome of TNBC patients with RD after NAC.
Materials and methods
Patients and tumor characteristics
This study included 44 TNBC patients with a median
age of 52 years (range 36–70). These patients received
anthracycline- and taxane-based combination chemotherapy prior to
surgery at Saitama Cancer Center, Saitama, Japan, between July 2004
and July 2008. The characteristics of the patients and their tumors
are listed in Table I. The mean
follow-up period was 12 months (range 1–35). Patients at clinical
stages II, III and IV were enrolled. The 1 patient with clinical
stage IV disease had only ipsilateral subclavicular lymph node
involvement. Clinical tumor size, lymph node status and TNM status
were evaluated by clinical examination, mammography, breast
ultrasonography and magnetic resonance imaging. The tumor subtype
in 39 patients was invasive ductal carcinoma, not otherwise
specified (NOS), and the tumors in the other 5 patients comprised 3
metaplastic carcinomas, 1 apocrine carcinoma and 1 invasive
micropapillary carcinoma, respectively, on the basis of the WHO
criteria (14). The histological
grade of each tumor was assessed according to the criteria
described by Elston and Ellis (15), and all of the tumors were judged as
grade III.
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
| Characteristics | No. |
|---|
| Age range (median),
in years | 36–70 (52) |
| ≤40 | 5 |
| >40 | 39 |
| Menopausal
status | |
| Pre-menopausal | 21 |
| Menopausal | 23 |
| Clinical tumor
size | |
| T1 | 3 |
| T2 | 24 |
| T3 | 11 |
| T4 | 6 |
| Clinical lymph node
status | |
| N0 | 8 |
| N1 | 26 |
| N2 | 5 |
| N3 | 4 |
| M1 (ipsilateral
subclavicular lymph node) | 1 |
| Clinical TNM
stage | |
| IIA | 9 |
| IIB | 15 |
| IIIA | 11 |
| IIIB | 4 |
| IIIC | 4 |
| IV | 1 |
| Histology | |
| Invasive ductal
carcinoma, NOS | 39 |
| Metaplastic
carcinoma | 3 |
| Apocrine
carcinoma | 1 |
| Invasive
micropapillary carcinoma | 1 |
| Regimens of
neoadjuvant chemotherapy | |
| 4 cycles of AC
(60a, 600a) and 4 cycles of T
(70a) | 35b |
| 4 cycles of AC
(60a, 600a) and 12 cycles of P
(80a) | 1 |
| 4 cycles of AP
(50a, 150a) and 4 cycles of T
(70a) or 12 cycles of
P (80a) | 5 |
| 4 cycles of EC
(75a, 600a) and 4 cycles of T
(70a) or 12 cycles of
P (80a) | 2 |
| 4 cycles of CEF
(500a, 100a, 500a) and 4 cycles of T
(70a) | 1 |
| Type of
surgery | |
| Breast-conserving
therapy | 41 |
| Mastectomy | 3 |
Treatment
Thirty-three patients received 4 cycles of
doxorubicin (60 mg/mm2) and cyclophosphamide (600
mg/mm2) followed by 4 cycles of docetaxel (70
mg/mm2). The treatment regimens in the other patients
are described in Table I. After
completion of NAC, 41 patients underwent breast-conserving therapy
and the others underwent mastectomy. Postoperative radiotherapy was
performed for the 41 patients who received breast-conserving
therapy. Among the 10 patients whose tumors were diagnosed as
having a positive surgical margin (tumor cells being observed
within 5 mm from the surgical margin by histological examination),
2 underwent additional breast-conserving therapy and the others
received additional booster radiotherapy.
Definition of triple-negative breast
cancer
Expression of ER and PR was evaluated by
immunohistochemistry. Tumors were interpreted as negative for ER
and PR when <10% of all tumor cells showed nuclear staining.
HER2 was considered to be negative when the HER2 score was 0 or 1+
by immunohistochemistry, or when the HER2/CEP17 gene copy ratio was
<1.8 by FISH analysis in tumors, the HER2 score of which was 2+.
TNBCs were defined as ER-negative, PR-negative and HER2-negative
breast cancers.
Assessment of pathological response and
definition of pathological complete response
The response grade of each tumor was determined by
at least three of the authors (K.S., H.O. and M.K.). The degree of
pathological response to NAC was evaluated according to the
response criteria of the Japanese Breast Cancer Society (16). pCR was defined as complete
disappearance of invasive carcinoma cells in both the breast and
axillary lymph nodes after NAC. Residual intraductal carcinoma was
included in the pCR category (13).
Clinicopathological studies for
prediction of pathological complete response to neoadjuvant
chemotherapy
The relationships between pathological response and
clinicopathological characteristics (age, menopausal status,
clinical tumor size, lymph node status, TNM stage, histological
classification, morphological features and immunohistochemical
parameters) were analyzed in order to identify factors that are
closely correlated with pCR to NAC. For the histological analysis,
we focused our attention on the 39 invasive ductal carcinomas,
since the other five tumor types are biologically distinct from
conventional invasive ductal carcinomas. We examined the 39
pre-treatment core-needle biopsy specimens to detect markers that
were significantly correlated with a good response to NAC. The
morphological features evaluated were central necrosis, central
fibrosis, lymphoid stroma and pushing margin. These features are
frequently found in ER-negative breast carcinomas and
basal-like-subtype breast carcinomas (overlapped with TNBC)
(17,18). The immunohistochemical parameters
evaluated were CK5/6, EGFR, Ki-67, p53, breast cancer
susceptibility protein 1 (BRCA1) and topoisomerase IIα (TOPO IIα).
The methods used for immunohistochemistry were previously described
(19). The clone designations,
companies that supplied the primary antibodies and dilution factors
used were: CK5/6 (D5/16B4; Dako Japan; 1:50), Ki-67 (MIB-1; Dako
Japan; 1:50), p53 (DO7; Dako Japan; 1:100), BRCA1 (MS110; EMD
Bioscience Calbiochem; 1:50) and TOPO IIα (Ki-S1; Dako Japan;
1:100). EGFR was stained using an EGFRpharmDx kit (Dako Japan) in
accordance with the manufacturer’s instructions. Cases were
interpreted as positive for CK5/6 and EGFR when any of the tumor
cells had cytoplasmic and membranous staining, respectively. The
basal-like subtype was defined as CK5/6-and/or EGFR-positive among
the TNBCs (11). The Ki-67
labeling index (LI) was defined as the ratio of MIB-1-stained tumor
cells to all tumor cells counted, multiplied by 100. To evaluate
the Ki-67 LI, stained tumor cells were counted in at least three
high-power fields that showed the highest positivity in each
section. Since TNBC commonly shows a high Ki-67 LI, we used a Ki-67
LI of 50% as a cut-off point to define each tumor as having a high
or low Ki-67 LI; this value was almost equal to the mean index of
TNBC observed at our institution. For the expression of p53, BRCA1
and TOPO IIα, nuclear staining cutoff values of 25 (12), 10 (20) and 25% (2) of all tumor cells, respectively, were
used to define positivity.
Clinicopathological studies for the
prediction of outcome after neoadjuvant chemotherapy
For this analysis, from 28 TNBC patients with RD 1
patient with stage IV disease was excluded. Of the 27 patients with
RD, the relationships between disease-free survival (DFS) and
various clinicopathological characteristics were assessed to
identify the factors related to outcome after NAC. We analyzed the
surgical materials resected after NAC to obtain data on
pathological parameters (tumor size, lymph node status, TNM status,
grade of pathological response and lymphovascular invasion).
Statistical analysis
Fisher’s exact test was conducted to analyze the
associations between pathological response to NAC and
clinicopathological characteristics. The Kaplan-Meier method was
used to estimate the DFS and overall survival (OS) survivorship
functions. Survival curves of the pCR and RD groups were compared
using the log-rank test. The univariate Cox proportional hazards
model was used to derive factors that were predictive of
unfavorable DFS in patients with RD after NAC.
Results
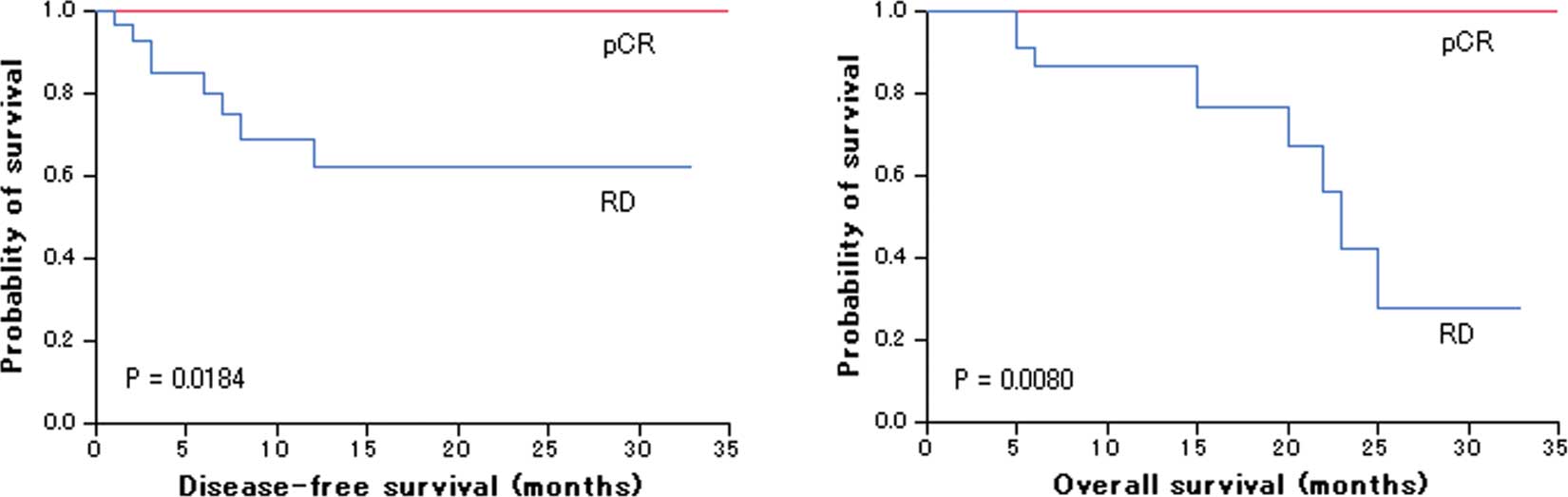
Pathological response and survival
analysis
No residual invasive carcinoma cells in the breast
were confirmed histologically in 19 of the 44 patients (43%) after
NAC. Three of the 19 patients, however, had residual carcinoma
cells in the resected lymph nodes. As a result, 16 of the 44
patients (36%) achieved a pCR. All of the patients with pCR were
alive and well during their follow-up periods (mean 17.5; range
1–35 months). On the other hand, 8 of the 28 patients (29%) with RD
relapsed during follow-up (mean 11; range 2–33 months) and died of
breast cancer within 2 years after surgery. Log-rank test showed
that patients with pCR had a significantly more favorable outcome
than patients with RD (DFS, P=0.00184; OS, P=0.0080) (Fig. 1).
Factors predictive of a pathological
response to neoadjuvant chemotherapy
Although the associations between pathological
response to NAC and the clinicopathological characteristics (age,
menopausal status, clinical tumor size, lymph node status, TNM
stage and histological classification) were evaluated, none of the
characteristics was significantly correlated with pathological
response, as represented in Table
II.
 | Table II.Correlations between pathological
response and clinicopathological characteristics. |
Table II.
Correlations between pathological
response and clinicopathological characteristics.
|
Characteristics | pCR, no. (%) | RD, no. (%) | P-valuea |
|---|
| Age, in years | | | |
| ≤40 | 1 (20) | 4 (80) | 0.6380 |
| >40 | 15 (38) | 24 (62) | |
| Menopausal
status | | | |
|
Pre-menopausal | 7 (33) | 14 (67) | 0.7606 |
| Menopausal | 9 (39) | 14 (61) | |
| Clinical tumor
size | | | |
| T1, T2 | 9 (33) | 18 (67) | 0.7495 |
| T3, T4 | 7 (41) | 10 (59) | |
| Clinical lymph node
status | | | |
| Negative | 2 (25) | 6 (75) | 0.6895 |
| Positive | 14 (39) | 22 (61) | |
| Clinical TNM
stage | | | |
| IIA, IIB | 9 (38) | 15 (63) | >0.9999 |
| IIIA, IIIB, IIIC,
IV | 7 (35) | 13 (65) | |
| Histology | | | |
| Invasive ductal
carcinoma, NOS | 15 (38) | 24 (62) | 0.6380b |
| Metaplastic
carcinoma | 0 (0) | 3 (100) | |
| Apocrine
carcinoma | 1 (100) | 0 (0) | |
| Invasive
micropapillary carcinoma | 0 (0) | 1 (100) | |
In the 39 invasive ductal carcinomas that were
examined histologically, the only factor that was significantly
associated with response to NAC was immunohistochemical
overexpression of p53 (Table III;
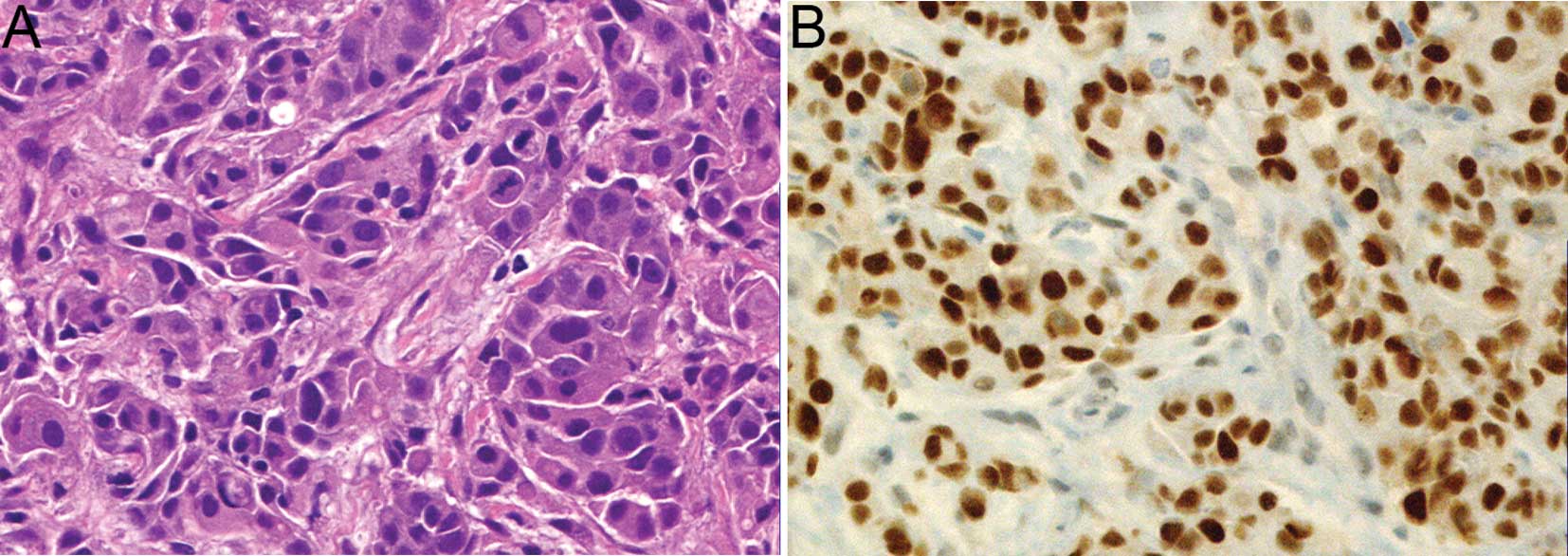
P=0.0484). The representative histology and immunohistochemistry
for p53 of a tumor that disappeared in response to NAC are shown in
Fig. 2. In addition, it was
suggested that an absence of central necrosis was correlated with a
higher pCR rate (P=0.0832). The pCR rate for basal-like subtype
cancers, accounting for 34 of the 39 invasive ductal carcinomas
(87%), was similar to that for non-basal-like subtype cancers.
Although overexpression of TOPO IIα was observed in 27 of the 39
invasive ductal carcinomas (69%), no significant correlation
between overexpression and the rate of pathological response to NAC
was observed.
 | Table III.Correlations between pathological
response and histological features, including immunohistochemical
parameters. |
Table III.
Correlations between pathological
response and histological features, including immunohistochemical
parameters.
|
Characteristics | pCR, no. (%) | RD, no. (%) | P-valuea |
|---|
| Central
necrosis | | | |
| Positive | 2 (17) | 10 (83) | 0.0832 |
| Negative | 13 (48) | 14 (52) | |
| Central
fibrosis | | | |
| Positive | 3 (30) | 7 (70) | 0.7110 |
| Negative | 12 (41) | 17 (59) | |
| Lymphoid
stroma | | | |
| Positive | 3 (60) | 2 (40) | 0.3541 |
| Negative | 12 (35) | 22 (65) | |
| Pushing margin | | | |
| Positive | 11 (35) | 20 (65) | 0.6857 |
| Negative | 4 (50) | 4 (50) | |
| Basal-like
phenotype | | | |
| Yes | 13 (38) | 21 (62) | >0.9999 |
| No | 2 (40) | 3 (60) | |
| Ki-67 LI | | | |
| High LI | 11 (39) | 17 (61) | >0.9999 |
| Low LI | 4 (36) | 7 (64) | |
| p53 | | | |
| Positive | 11 (55) | 9 (45) | 0.0484 |
| Negative | 4 (21) | 15 (79) | |
| BRCA1 | | | |
| Positive | 4 (27) | 11 (73) | 0.3172 |
| Negative | 11 (46) | 13 (54) | |
| TOPO IIα | | | |
| Positive | 11 (41) | 16 (59) | 0.7342 |
| Negative | 4 (33) | 8 (67) | |
Factors predictive of disease-free
survival among the 27 patients with residual disease after
neoadjuvant chemotherapy
Lymphovascular invasion was found to significantly
correlate with shorter DFS (hazard ratio, 13.333; 95% CI
1.587–111.111; P=0.0171), as shown in Table IV.
 | Table IV.Correlations between disease-free
survival after surgery and clinicopathological characteristics in
patients with residual disease. |
Table IV.
Correlations between disease-free
survival after surgery and clinicopathological characteristics in
patients with residual disease.
|
Characteristics | Total no. | Relapse no.
(%) | Hazard ratio | 95% CI | P-valuea |
|---|
| Age, in years | | | | | |
| ≤40 | 4 | 1 (25) | 0.800 | 0.095–6.704 | 0.8366 |
| >40 | 23 | 6 (26) | 1 | | |
| Menopausal
status | | | | | |
|
Pre-menopausal | 14 | 3 (21) | 0.652 | 0.145–2.935 | 0.5770 |
| Menopausal | 13 | 4 (31) | 1 | | |
| Clinical tumor
sizeb | | | | | |
| T1, T2 | 18 | 5 (28) | 1.371 | 0.265–7.087 | 0.7066 |
| T3, T4 | 9 | 2 (22) | 1 | | |
| Pathological tumor
sizec | | | | | |
| T0, T1, T2 | 24 | 5 (21) | 0.316 | 0.061–1.637 | 0.1698 |
| T3, T4 | 3 | 2 (67) | 1 | | |
| Clinical lymph node
statusb | | | | | |
| Positive | 21 | 6 (29) | 1.541 | 0.185–12.821 | 0.6898 |
| Negative | 6 | 1 (17) | 1 | | |
| Pathological lymph
node statusc | | | | | |
| Positive | 18 | 6 (33) | 2.188 | 0.259–18.519 | 0.4722 |
| Negative | 9 | 1 (11) | 1 | | |
| Clinical TNM
stageb | | | | | |
| IIA, IIB | 15 | 2 (13) | 0.304 | 0.059–1.578 | 0.1565 |
| IIIA, IIIB,
IIIC | 12 | 5 (42) | 1 | | |
| Pathological TNM
stagec | | | | | |
| I, IIA, IIB | 22 | 4 (18) | 0.373 | 0.083–1.672 | 0.1974 |
| IIIA, IIIB,
IIIC | 5 | 3 (60) | 1 | | |
| Histology | | | | | |
| Invasive ductal
carcinoma, NOS | 23 | 5 (22) | 0.488 | 0.094–2.525 | 0.3918 |
| Others | 4 | 2 (50) | 1 | | |
| Grade of
pathological response | | | | | |
| 3d, 2b, 2a | 7 | 2(29) | 1.183 | 0.229–6.135 | 0.8405 |
| 1b, 1a | 20 | 5 (25) | 1 | | |
| Lymphovascular
invasion after NAC | | | | | |
| Positive | 10 | 6 (60) | 13.333 | 1.587–111.111 | 0.0171 |
| Negative | 17 | 1 (6) | 1 | | |
Discussion
In the present study we showed that p53
overexpression in the TNBCs examined was strongly correlated with
increased susceptibility of the cancer to NAC. This finding is in
line with data obtained from European TNBC patients who received
NAC (21). Among previous analyses
of breast cancers with various ER and HER2 statuses, some have
shown that p53 abnormalities, including gene mutation, LOH or
immunohistochemical overexpression, are significantly associated
with resistance to anthracycline-based chemotherapy (22,23),
whereas others have not supported this conclusion (24–26).
Although the relationship between p53 abnormality and sensitivity
to NAC seems to be unproven among breast cancers as a whole, it is
likely that immunohistochemical overexpression of p53 protein is a
reliable indicator of a good response to NAC in TNBC. It has also
been demonstrated that p53 overexpression or mutation is present
more frequently in TNBC than in non-TNBC (2,3,27).
From a clinical viewpoint, this suggests that more TNBC patients
than non-TNBC patients may achieve a pCR to NAC. However, as our
study population was small, the results need to be validated in a
larger number of TNBC patients.
Normal p53 protein induces G1 arrest and repairs
damage to DNA, or causes apoptosis in cells with DNA damage. In the
present study, TNBCs that were positive for p53 overexpression,
many of which were considered to have lost their normal p53
function, showed a much better response to NAC than those that were
negative, many of which would have normal p53 function. TNBC cells
that have lost their p53 function appear to accumulate DNA damage,
resulting in cell death during or after NAC. In other words, a lack
of normal p53 function in TNBC cells appears to be one of the
factors determining susceptibility to NAC.
In the present study, all of the TNBC patients with
pCR were alive and well during their follow-up periods. On the
other hand, 8 of the 28 patients with RD after NAC, whose tumors
showed various degrees of response to NAC, relapsed within 2 years
after surgery and died of the disease. TNBC patients with RD after
NAC, which constitute 70–80% of TNBC cases, are likely to relapse
soon after completion of their therapy. Therefore, the clinical
outcome for TNBC as a whole remains poor, irrespective of the high
pCR rate (1,8,12).
We found that the presence of lymphovascular invasion in the
residual tumor tissues after NAC was significantly associated with
a poorer outcome in patients with RD. Nogi et al (28) reported that EGFR-positive TNBC
patients had a worse outcome after NAC compared to patients with
EGFR-negative tumors. In our study, none of the immunohistochemical
markers examined, including CK5/6, EGFR, Ki-67, p53, BRCA1 and TOPO
IIα, were positively correlated with outcome in patients with RD
(data not shown). Since little is known about the prognostic
factors of TNBC patients with RD after NAC, the factors strongly
influencing outcome in such patients remain to be clarified.
TOPO IIα is known to be a target molecule of
anthracyclines. Approximately 40% of HER2-positive breast cancers
have been shown to have a TOP2A gene amplification (29), as the gene is located next to the
HER2 gene on chromosome 17q12-q21. Although very few HER2-negative
breast cancers have a TOP2A gene amplification, previous studies
have indicated that 80–90% of TNBCs are immunohistochemically
positive for TOPO IIα protein (2,30).
The finding that 69% of the TNBCs we examined demonstrated TOPO IIα
protein overexpression is compatible with previous data. Although
it is reported that TOPO IIα-positive breast cancers, i.e., those
showing a gene amplification and/or protein overexpression, are
susceptible to anthracycline (29,31,32),
we did not observe a prominent correlation between TOPO IIα protein
expression and pathological response to NAC. On the other hand,
TOPO IIα protein expression is apparently associated with the
growth activity of breast cancer cells (33,34).
In our study, the expression of TOPO IIα protein was significantly
correlated with the Ki-67 LI (data not shown) of the tumor cells.
Thus, high positivity for TOPO IIα in TNBC seems to reflect the
high proliferative activity of the tumor cells.
In conclusion, immunohistochemical overexpression of
p53 in TNBC cells has been shown to be strikingly correlated with a
pCR; p53 overexpression may thus be a marker of a more favorable
response to anthracycline- and taxane-based NAC. TNBC patients with
residual disease after NAC, particularly those with lymphovascular
invasion, often relapsed and died of the cancer. There is an urgent
need to develop effective therapy for TNBCs that are resistant to
anthracycline- and taxane-based NAC.
References
|
1.
|
Liedtke C, Mazouni C, Hess KR, et al:
Response to neoadjuvant therapy and long-term survival in patients
with triple-negative breast cancer. J Clin Oncol. 26:1275–1281.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Tan DS, Marchió C, Jones RL, Savage K,
Smith IE, Dowsett M and Reis-Filho JS: Triple negative breast
cancer: molecular profiling and prognostic impact in adjuvant
anthracycline-treated patients. Breast Cancer Res Treat. 111:27–44.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rakha EA, El-Sayed ME, Green AR, Lee AH,
Robertson JF and Ellis IO: Prognostic markers in triple-negative
breast cancer. Cancer. 109:25–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: a population-based study from the California Cancer
Registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar
|
|
5.
|
Haffty BG, Yang Q, Reiss M, Kearney T,
Higgins SA, Weidhaas J, Harris L, Hait W and Toppmeyer D:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Carey LA, Perou CM, Livasy CA, et al:
Race, breast cancer subtypes, and survival in the Carolina Breast
Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sørlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001.PubMed/NCBI
|
|
10.
|
Sorlie T, Tibshirani R, Parker J, et al:
Repeated observation of breast tumor subtypes in independent gene
expression data sets. Proc Natl Acad Sci USA. 100:8418–8423. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nielsen TO, Hsu FD, Jensen K, et al:
Immunohistochemical and clinical characterization of the basal-like
subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Keam B, Im SA, Kim HJ, et al: Prognostic
impact of clinicopathologic parameters in stage II/III breast
cancer treated with neoadjuvant docetaxel and doxorubicin
chemotherapy: paradoxical features of the triple negative breast
cancer. BMC Cancer. 7:2032007. View Article : Google Scholar
|
|
13.
|
Mazouni C, Peintinger F, Wan-Kau S, Andre
F, Gonzalez Angulo AM, Symmans WF, Meric-Bernstam F, Valero V,
Hortobagyi GN and Pusztai L: Residual ductal carcinoma in situ in
patients with complete eradication of invasive breast cancer after
neoadjuvant chemotherapy does not adversely affect patient outcome.
J Clin Oncol. 25:2650–2655. 2007. View Article : Google Scholar
|
|
14.
|
Tavassoli FA and Devilee P; World Health
Organization: Classification of Tumours: Pathology and Genetics of
Tumours of the Breast and Female Genital Organs. IARC Press; Lyon:
2003
|
|
15.
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar
|
|
16.
|
Kurosumi M: Significance and problems in
evaluations of pathological responses to neoadjuvant therapy for
breast cancer. Breast Cancer. 13:254–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Putti TC, El-Rehim DM, Rakha EA, Paish CE,
Lee AH, Pinder SE and Ellis IO: Estrogen receptor-negative breast
carcinomas: a review of morphology and immunophenotypical analysis.
Mod Pathol. 18:26–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Livasy CA, Karaca G, Nanda R, Tretiakova
MS, Olopade OI, Moore DT and Perou CM: Phenotypic evaluation of the
basal-like subtype of invasive breast carcinoma. Mod Pathol.
19:264–271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kono S, Kurosumi M, Simooka H, Kawanowa K,
Ninomiya J, Takei H, Suemasu K and Kuroda Y: Immunohistochemical
study of the relationship between Ki-67 labeling index of
proliferating cells of gynecomastia, histological phase and
duration of disease. Pathol Int. 56:655–658. 2006. View Article : Google Scholar
|
|
20.
|
Yang Q, Sakurai T, Mori I, Yoshimura G,
Nakamura M, Nakamura Y, Suzuma T, Tamaki T, Umemura T and Kakudo K:
Prognostic significance of BRCA1 expression in Japanese sporadic
breast carcinomas. Cancer. 92:54–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bidard FC, Matthieu MC, Chollet P,
Raoefils I, Abrial C, Dômont J, Spielmann M, Delaloge S, André F
and Penault Llorca F: p53 status and efficacy of primary
anthracyclines/alkylating agent-based regimen according to breast
cancer molecular classes. Ann Oncol. 19:1261–1265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Geisler S, Lønning PE, Aas T, Johnsen H,
Fluge O, Haugen DF, Lillehaug JR, Akslen LA and Børresen-Dale AL:
Influence of TP53 gene alterations and c-erbB-2 expression on the
response to treatment with doxorubicin in locally advanced breast
cancer. Cancer Res. 61:2505–2512. 2001.PubMed/NCBI
|
|
23.
|
Aas T, Børresen AL, Geisler S,
Smith-Sørensen B, Johnsen H, Varhaug JE, Akslen LA and Lønning PE:
Specific P53 mutations are associated with de novo resistance to
doxorubicin in breast cancer patients. Nat Med. 2:811–814. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Mieog JS, van der Hage JA, van de Vijuer
MJ and van de Velde CJ; Cooperating Investigators of the EORTC:
Tumour response to preoperative anthracycline-based chemotherapy in
operable breast cancer: the predictive role of p53 expression. Eur
J Cancer. 42:1369–1379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Colleoni M, Orvieto E, Nolé F, et al:
Prediction of response to primary chemotherapy for operable breast
cancer. Eur J Cancer. 35:574–579. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Bertheau P, Turpin E, Rickman DS, Espié M,
de Reyniès A, Feugeas JP, Plassa LF, Soliman H, Varna M, de
Roquancourt A, Lehmann-Che J, Beuzard Y, Marty M, Misset JL, Janin
A and de Thé H: Exquisite sensitivity of TP53 mutant and basal
breast cancers to a dose-dense epirubicin-cyclophosphamide regimen.
PLoS Med. 4:e902007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Umemura S, Takekoshi S, Suzuki Y, Saitoh
Y, Tokuda Y and Osamura RY: Estrogen receptor-negative and human
epidermal growth factor receptor 2-negative breast cancer tissue
have the highest Ki-67 labeling index and EGFR expression: gene
amplification does not contribute to EGFR expression. Oncol Rep.
14:337–343. 2005.
|
|
28.
|
Nogi H, Kobayashi T, Suzuki M, Tabei I,
Kawase K, Toriumi Y, Fukushima H and Uchida K: EGFR as paradoxical
predictor of chemosensitivity and outcome among triple-negative
breast cancer. Oncol Rep. 21:413–417. 2009.PubMed/NCBI
|
|
29.
|
Tanner M, Isola J, Wiklund T, Erikstein B,
Kellokumpu-Lehtinen P, Malmström P, Wilking N, Nilsson J and Bergh
J: Topoisomerase IIalpha gene amplification predicts favorable
treatment response to tailored and dose-escalated
anthracycline-based adjuvant chemotherapy in HER-2/neu-amplified
breast cancer: Scandinavian Breast Group Trial 9401. J Clin Oncol.
24:2428–2436. 2006. View Article : Google Scholar
|
|
30.
|
Arriola E, Rodriguez-Pinilla SM, Lambros
MB, Jones RL, James M, Savage K, Smith IE, Dowsett M and Reis-Filho
JS: Topoisomerase II alpha amplification may predict benefit from
adjuvant anthracyclines in HER2 positive early breast cancer.
Breast Cancer Res Treat. 106:181–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
MacGrogan G, Rudolph P, Mascarel Id I, et
al: DNA topoisomerase IIalpha expression and the response to
primary chemotherapy in breast cancer. Br J Cancer. 89:666–671.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Järvinen TA, Tanner M, Rantanen V, Bärlund
M, Borg A, Grénman S and Isola J: Amplification and deletion of
topoisomerase IIalpha associate with ErbB-2 amplification and
affect sensitivity to topoisomerase II inhibitor doxorubicin in
breast cancer. Am J Pathol. 156:839–847. 2000.PubMed/NCBI
|
|
33.
|
Lynch BJ, Guinee DG Jr and Holden JA:
Human DNA topoisomerase II-alpha: a new marker of cell
proliferation in invasive breast cancer. Hum Pathol. 28:1180–1188.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Järvinen TA, Kononen J, Pelto-Huikko M and
Isola J: Expression of topoisomerase IIalpha is associated with
rapid cell proliferation, aneuploidy, and c-erbB2 overexpression in
breast cancer. Am J Pathol. 148:2073–2082. 1996.PubMed/NCBI
|