Introduction
Homeobox containing 1 (HMBOX1), a novel
transcription factor, contains a homeobox domain in the N-terminus
and an HNF1-N domain in the C-terminus. It is a member of the
homeobox family of genes, which is characterized by the presence of
a DNA sequence, the homeobox (1).
Phylogenetic analyses have confirmed that HMBOX1 is more distantly
related to HNF1A and HNF1B and has an atypical homeo-domain
possessing a 21-amino acid insertion between the second and third
helix (2,3). Zhang et al (3) previously cloned a splicing variant of
HMBOX1, designated HMBOX1b, from a human pancreatic cDNA library.
Compared to HMBOX1, HMBOX1b encodes a 304-amino acid protein that
shares the N-terminal region but has no homeo-domain and C-terminal
region.
HMBOX1 is highly conserved in humans, mice, rats,
chickens and xenopus (2),
and is detected in both the cytoplasm and nucleus of 10 normal
human tissues, including the cerebrum, pancreas, kidney and liver
(4). Co-transfection of HEK-293T
cells with the pM-HMBOX1 plasmid and reporter plasmid
pGAL45tkLUC indicates that the HMBOX1 protein may be a
transcription repressor, while HMBOX1b only retains faint
transcriptional repressive activity (2,3). We
also found that HMBOX1 exhibits negative regulatory effects on
natural killer cell activation (unpublished data).
In order to clarify the mechanisms of HMBOX1, we
generated two specific mouse anti-human HMBOX1 monoclonal
antibodies (mAbs), 2A5F4 and 4A4F2. The specificities of the mAbs
were verified in HEK-293T cells, which were transformed by
pcDNA3.1-HMBOX1 or siRNA/HMBOX1 (4). These two anti-HMBOX1 antibodies were
used to investigate the protein expression profile of HMBOX1 in
various human cancer tissues and cell lines by immunoanalytical
methods, including immunoblotting, immunofluorescence and
immunohistochemical assays. Abnormal expression of HMBOX1 was
detected in the different types of carcinoma tissue.
Materials and methods
Cell lines and culture
The human hepatocarcinoma cell lines H7402 and HepG2
and the T-lymphocyte cell line Jurkat were cultured in RPMI-1640
medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 10%
calf serum (Sijiqing Co, Hangzhou, China) at 37°C in 5%
CO2.
Antibodies against HMBOX1
Production of the mAbs against HMBOX1 was performed
using a hybridoma technique. As previously described (4), the HMBOX1 fusion protein mixed with
acid-treated naked Salmonella minnesota R595 bacteria was
used to intraperitoneally immunize 8-week-old BALB/c female mice at
2-week intervals. When the titer of the mouse serum was higher than
1:105, cell fusion and cloning programs were performed.
Two hybridoma lines, 2A5F4 and 4A4F2, were obtained, and both
stably produced anti-HMBOX1 antibodies. Anti-HMBOX1 mAbs were
prepared from the ascetic fluid of the BALB/c mice.
The anti-HMBOX1 antibody was purchased from Abcam
Inc. (Cambridge, MA, USA; http://www.abcam.com/HMBOX1-antibody-ab50392.html). It
is a rabbit anti-mouse polyclonal antibody that is used in Western
blot analysis and ELISA assays. It was used as a positive control
Ab in this study.
Western blot analyses
Cell extracts were separated by SDS-PAGE on a 12%
polyacrylamide gel and then transferred onto PVDF membranes
(Millipore, Billerica, MA, USA) by electroblotting. The membranes
were blocked with 5% nonfat milk in TBS/0.1% Tween-20 and then
incubated with mAb 2A5F4, mAb 4A4F2 or the control Ab against
HMBOX1 for 2 h at room temperature (RT), and subsequently washed
with TBST 3 times and incubated for 1 h with anti-murine
antibody-conjugated horseradish peroxidase (HRP) (Dako, Glostrup,
Denmark) or anti-rabbit antibody-conjugated HRP (Cell Signaling
Technology, Danvers, MA, USA). After washing 5 times with TBST, the
proteins were detected with the enhanced chemiluminescence system
(Pierce Biotechnology, Rockford, IL, USA).
Immunoprecipitation
Cell extracts were prepared by resuspending
1×107 cells in ice-cold gentle lysis buffer [20 mM
Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM PMSF, 1
mM NaVO3, 50 mM NaF, 1% NP-40 and protease inhibitors]
for 30 min on ice. The extracts were then clarified by
centrifugation (16,000 x g for 15 min at 4°C), and the supernatant
was collected. After incubation with protein A-Sepharose beads
(GenScript, Nanjing, China) for 10 min at 4°C, the supernatant was
cleared and concentrated using the Bradford method while BSA
(Bio-Rad Laboratories, Hercules, CA, USA) was used as a standard.
The cleared extract was incubated at 4°C overnight with mAb 2A5F2
or mAb 4A4F2 at a dose of 5 μg/500 μg total protein in 500 μl and
then incubated with 50 μl protein A-Sepharose beads at 4°C for 1 h.
The immune complexes were recovered by centrifugation, washed 3
times with PBS buffer, and solubilized in loading buffer by heating
at 100°C for 5 min. Immunoprecipitated complexes were
electrophoresed on a 12% reduced SDS polyacrylamide gel,
transferred to a PVDF membrane, and immunoblotted as previously
described (5).
Immunostaining and flow cytometry
The hepatic cell lines were adjusted to
1×106 cells/ml, fixed in 4% cold paraformaldehyde for 10
min at RT, and permeabilized with vigorous overtaxing in 500 μl
ice-cold 0.1% saponin per 106 cells. The primary
antibody against HMBOX1 (mAb 2A5F4 or mAb 4A4F2) was then added at
a dilution of 1:50, and the cells were incubated at 4°C overnight.
After washing three times with PBS, cells were resuspended in 100
μl PBS and incubated for 1 h with FITC-conjugated anti-mouse IgG
antibody at a dilution of 1:100 (eBioscience, San Diego, CA, USA).
Fluorescence was assayed using a flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA). The isotype-matched control sample was
included as the standard control.
Immunohistochemistry
The immunohistochemical SP staining method was used
to detect the HMBOX1 protein levels in hepatocytes growing on glass
chips or a tissue microarray (OD-CT-Com03-002; Shanghai Biochip,
China). The sections were incubated at 4°C overnight with mAb 2A5F4
or mAb 4A4F2 in a humidified chamber. The mouse SP detection and
DAB envision systems (both from Zimbio, Beijing, China) were
applied.
Results
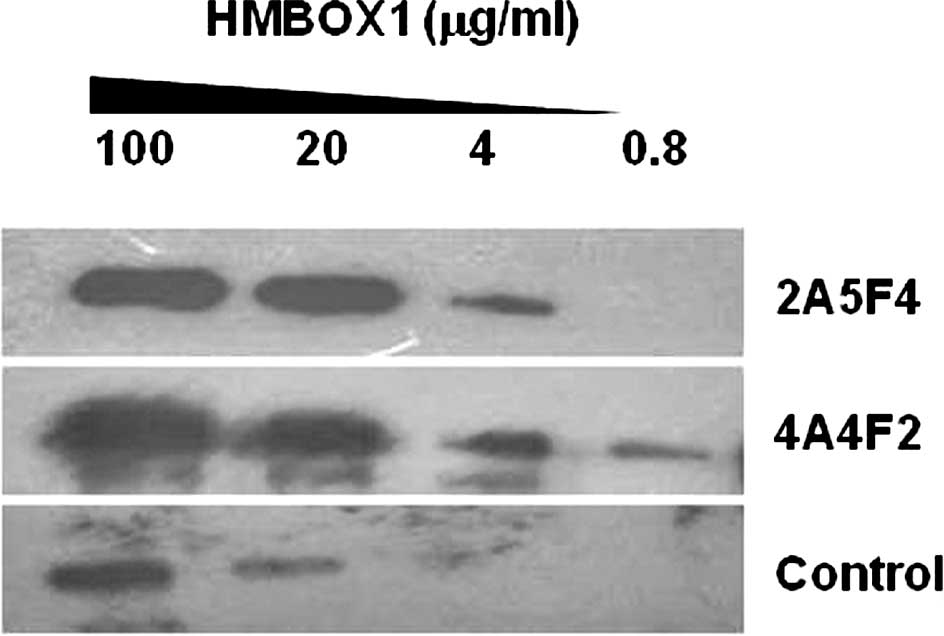
Use of 2A5F4 mAb and 4A4F2 mAb in Western
blot analysis
To verify the specificity and sensitivity of mAb
2A5F4 and mAb 4A4F2 against HMBOX1, the HMBOX1 fusion protein was
gradient diluted and detected by Western blot analysis with mAb
2A4F4 and mAb 4A4F2. As shown in Fig.
1, the lowest concentration of the HMBOX1 protein detected was
0.8 μg/ml. Thus, the specificity and sensitivity of mAb 2A5F4 and
mAb 4A4F2 were found to be comparable with that of the commercial
HMBOX1 antibody from Abcam Inc.
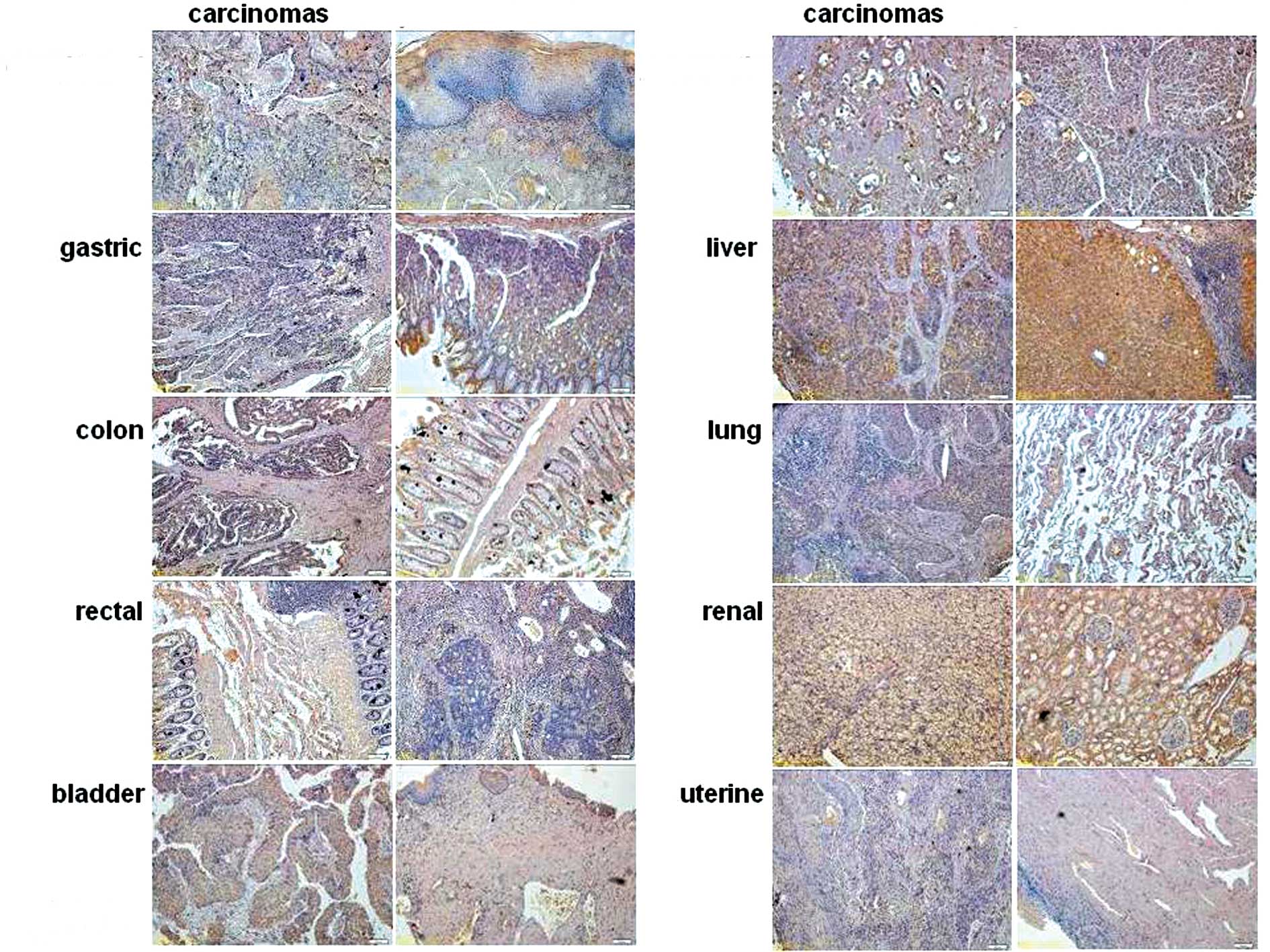
Abnormal expression of HMBOX1 in
carcinoma tissues
Previous research has confirmed that HMBOX1 is
widely expressed in human tissues (4). Here, the expression profile of HMBOX1
was further identified in various human tumors. As shown in
Fig. 2, a tissue microarray was
used to analyze the expression of HMBOX1. The expression level of
HMBOX1 was found to be dramatically decreased in liver cancer
compared with that in adjacent normal tissue, whereas HMBOX1 in
kidney tissue was mainly expressed in the renal tubule, and the
expression level of HMBOX1 was much higher in clear-cell carcinoma
of the kidney originating from the renal tubule. Additionally,
there was no significant change in HMBOX1 expression in the other
cancer tissues tested. Similar to the results reported by Chen
et al (2), high levels of
HMBOX1 protein were detected not only in pancreatic cancer but also
in adjacent normal tissue.
Expression of HMBOX1 in the
hepatocellular cell lines H7402 and HepG2
Cell lines are commonly used as in vitro
models in scientific research. Thus, the expression of HMBOX1 was
further evaluated in the human hepatocellular carcinoma cell lines
H7402 and HepG2 by immunoanalytical methods. First, the HMBOX1
protein was found to be localized in the nucleus and cytoplasm as
detected by Western blot and immunohistochemical analyses (Fig. 3A and C). Jurkat, a T-lymphocyte
cell line, was used as a positive control. Similar to the control
Ab, positive immunostaining for HMBOX1 was observed in both the
H7402 and HepG2 cells using mAb 4A4F2 (Fig. 3A), which is consistent with
previous reports (4). Second,
immunoprecipitation was performed with mAb 2A5F4 and mAb 4A4F2. As
shown in Fig. 3B, mAb 2A5F4 was
found to immunoprecipitate two different variants of HMBOX1, HMBOX1
(410aa, 47 kDa) and HMBOX1b (304aa, 35 kDa), from the H7402 and
HepG2 cells, while mAb 4A4F2 did not. One possible explanation was
that the type of mAb 4A4F2 used was IgM (4), which has poor affinity to protein
A-Sepharose beads (6), and thus
the immune complex could not be precipitated. Finally, mAb 2A5F4
and mAb 4A4F2 were also used for immunohisto chemical (Fig. 3C) and flow cytometric (Fig. 3D) analyses, and as expected no
significant difference in expression was noted between the cell
lines. These findings demonstrated that the HMBOX1 protein was
expressed in H7402 and HepG2 cells, and that these cell lines are
suitable for investigating the mechanisms of HMBOX1 in HCC
development in vitro.
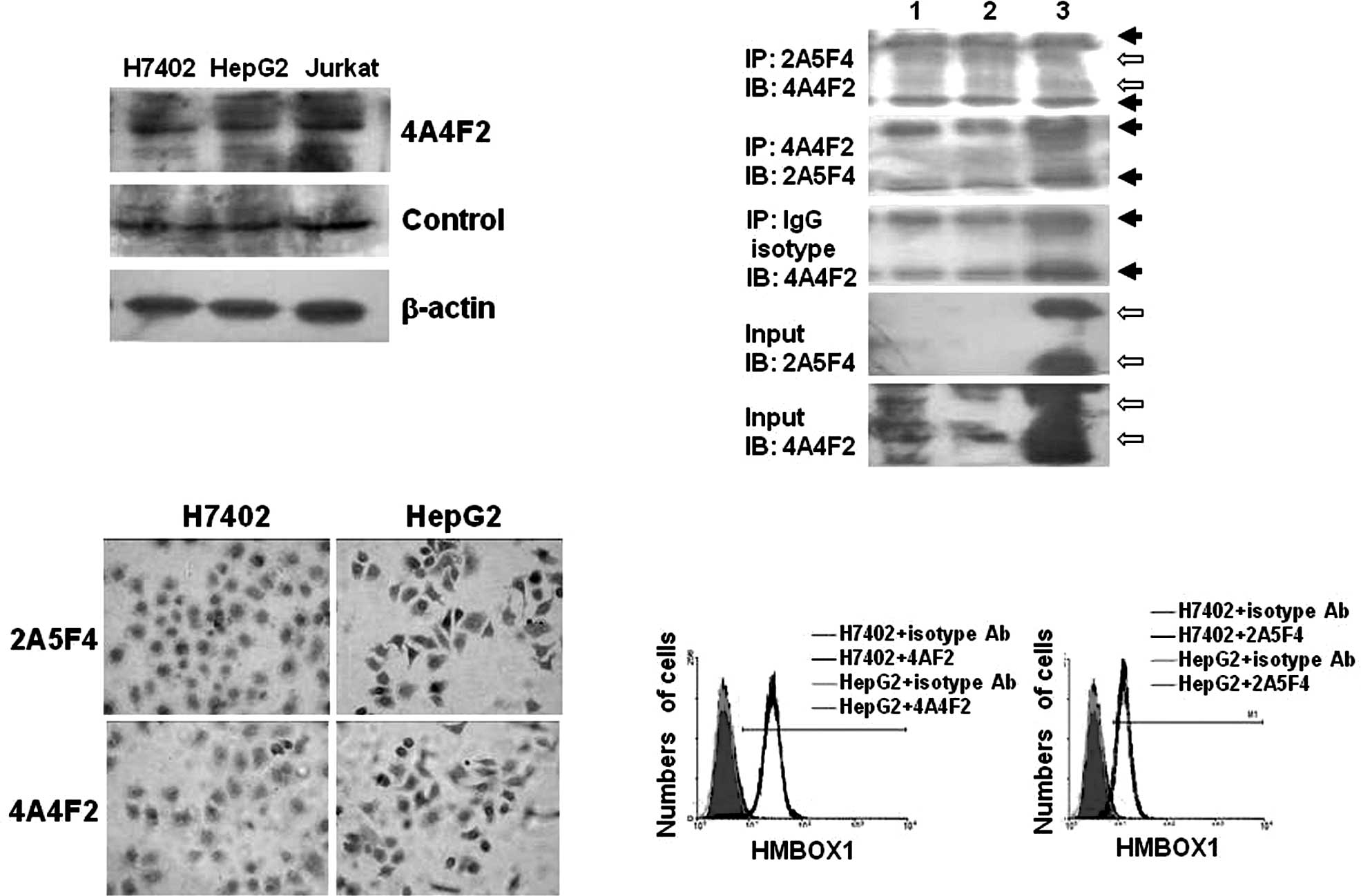 | Figure 3.Expression of HMBOX1 in the
hepatocellular cell lines H7402 and HepG2. (A) Western blot
analysis was performed using mAb 4A4F2 or the control Ab. Jurkat
cells were used as positive control cells, and the β-actin
messenger was used as the internal control. (B) Immunoprecipitation
analysis. Cell lysates from hepatocellular cell lines (lane 1,
H7402; lane 2, HepG2) and fused HMBOX1 proteins (lane 3) were
pulled down by mAb 2A5F4, mAb 4A4F2 or the Ig isotype antibody,
respectively. Complexes and input (non-immunoprecipitated whole
cell extract) were detected by Western blot analysis using mAb
4A4F2 or mAb 2A5F4 as described in Materials and methods. ➙, IgH
chain or IgL chain; ⇨, HMBOX1. (C) Immunohistochemical method.
Hepatocytes (H7402 and HepG2) growing on glass chips were
immunostained using mAb 4A4F2 or mAb 2A5F4 as primary antibody, and
the mouse SP detection system and the DAB envision system were
applied. Original magnification, x400. (D) Flow cytometric assay.
The hepatocellular cell lines H7402 and HepG2 were labeled with mAb
2A5F4 or mAb 4A4F2, and FITC-labeled secondary antibody was applied
before FACS analysis. Isotype antibodies were used as the
control. |
Discussion
In the present study, the protein expression profile
of HMBOX1 was analyzed in different types of human cancer tissues
and cell lines. Abnormal expression of HMBOX1 was noted in several
carcinomas, particularly in liver and renal cancer. It was
previously suggested that HMBOX1 may be correlated with
tumorigenesis. Armendariz and Krauss (7) and Van Wering et al (8) reported that hepatoma differentiation
and the suppression of hepato-specific gene expression are usually
accompanied by a decrease in the expression of HNF1α, a member of
the homeobox genes. Therefore, HMBOX1 may potentially be an
important negative regulator of tumor pathobiology.
Homeobox family genes are capable of interacting
with multiple coactivators, leading to a strong enhancement of
transcription. For example, HNF1α can physically interact with
PCBD1, HATs, CBP, RAC3, GATA5, Neurog3 and Cdx2 (8–12).
Thus, further functional research of HMBOX1 may be of value. As mAb
2A5F4 and mAb 4A4F2 can be used for immunoprecipitation and
immunofluorescence, potential coactivators of HMBOX1 may also be
identified with antibodies against HMBOX1.
In the present study, high expression levels of
HMBOX1 were detected in hepatic tissue, the renal tubule and
pancreatic tissue. These tissues are closely related with
glycometabolism, and previous studies have demonstrated that
heterozygous mutations in genes coding for HNF1 homeobox A (HNF1A),
hepatocyte nuclear factor-4 A (HNF4A), and HNF1 homeobox B (HNF1B)
can cause maturity-onset diabetes of the young (MODY). MODY is
characterized by progressive β-cell failure and inability to
increase insulin secretion in response to hyperglycemia (13). Thus, HMBOX1 may play a role in the
process of glycometabolism, and may be related to MODY. To
conclude, the mechanism of HMBOX1 has yet to be elucidated and may
pose a challenge for future research.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (30901307,
30972962) and the Ministry of Science and Technology of China
(2007AA021000, 2007AA021109, 2006CB504303 and 2008ZX10002-008).
References
|
1.
|
Lawrence PA and Morata G: Homeobox genes:
their function in Drosophila segmentation and pattern
formation. Cell Mol Immunol. 78:181–189. 1994.PubMed/NCBI
|
|
2.
|
Chen S, Saiyin H, Zeng X, et al: Isolation
and functional analysis of human HMBOX1, a homeobox containing
protein with transcriptional repressor activity. Cytogenet Genome
Res. 114:131–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zhang M, Chen S, Li Q, Ling Y, Zhang J and
Yu L: Characterization of a novel human HMBOX1 splicing variant
lacking the homeodomain and with attenuated transcription repressor
activity. Mol Biol Rep. 37:2767–2772. 2010. View Article : Google Scholar
|
|
4.
|
Dai J, Wu L, Zhang C, Zheng X, Tian Z and
Zhang J: Recombinant expression of a novel human transcriptional
repressor HMBOX1 and preparation of anti-HMBOX1 monoclonal
antibody. Cell Mol Immunol. 6:261–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tsangaridou E, Polioudaki H, Sfakianaki R,
et al: Differential detection of nuclear envelope autoantibodies in
primary biliary cirrhosis using routine and alternative methods.
BMC Gastroenterol. 10:282010. View Article : Google Scholar
|
|
6.
|
Balint JP Jr, Ikeda Y, Nagai T and Terman
DS: Isolation of human and canine IgM utilizing protein A affinity
chromatography. Immunol Commun. 10:533–540. 1981.PubMed/NCBI
|
|
7.
|
Armendariz AD and Krauss RM: Hepatic
nuclear factor 1-alpha: inflammation, genetics, and
atherosclerosis. Curr Opin Lipidol. 20:106–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Van Wering HM, Huibregtse IL, van der Zwan
SM, et al: Physical interaction between GATA-5 and hepatocyte
nuclear factor-1alpha results in synergistic activation of the
human lactasephlorizin hydrolase promoter. J Biol Chem.
277:27659–27667. 2002.
|
|
9.
|
Sourdive DJ, Transy C, Garbay S and Yaniv
M: The bifunctional DCOH protein binds to HNF1 independently of its
4-alpha-carbinolamine dehydratase activity. Nucleic Acids Res.
25:1476–1484. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Soutoglou E, Papafotiou G, Katrakili N and
Talianidis I: Transcriptional activation by hepatocyte nuclear
factor-1 requires synergism between multiple coactivator proteins.
J Biol Chem. 275:12515–12520. 2000. View Article : Google Scholar
|
|
11.
|
Dohda T, Kaneoka H, Inayoshi Y, Kamihira
M, Miyake K and Iijima S: Transcriptional coactivators CBP and p300
cooperatively enhance HNF-1alpa-mediated expression of the albumin
gene in hepatocytes. J Biochem. 136:313–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Smith SB, Gasa R, Watada H, Wang J, Grimen
SC and German MS: Neurogenin3 and hepatic nuclear factor 1
cooperate in activating pancreatic expression of Pax4. J Biol Chem.
278:28254–28259. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Owen K and Hattersley AT: Maturity-onset
diabetes of the young: from clinical description to molecular
genetic characterization. Best Pract Res Clin Endocrinol Metab.
15:309–323. 2001. View Article : Google Scholar : PubMed/NCBI
|