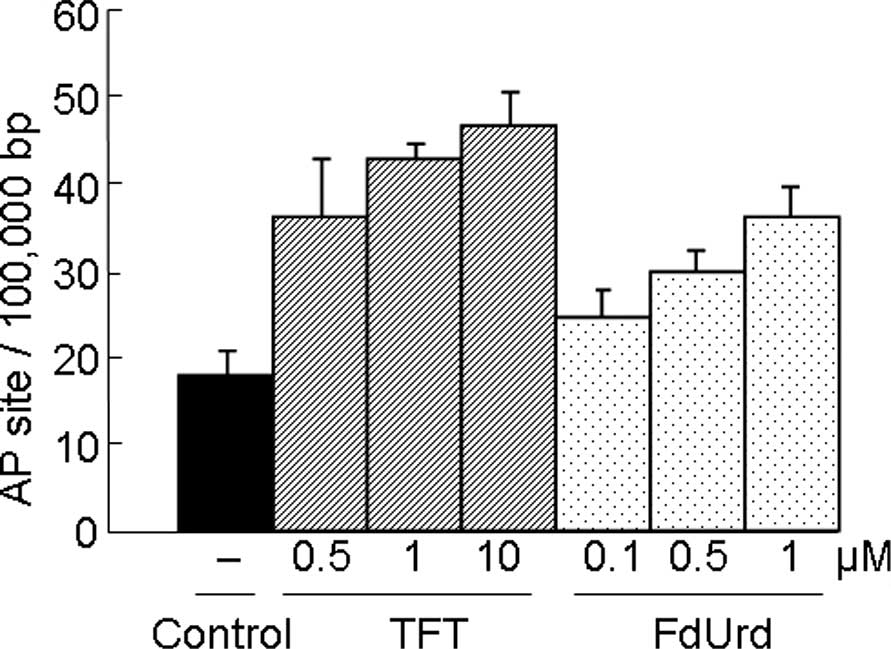
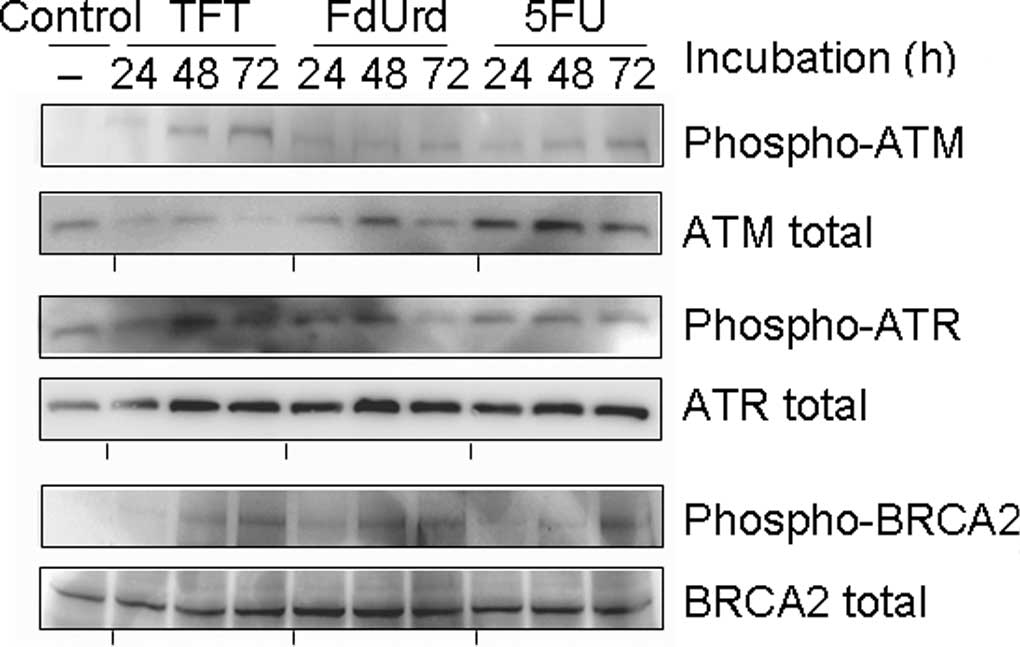
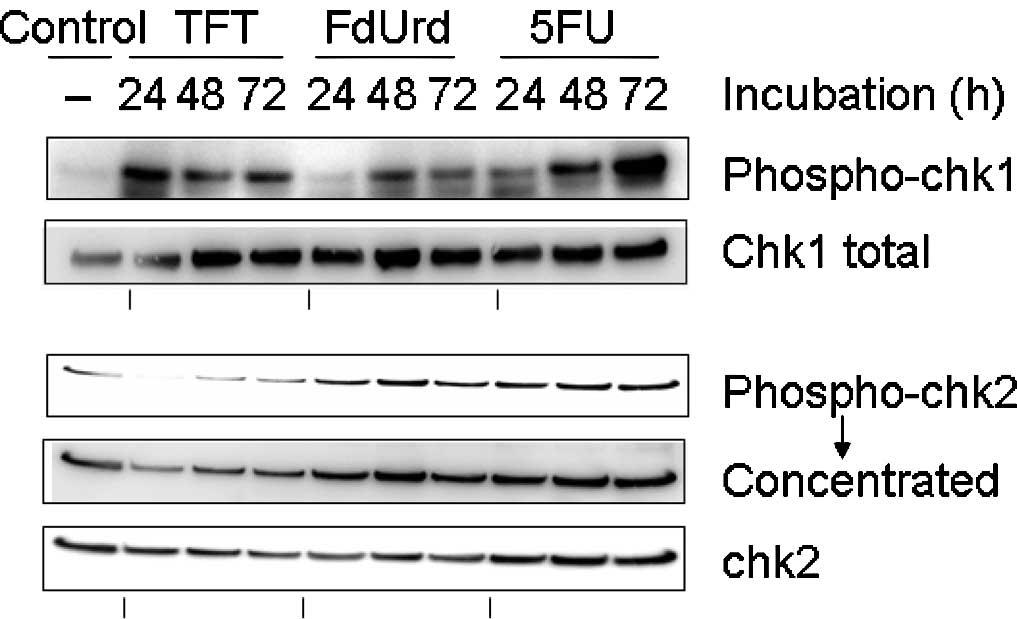
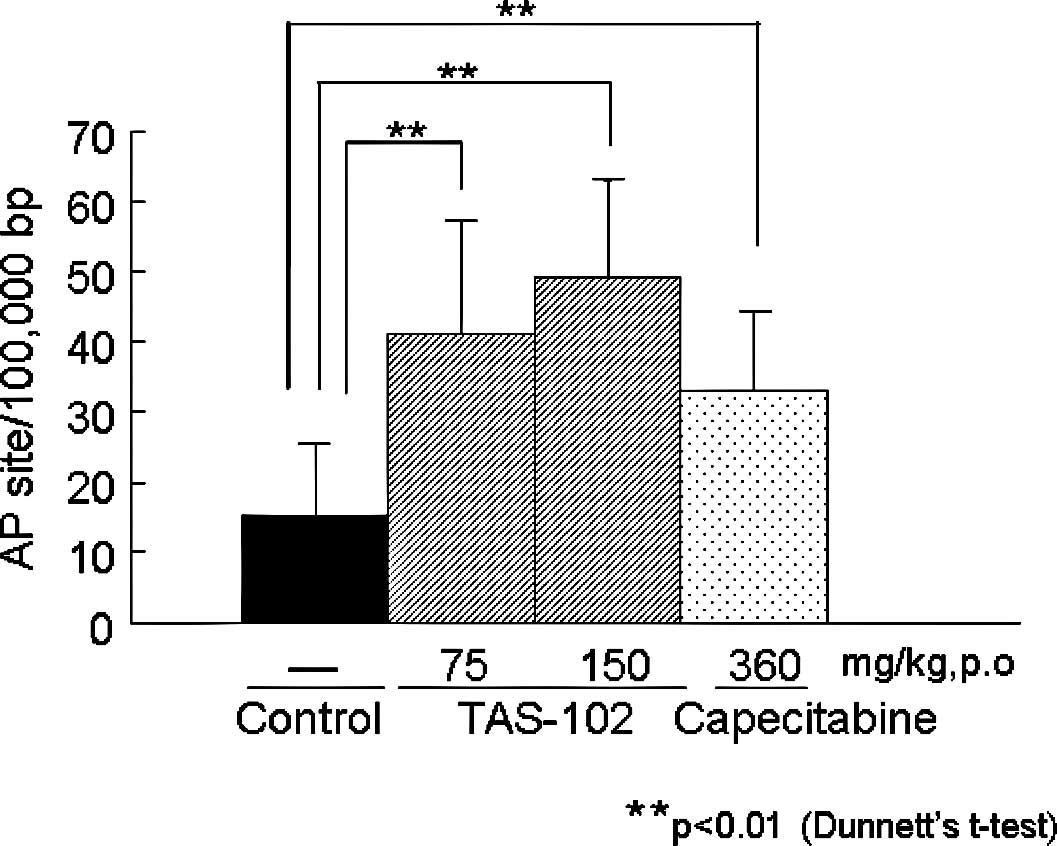
|
1.
|
Heidelberger C, Parsons DG and Remy DC:
Syntheses of 5-trifluoromethyluracil and
5-trifluoromethyl-2′-deoxyuridine. J Med Chem. 7:1–5. 1964.
|
|
2.
|
Bresnick E and Wiliams SS: Effects of
5-trifluoromethyldeoxy-uridine upon deoxythymidine kinase. Biochem
Pharmacol. 16:503–507. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Reyers P and Heidelberger C: Fluorinated
pyrimidines: XXVI. Mammalian thymidylate synthetase: its mechanism
of action and inhibition by fluorinated nucleotides. Mol Pharmacol.
1:14–30. 1965.
|
|
4.
|
Eckstein JW, Foster PG, Finer-Moore J,
Wataya Y and Santi DV: Mechanism-based inhibition of thymidylate
synthase by 5-(trifluoromethyl)-2′-deoxyuridine 5′-monophosphate.
Biochemistry. 3:15086–15094. 1994.PubMed/NCBI
|
|
5.
|
Umeda M and Heidelberger C: Comparative
studies of fluorinated pyrimidines with various cell lines. Cancer
Res. 28:2529–2538. 1968.PubMed/NCBI
|
|
6.
|
Murakami Y, Kazuno H, Emura T, Tsujimoto
H, Suzuki N and Fukushima M: Different mechanisms of acquired
resistance to fluorinated pyrimidines in human colorectal cancer
cells. Int J Oncol. 17:277–283. 2000.PubMed/NCBI
|
|
7.
|
Emura T, Nakagawa F, Fujioka A, Ohshimo H,
Yokogawa T, Okabe H and Kitazato K: An optimal dosing schedule for
a novel combination antimetabolite, TAS-102, based on its
intracellular metabolism and its incorporation into DNA. Int J Mol
Med. 13:249–255. 2004.PubMed/NCBI
|
|
8.
|
Emura T, Suzuki N, Yamaguchi M, Ohshimo H
and Fukushima M: A novel combination antimetabolite, TAS-102,
exhibits antitumor activity in FU-resistant human cancer cells
through a mechanism involving FTD incorporation in DNA. Int J
Oncol. 25:571–578. 2004.
|
|
9.
|
Suzuki N, Emura T and Fukushima M: Mode of
action of trifluorothymidine (TFT) against DNA replication and
repair enzymes. Int J Oncol. (In press).
|
|
10.
|
Nakamura J, Walger VE, Upton PB, Chiang
SY, Kow YW and Swenberg JA: Highly sensitive apurinic/apyrimidinic
site assay can detect spontaneous and chemically induced
depurination under physiological conditions. Cancer Res.
58:222–225. 1998.
|
|
11.
|
Nishikawa T, Munshi A, Story MD, Ismail S,
Stevens C, Chada S and Meyn RE: Adenoviral-mediated mda-7
expression suppresses DNA repair capacity and radiosensitizes
non-small-cell lung cancer cells. Oncogene. 16:7125–7131. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hartman KY and Heidelberger C: Studies on
fluorinated pyrimidines: XIII. Inhibition of thymidylate synthase.
J Biol Chem. 236:3006–3013. 1961.PubMed/NCBI
|
|
13.
|
Santi DV and McHenry CS:
5-Fluoro-2′-deoxyuridinylate: covalent complex with thymidylate
synthase. Proc Natl Acad Sci USA. 69:1855–1857. 1972.
|
|
14.
|
Glazer RI and Lloyd LS: Association of
cell lethality with incorporation of 5-fluorouracil and
5-fluorouridine into nuclear RNA in human colon carcinoma cells in
culture. Mol Pharmacol. 21:468–473. 1982.PubMed/NCBI
|
|
15.
|
Greenhalgh DA and Parish JH: Effect of
5-fluorouracil on cytotoxicity and RNA metabolism in human colonic
carcinoma cells. Cancer Chemother Pharmacol. 25:37–44. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Rogers WI, Hartman AC, Palm PE, Okstein C
and Kensler CJ: The fate of 5-trifluoromethyl-2′-deoxyuridine in
monkeys, dogs, mice, and tumor-bearing mice. Cancer Res.
29:953–961. 1969.PubMed/NCBI
|
|
17.
|
Dexter DL, Wolberg WH, Ansfield FJ, Helson
L and Heidelberger C: The clinical pharmacology of
5-trifluoromethyl-2′-deoxyuridine. Cancer Res. 32:247–53. 1972.
|
|
18.
|
Suzuki N and Fukushima M: Simple and rapid
enzymatic method for the synthesis of single-strand
oligonucleotides containing trifluorothymidine. Nucleosides
Nucleotides Nucleic Acids. 29:896–904. 2010. View Article : Google Scholar
|
|
19.
|
Bijnsdorp IV, Kruyt FA, Gokoel S,
Fukushima M and Peters GJ: Synergistic interaction between
trifluorothymidine and docetaxel is sequence dependent. Cancer Sci.
99:2302–2308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Backus HH, Pinedo HM, Wouters D, Kuiper
CM, Jansen G, van Groeningen CJ and Peters GJ: Differences in the
induction of DNA damage, cell cycle arrest, and cell death by
5-fluorouracil and antifolates. Oncol Res. 12:231–239.
2000.PubMed/NCBI
|
|
21.
|
Brown EJ and Baltimore D: Essential and
dispensable roles of ATR in cell cycle arrest and genome
maintenance. Genes Dev. 17:615–628. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Morrison C, Sonoda E, Takao N, Shinohara
A, Yamamoto K and Takeda S: The controlling role of ATM in
homologous recombinational repair of DNA damage. EMBO J.
19:463–471. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Beucher A, Birraux J, Tchouandong L,
Barton O, Shibata A, Conrad S, Goodarzi AA, Krempler A, Jeggo PA
and Löbrich M: ATM and Artemis promote homologous recombination of
radiation-induced DNA double-strand breaks in G2. EMBO J.
28:3413–3427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Branzei D and Foiani M: Regulation of DNA
repair throughout the cell cycle. Nat Rev Mol Cell Biol. 9:297–308.
2008. View
Article : Google Scholar : PubMed/NCBI
|