Introduction
Trifluorothymidine (TFT), which was originally
synthesized by Heidelberger et al (1), has been shown to exert a potent
suppressive effect on many transplanted tumors in mice. TFT is
reportedly phosphorylated by thymidine kinase to yield
TFT-monophosphate (2), which
directly inhibits thymidylate synthase (TS) in the absence of
reduced folate (3,4). The antimetabolite 5-fluorouracil
(5FU), which is currently used to treat patients with gastric,
colorectal, head/neck, breast and other types of cancer, is also
thought to exert its antitumor activity mainly by inhibiting TS via
the formation of a ternary-complex with methylenetetrahydrofolate
and 5-fluorodeoxyuridine monophosphate (FdUMP) derived from 5FU
(5). However, we found that when
tumor cells were treated with higher concentrations of TFT for
short periods, TFT was rapidly incorporated into the DNA and
exerted a cytocidal activity by causing DNA fragmentation rather
than by inhibiting TS in the tumor cells (6–8).
Based on these observations, TAS-102 was developed as a novel
anticancer drug composed of a combination of TFT and TPI, a
specific inhibitor of thymidine phosphorylase that strongly
inhibits the biodegradation of TFT. TPI is expected to reinforce
the uptake of TFT into tumor DNA, thereby promoting the function of
TFT against cancer cells with high expression levels of TS that
have a low sensitivity and/or are resistant to 5FU.
We previously reported (9) that no detectable excisions of TFT
paired to adenine were observed using uracil DNA glycosylases
(UDG), thymine DNA glycosylase (TDG), methyl-CpG binding domain 4
(MBD4) and HeLa whole cell extracts. However, TDG and MBD4 were
able to excise the TFT paired to guanine in DNA. Additional data
also indicated that the small-interfering RNA-mediated knockdown of
TDG or MBD4 significantly increased resistance to the cytotoxic
effects of 5-fluoro-2′-deoxyuridine (FdUrd), but not to those of
TFT. These data suggest that the inhibitory effects of TFT on DNA
replication and repair enzymes are apparently distinct from those
of 5FU and FdUrd.
In the present study, we showed that the
double-strand breaks induced by TFT in the DNA of tumor cells were
important for the exertion of the potent anticancer activity of
TFT.
Materials and methods
Materials
FdUrd and EMEM were obtained from Sigma-Aldrich
Japan (Tokyo, Japan). 5FU was purchased from Wako Pure Chemical
Industries, Ltd. (Osaka, Japan). TFT and TPI
[5-chloro-6-(2-iminopyrrolidin-1-yl) methyl-2,4
(1H,3H)-pyrimidinedione hydrochloride] were synthesized at Taiho
Pharmaceutical Co., Ltd. (Tokyo, Japan). Capecitabine, an oral 5FU
prodrug, was obtained from BePharm Ltd. (Shanghai, China). TAS-102
was prepared by mixing TFT and TPI at a molar ratio of 1:0.5 in
0.5% hydroxypropyl methylcellulose. Fetal calf serum (FCS) was
obtained from JRH Bioscience (Lenexa, KS, USA).
Cell lines and cultures
The HeLa human cervical cancer cell line was
purchased from Dai-Nippon Pharmaceutical Co., Ltd. (Osaka, Japan)
and cultured in EMEM medium with 10% FCS.
Apurinic/apyrimidinic site
measurements
The number of apurinic/apyrimidinic (AP) sites was
determined using a commercially available DNA damage quantification
kit (Dojindo Molecular Technology, Kumamoto, Japan). Briefly, a Get
pure DNA kit (Dojindo Molecular Technology) was used to extract and
purify the genomic DNA from the indicated HeLa cell line according
to the manufacturer's specifications. Purified DNA (1 μg)
was then incubated for 1 h at 37°C with an aldehyde reactive probe
(ARP) reagent (N'-aminooxymethyl-carbonyl-hydrazino-D-biotin),
which reacts specifically with the ring-open aldehyde form of an AP
site (10). After an overnight
fixation step, biotin-tagged AP sites were quantified using
colorimetric detection with peroxidase-conjugated streptavidin at
450 nm with a SpectraMax340 microplate spectrophotometer (Molecular
Devices).
Western blot analysis
Nuclear cell extracts were obtained using an NE-PER
Nuclear and Cytoplasmic Extraction kit (Thermo Fisher Scientific,
MA, USA). Western blot analysis was performed using
anti-phospho-ATM, anti-phospho-ATR, anti-phospho-chk1 and
anti-phospho-chk2 (Cell Signaling Technology, MA, USA), as well as
anti-phospho-BRCA2 (Millipore, CA, USA). A total protein control
for phosphorylated protein was performed using anti-ATM, anti-ATR,
anti-BRCA2, anti-chk1 and anti-chk2 (Cell Signaling
Technology).
Evaluation of antitumor activity
Five-week-old male nude mice (BALB/c nu/nu) were
purchased from Clea Japan, Inc. (Tokyo, Japan) and raised in the
specific pathogen-free animal quarters of our laboratory. The CO-3
human colorectal cancer cell line was obtained from the Central
Institute for Experimental Animals (Kawasaki, Japan). Tumor
specimens (∼3-mm cubic fragments) were transplanted subcutaneously
into the dorsal region of each animal. Approximately 1 week later,
the animals were grouped according to tumor size, so that the mean
and standard deviation of the tumor volume would be as uniform as
possible in all of the groups. The tumor volume was calculated
using the equation: (V) = 1/2 × S (shorter diameter)2 ×
L (longer diameter). TAS-102 and capecitabine were administered
orally once daily for 14 consecutive days. The rate of tumor growth
(IR) inhibition was regarded as a measure of the antitumor efficacy
and was calculated using the relative tumor volume (RTV) in the
drug-treated groups (T) compared to that in the control group (C)
using the following equation: IR (%) = (1 – T/C) × 100. All of the
protocols and procedures were approved by the Institutional Animal
Care and Use Committee of Taiho Pharmaceuticals.
Pulse field gel electrophoresis
To detect high molecular weight DNA fragmentation,
genomic DNA in the CO-3 tumor cells was extracted and purified
using a Get pure DNA kit, loaded with exactly 1.2% agarose and 10
μg of DNA in each well; the DNA was then separated using
pulse field gel electrophoresis (PFGE) using a CHEF-DR II system
(Bio-Rad Laboratories, Tokyo, Japan) at 1.5 V/cm for 20 h at 25°C
in 0.5X TBE buffer as previously described (11). A comparison of the DNA
concentrations among the different samples was performed using
ethidium bromide staining. The intensity of each band was
quantified fluorometrically, and the percentage of double-strand
break fragments vs. the number in an untreated control was
expressed for each lane.
Statistical analysis
Data concerning the relative tumor volume for all of
the mice in all groups were analyzed using a MultiStaff program
(Dunnett's t-test for comparison among multiple groups) to test for
the significance of inter-group differences in antitumor efficacy.
Data for groups in which premature deaths occurred were excluded
from this analysis.
Results
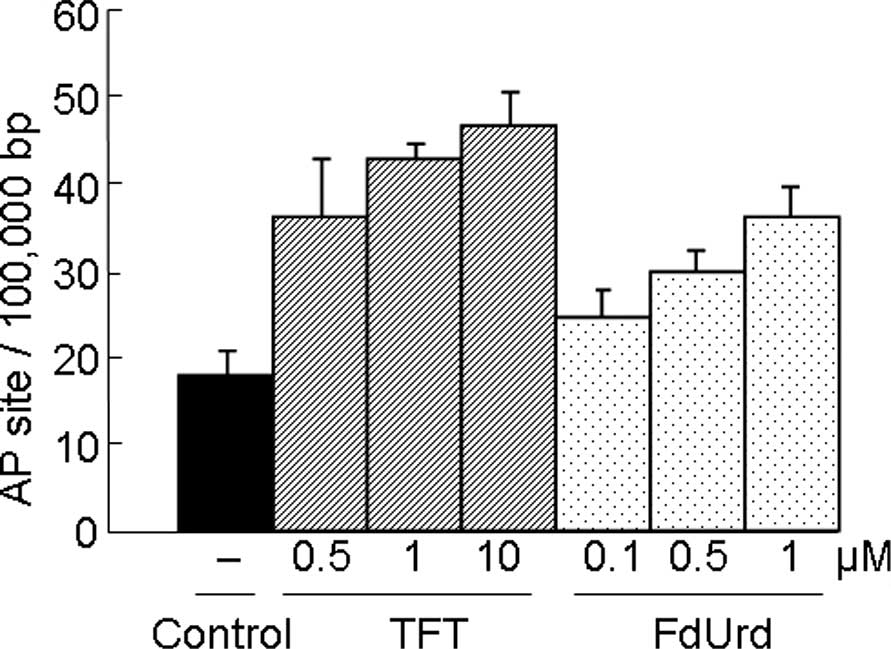
DNA damage by TFT or FdUrd on AP sites in
HeLa cellular DNA
Since AP sites are an intermediate of many DNA
N-glycosylases, the number of AP sites estimated using the APR
assay can be a useful indicator of the level of DNA damage in the
cells. Similar to FdUrd, TFT induced DNA damage in a
concentration-dependent manner (Fig.
1).
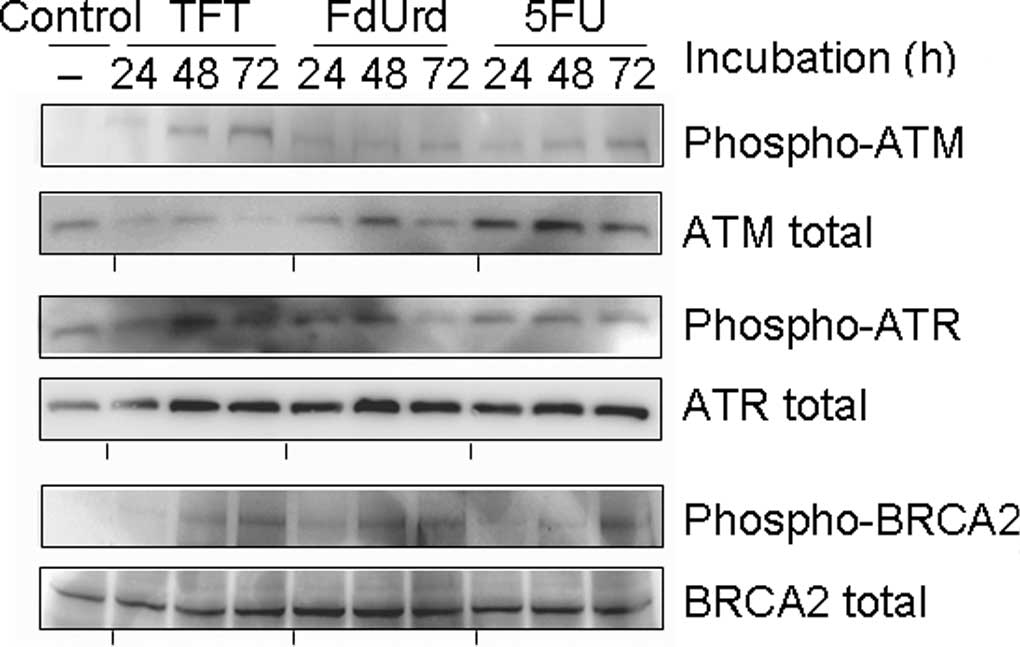
Phosphorylation of ATR, ATM and
BRCA2
To investigate the mechanism of TFT-induced DNA
damage, we measured the phosphorylation of ATR, ATM and BRCA2 using
Western blot analysis after 0, 24, 48 or 72 h of exposure to
IC50 concentrations of TFT, FdUrd or 5FU in HeLa cell
lysates (Fig. 2). The
phosphorylation of ATR was detected after 24 h of exposure to TFT,
but ATM and BRCA2 were detected after >48 h of exposure to TFT.
Exposure to FdUrd resulted in the weak phosphorylation of ATR at 24
h, while phosphorylated ATM was not observed between 24 and 72 h.
The phosphorylations of ATR, ATM and BRCA2 by 5FU was modestly
detected between 24 and 48 h.
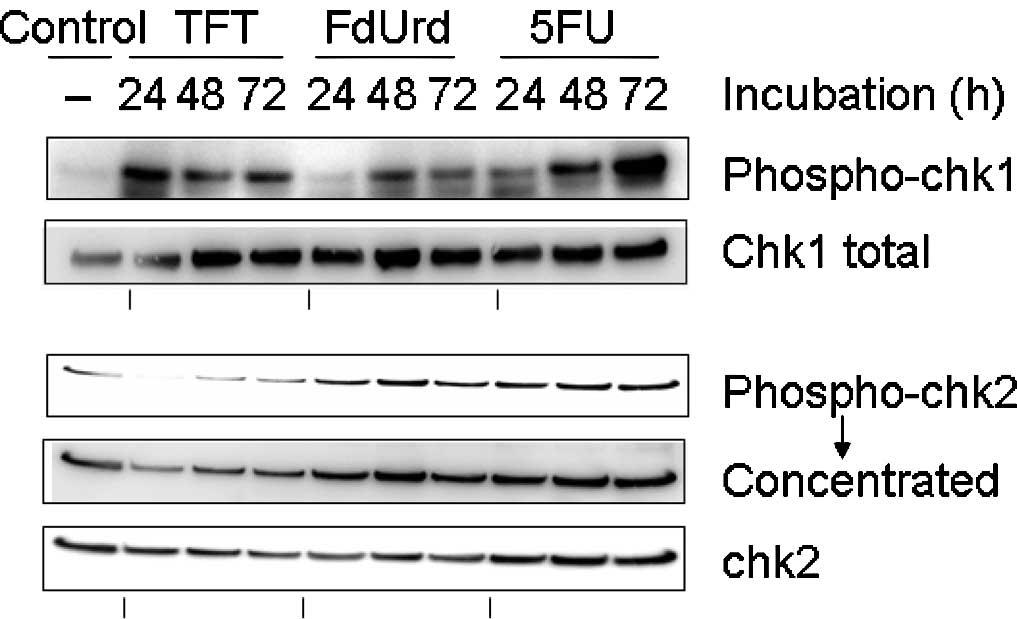
Phosphorylation of chk1 and chk2 as
observed using Western blot analysis
To investigate the checkpoint mechanism of
TFT-induced DNA damage, we measured the phosphorylation of chk1 and
chk2 using Western blot analysis after 0, 24, 48 or 72 h of
exposure to IC50 concentrations of TFT, FdUrd or 5FU in
HeLa cell lysates (Fig. 3). The
phosphorylation of chk1 protein was detected after 24 h of exposure
to TFT, while the phosphorylation of chk2 was only detected after
48 h of exposure to TFT. By contrast, exposure to FdUrd or 5FU did
not result in the phosphorylation of chk1 at 48 h.
Antitumor activity of TAS-102 and
capecitabine against colorectal cancer CO-3 xenografts in a nude
mouse model
The antitumor activities of TFT (as TAS-102) and
capecitabine against CO-3 human colorectal carcinoma are shown in
Table I. TFT was administered in
combination with TPI as TAS-102 to increase the TFT levels in the
serum. TAS-102 exhibited a dose-dependent and significant antitumor
effect when administered orally at a dose of 75 or 150 mg/kg
(Dunnett's t-test). Capecitabine also produced a significant
antitumor effect compared to that in the untreated control group
(Student's t-test). The weight loss at the end of the dosing period
relative to the body weight at the beginning of the experiment was
−7.86% in the untreated control group, and ranged from −10.35 to
−13.17% in the oral administration group. The weight loss in all of
the treatment groups did not differ significantly from that in the
untreated control group.
 | Table I.Antitumor effect of TAS-102 and
capecitabine in human colorectal cancer CO-3 cells. |
Table I.
Antitumor effect of TAS-102 and
capecitabine in human colorectal cancer CO-3 cells.
| Drug | Dose (mg/kg/day) | RTVa | IRb (%) | BWCc (%) |
|---|
| Control | - | 22.09±1.80 | - | −7.86 |
| TAS-102 | 75 | 12.27±0.86d | 44.5 | −10.35 |
| TAS-102 | 150 | 10.26±0.73d | 53.5 | −13.17 |
| Capecitabine | 360 | 13.62±0.97e | 38.4 | −11.06 |
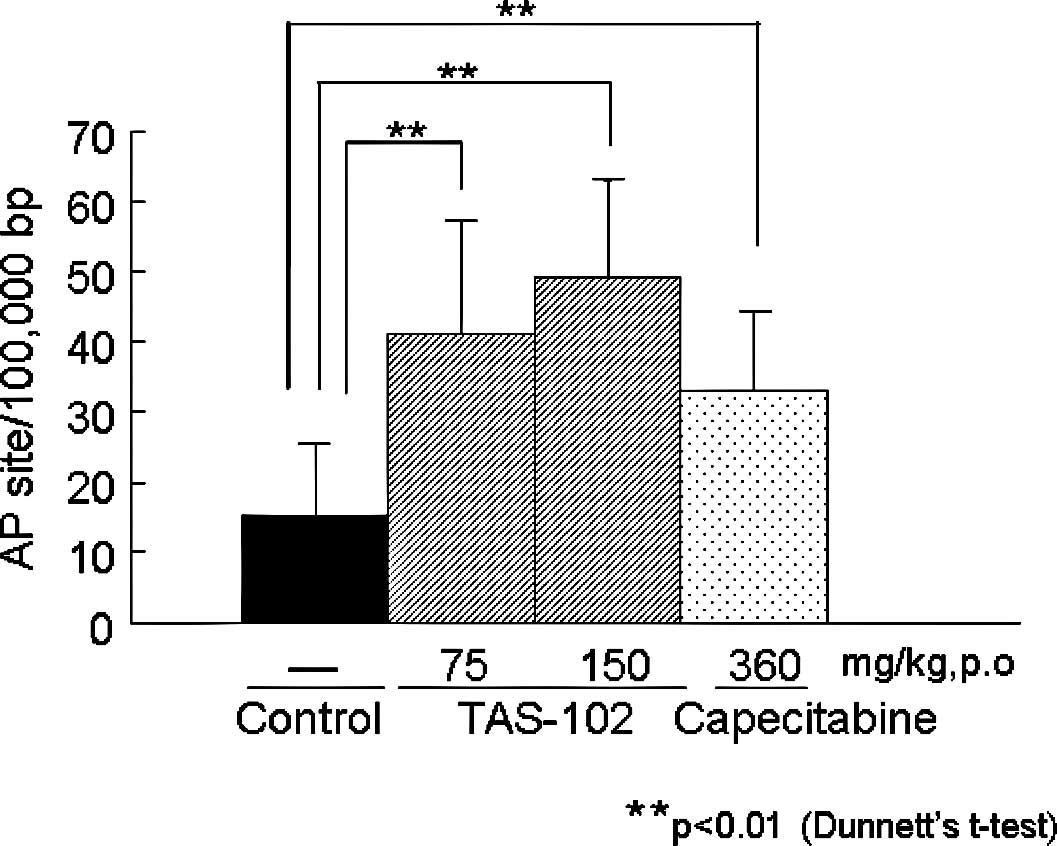
Effects of TAS-102 and capecitabine on AP
sites and damage to bases in cellular DNA
To investigate whether the incorporation of TFT into
DNA and the subsequent DNA damage may explain the potent antitumor
activity of TFT, we first measured the number of AP sites and the
amount of damage to the bases in cellular DNA from colorectal
cancer CO-3 xenografts in a nude mouse model (Fig. 4). Similar to the in vitro
activity (Fig. 1), the
administration of TAS-102 and capecitabine significantly increased
the number of AP sites and/or the amount of base damage formed in
the cellular DNA of the CO-3 xenografts (Dunnett's t-test). These
results for TFT-induced DNA damage are consistent with those of a
previous report (8). The numbers
of AP sites induced by TAS-102 (75 or 150 mg/kg) and capecitabine
(360 mg/kg) were 40.9±16.3, 49.1±13.8 and 33.1±11.3 AP
sites/100,000 bp, respectively; these values were higher than that
for the untreated control group (15.2±10.2 AP sites/100,000
bp).
Effects of TAS-102 and capecitabine on
double-strand DNA breaks
We previously reported that the DNA fragmentation
rate for small molecular weight DNA in a TAS-102-treated group was
significantly higher than that in an untreated control group in
vivo (8). Therefore, in the
present experiment, we measured the number of double-strand breaks
in large molecular weight DNA using PFGE to investigate TFT-induced
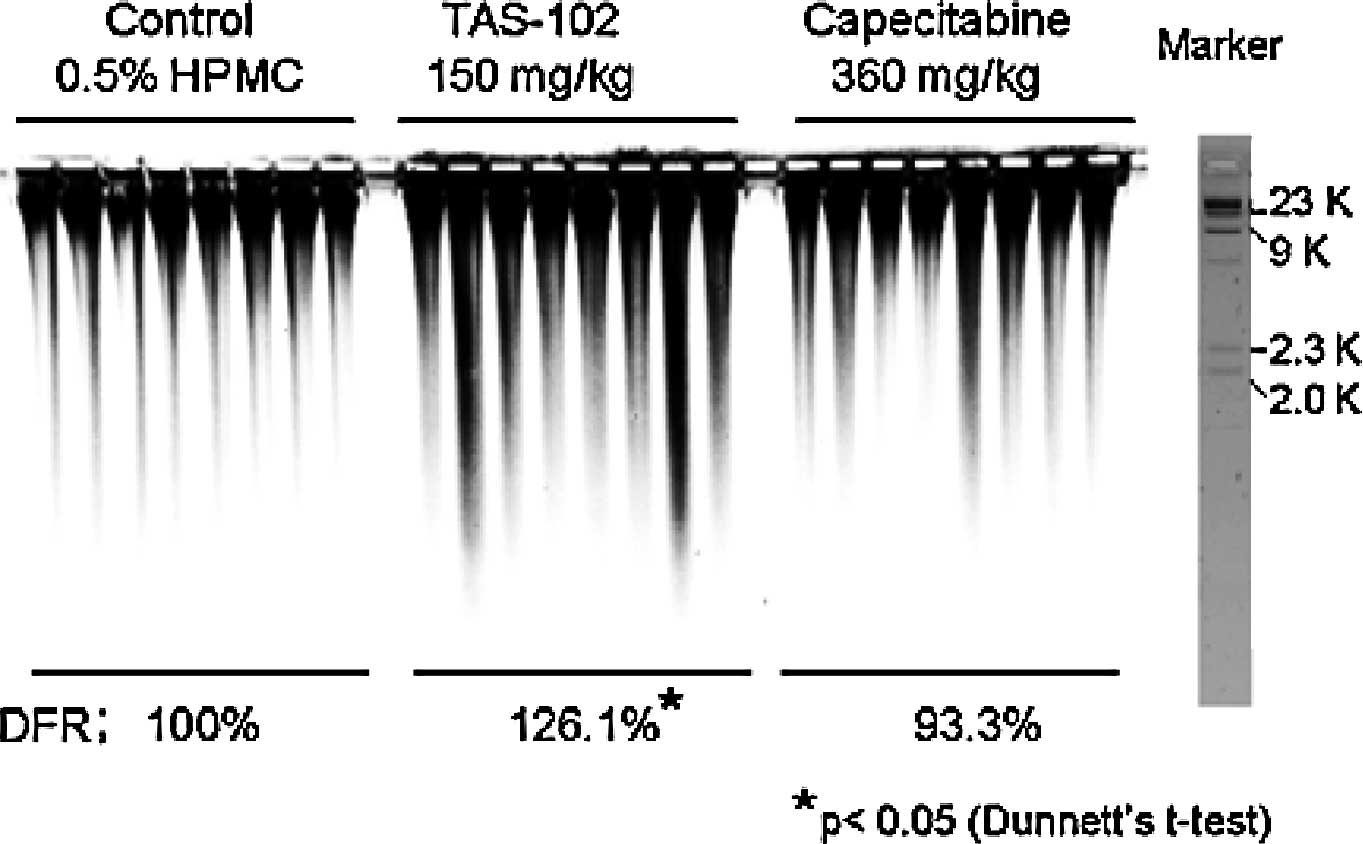
DNA damage in detail (Fig. 5).
PFGE revealed more marked DNA smearing (>2 kbp) in the
TAS-102-treated group than in any other group. The relative DNA
fragmentation rates (DFR), compared to the control group for each
fragmented area, were 126.1% in the TAS-102-treated group (150
mg/kg) and 93.3% in the capecitabine-treated group (360 mg/kg). The
DFR% in the TAS-102-treated group was significantly higher than
that in the control group. By contrast, the DFR% in the
capecitabine-treated group did not differ significantly from that
in the untreated control group.
Discussion
5FU is widely used as an antitumor pyrimidine for
the treatment of patients with gastric, colorectal, head and neck
and breast cancers. The cytotoxic mechanism of 5FU involves the
inhibition of TS activity by the formation of a ternary complex
with 5-fluoro-2′-deoxyuridine-5′-monophosphate and
methylenetetrahydrofolate as well as its incorporation into RNA and
the subsequent inhibition of RNA maturation (12–15).
Similar to 5FU, TFT reportedly exerts its cytocidal action by
inhibiting TS (5) and/or through
its incorporation into DNA (6,7). We
previously demonstrated that when tumor cells are treated with high
concentrations of TFT for short periods, TFT mainly manifests its
antitumor activity though the induction of DNA fragmentation after
its incorporation into the DNA of cancer cells (8). Therefore, we further examined the
mode of action of TFT-induced DNA damage in cancer cells.
When HeLa cells were treated with TFT, β-elimination
in the genomic DNA was detected in a dose-dependent manner
(Fig. 1).
We showed in a previous report (9) that no detectable excisions of TFT
paired to adenine were observed using UDG, TDG and MBD4, and HeLa
whole cell extracts. However, TDG and MBD4 were able to excise the
TFT paired to guanine in DNA. Although the precise mechanism for
TFT-induced β-elimination in genomic DNA remains to be elucidated,
the present results suggest the following points.
First, the majority of TFT is incorporated at
T-sites (T/A, not T/G base pairs) in the cellular DNA. However, as
TFT is massively incorporated into DNA, miss-incorporated TFT that
has bound to guanine may be excised by TDG or MBD4.
Second, DNA-incorporated TFT may be converted into
new structures, such as 5-carboxy-2′-deoxyuridine residues
(16,17). The reactivity of the
trifluoromethyl group of 5-trifluoro-methyluracil towards
nucleophiles has been well documented (18). The metabolism of TFT incorporated
into DNA requires further investigation. The repair of single- and
double-strand breaks is critical for genetic integrity and cell
survival. We showed that the phosphorylation of ATR and chk1
proteins in HeLa cells was detected after exposure to
IC50 concentrations of TFT for more than 24 h, while the
phosphorylation of ATM, BRCA2 and chk2 proteins in HeLa cells was
only detected after more than 48 h of exposure to the same
concentration of TFT (Fig. 3). The
phosphorylation rates for chk1 and chk2 were consistent with those
in a previous report (19). Thus,
TFT apparently induces cell cycle arrest during the G2/M-phase of
the cell cycle, while 5FU reportedly arrests cells during the G1 or
S-phase (20). The G2/M-phase
arrest induced by TFT may be accompanied by the activation of chk1,
probably in an ATR-dependent manner during the initial step, as ATR
initiates checkpoint responses to various agents that stall
replication forks and damage DNA in multiple ways (21). The ATM kinase is activated by
double-strand breaks, such as ionizing radiation. Furthermore, the
ATM signals induce the association of homologous recombination
repair components, such as Rad51, Xrcc3, BRCA2 and H2AX (22,23).
Our results suggest that single-strand breaks induced by the
incorporation of TFT into DNA may lead to to double-strand breaks
when the cells progress to a subsequent DNA replication phase
(24). In an in vivo
experiment, TFT (as TAS-102) showed a more potent antitumor
activity than the 5FU-derivative capecitabine when evaluated using
equitoxic doses. Unlike capecitabine, this antitumor potency of TFT
may originate from the double-strand breaks, rather than the
single-strand breaks, in DNA. The presently reported functional
mechanisms of TFT-induced DNA damage may be responsible for the
potent anticancer activity of TFT against 5FU-resistant tumors.
References
|
1.
|
Heidelberger C, Parsons DG and Remy DC:
Syntheses of 5-trifluoromethyluracil and
5-trifluoromethyl-2′-deoxyuridine. J Med Chem. 7:1–5. 1964.
|
|
2.
|
Bresnick E and Wiliams SS: Effects of
5-trifluoromethyldeoxy-uridine upon deoxythymidine kinase. Biochem
Pharmacol. 16:503–507. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Reyers P and Heidelberger C: Fluorinated
pyrimidines: XXVI. Mammalian thymidylate synthetase: its mechanism
of action and inhibition by fluorinated nucleotides. Mol Pharmacol.
1:14–30. 1965.
|
|
4.
|
Eckstein JW, Foster PG, Finer-Moore J,
Wataya Y and Santi DV: Mechanism-based inhibition of thymidylate
synthase by 5-(trifluoromethyl)-2′-deoxyuridine 5′-monophosphate.
Biochemistry. 3:15086–15094. 1994.PubMed/NCBI
|
|
5.
|
Umeda M and Heidelberger C: Comparative
studies of fluorinated pyrimidines with various cell lines. Cancer
Res. 28:2529–2538. 1968.PubMed/NCBI
|
|
6.
|
Murakami Y, Kazuno H, Emura T, Tsujimoto
H, Suzuki N and Fukushima M: Different mechanisms of acquired
resistance to fluorinated pyrimidines in human colorectal cancer
cells. Int J Oncol. 17:277–283. 2000.PubMed/NCBI
|
|
7.
|
Emura T, Nakagawa F, Fujioka A, Ohshimo H,
Yokogawa T, Okabe H and Kitazato K: An optimal dosing schedule for
a novel combination antimetabolite, TAS-102, based on its
intracellular metabolism and its incorporation into DNA. Int J Mol
Med. 13:249–255. 2004.PubMed/NCBI
|
|
8.
|
Emura T, Suzuki N, Yamaguchi M, Ohshimo H
and Fukushima M: A novel combination antimetabolite, TAS-102,
exhibits antitumor activity in FU-resistant human cancer cells
through a mechanism involving FTD incorporation in DNA. Int J
Oncol. 25:571–578. 2004.
|
|
9.
|
Suzuki N, Emura T and Fukushima M: Mode of
action of trifluorothymidine (TFT) against DNA replication and
repair enzymes. Int J Oncol. (In press).
|
|
10.
|
Nakamura J, Walger VE, Upton PB, Chiang
SY, Kow YW and Swenberg JA: Highly sensitive apurinic/apyrimidinic
site assay can detect spontaneous and chemically induced
depurination under physiological conditions. Cancer Res.
58:222–225. 1998.
|
|
11.
|
Nishikawa T, Munshi A, Story MD, Ismail S,
Stevens C, Chada S and Meyn RE: Adenoviral-mediated mda-7
expression suppresses DNA repair capacity and radiosensitizes
non-small-cell lung cancer cells. Oncogene. 16:7125–7131. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hartman KY and Heidelberger C: Studies on
fluorinated pyrimidines: XIII. Inhibition of thymidylate synthase.
J Biol Chem. 236:3006–3013. 1961.PubMed/NCBI
|
|
13.
|
Santi DV and McHenry CS:
5-Fluoro-2′-deoxyuridinylate: covalent complex with thymidylate
synthase. Proc Natl Acad Sci USA. 69:1855–1857. 1972.
|
|
14.
|
Glazer RI and Lloyd LS: Association of
cell lethality with incorporation of 5-fluorouracil and
5-fluorouridine into nuclear RNA in human colon carcinoma cells in
culture. Mol Pharmacol. 21:468–473. 1982.PubMed/NCBI
|
|
15.
|
Greenhalgh DA and Parish JH: Effect of
5-fluorouracil on cytotoxicity and RNA metabolism in human colonic
carcinoma cells. Cancer Chemother Pharmacol. 25:37–44. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Rogers WI, Hartman AC, Palm PE, Okstein C
and Kensler CJ: The fate of 5-trifluoromethyl-2′-deoxyuridine in
monkeys, dogs, mice, and tumor-bearing mice. Cancer Res.
29:953–961. 1969.PubMed/NCBI
|
|
17.
|
Dexter DL, Wolberg WH, Ansfield FJ, Helson
L and Heidelberger C: The clinical pharmacology of
5-trifluoromethyl-2′-deoxyuridine. Cancer Res. 32:247–53. 1972.
|
|
18.
|
Suzuki N and Fukushima M: Simple and rapid
enzymatic method for the synthesis of single-strand
oligonucleotides containing trifluorothymidine. Nucleosides
Nucleotides Nucleic Acids. 29:896–904. 2010. View Article : Google Scholar
|
|
19.
|
Bijnsdorp IV, Kruyt FA, Gokoel S,
Fukushima M and Peters GJ: Synergistic interaction between
trifluorothymidine and docetaxel is sequence dependent. Cancer Sci.
99:2302–2308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Backus HH, Pinedo HM, Wouters D, Kuiper
CM, Jansen G, van Groeningen CJ and Peters GJ: Differences in the
induction of DNA damage, cell cycle arrest, and cell death by
5-fluorouracil and antifolates. Oncol Res. 12:231–239.
2000.PubMed/NCBI
|
|
21.
|
Brown EJ and Baltimore D: Essential and
dispensable roles of ATR in cell cycle arrest and genome
maintenance. Genes Dev. 17:615–628. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Morrison C, Sonoda E, Takao N, Shinohara
A, Yamamoto K and Takeda S: The controlling role of ATM in
homologous recombinational repair of DNA damage. EMBO J.
19:463–471. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Beucher A, Birraux J, Tchouandong L,
Barton O, Shibata A, Conrad S, Goodarzi AA, Krempler A, Jeggo PA
and Löbrich M: ATM and Artemis promote homologous recombination of
radiation-induced DNA double-strand breaks in G2. EMBO J.
28:3413–3427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Branzei D and Foiani M: Regulation of DNA
repair throughout the cell cycle. Nat Rev Mol Cell Biol. 9:297–308.
2008. View
Article : Google Scholar : PubMed/NCBI
|