Introduction
It is a commonly held belief that ductal carcinoma
in situ (DCIS) is the precursor of invasive breast lesions
(1–3). The epithelium that lines the mammary
ducts of the normal human breast and encloses in situ breast
carcinomas, is physically separated from the stroma by both a
myoepithelial (ME) cell layer and basement membrane (4–8).
Most tumor epithelial cells must pass from the lumen of the duct
through the ME cell layer, which makes the physical disruption of
the myoepithelium a prerequisite for breast tumor invasion
(9). Our previous studies noted
the correlation between alterations of the myoepithelium and that
of adjacent epithelial cells. Approximately 15% of pre-invasive
breast tumors have been found to be associated with focal
myoepithelial cell layer disruptions (FMCLDs) (9–13).
At or near these breaks in the myoepithelium, tumor cells are
generally arranged as finger-like projections, budding from these
disruptions. The cells overlying these disruptions have
significantly less estrogen receptor (ER) expression, and also show
greater proliferation, genetic instability and invasion-related
gene expression than their morphologically similar counterparts
within the same duct (9–13). It is well known that cell adhesion
and motility-related molecules may play a critical role in the
forward progression of breast carcinoma. These molecules include
α-smooth muscle actin (SMA), epithelial (E)-cadherin, integrins,
talin, focal adhesion kinase (FAK) and vinculin (14–16).
The present study attempted to elucidate the
histological and ultrastructural features of tumor cells that
overlie FMCLDs, with respect to the uniqueness of vinculin, talin,
E-cadherin, integrins and FAK, and to determine whether they may be
qualified as biomarkers for early detection of breast tumor
invasion.
Materials and methods
Breast cancer samples
Formalin-fixed paraffin-embedded (FFPE) blocks of
breast cancer tissue were obtained from Weifang People's Hospital,
First Hospital of Jilin University and China-Japan Union Hospital
of Jilin University. Consecutive sections (4–5 μm) were cut and
placed on positively charged microscope slides. All cases were DCIS
as determined by H&E staining. We screened 17 ER-positive cases
to assess the molecules related to cell adhesion and motility, and
selected 2 cases with combined DCIS and invasive lesions to compare
to those of pure DCIS. The characteristics of the patients are
listed in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Case | Age (years) | Tumor type | ER |
|---|
| 1 | 58 | DCIS, grade I | ++ |
| 2 | 60 | DCIS, grade II | +++ |
| 3 | 44 | DCIS, grade II | ++ |
| 4 | 57 | DCIS, grade I | ++ |
| 5 | 43 | DCIS, grade I | +++ |
| 6 | 37 | DCIS, grade II | ++ |
| 7 | 62 | DCIS, grade I | +++ |
| 8 | 51 | DCIS, grade II | +++ |
| 9 | 47 | DCIS, grade III | + |
| 10 | 53 | DCIS, grade I | +++ |
| 11 | 48 | DCIS, grade II | ++ |
| 12 | 53 | DCIS, grade II | ++ |
| 13 | 45 | DCIS, grade II | +++ |
| 14 | 42 | DCIS, grade I | +++ |
| 15 | 48 | DCIS, grade II | ++ |
| 16 | 53 | DCIS, grade II | + |
| 17 | 49 | DCIS, grade I | +++ |
| 18 | 52 | DCIS and invasive
tumor | + |
| 19 | 47 | DCIS and invasive
tumor | − |
Reagents and procedures
Antibodies reported to be associated with motility
and adhesion of epithelial cells were selected for this study
(Table II). These included
antibodies against α-SMA, FAK, integrin β1, talin, vinculin and
E-cadherin. ER antibody was used to screen the ER-positive cases
and assess the ER-negative cell clusters overlying FMCLDs. α-SMA
antibody was used to examine the disruption of the ME cell layer.
The immunohistochemical staining kit was purchased from Maxin
Biocompany (Fuzhou, China). The double immunohistochemical staining
kit was purchased from Zhongshan Biocompany (Beijing, China).
 | Table II.Antibodies used for
immunohistochemical staining. |
Table II.
Antibodies used for
immunohistochemical staining.
| Antibody | Catalog no. | Antigen
retrieval | Manufacturer |
|---|
| α-smooth muscle
actin | ZM-0003 | No | Zhongshan (Beijing,
China) |
| Estrogen
receptor | M3634 | Yes | Dako (Carpinteria,
CA, USA) |
| Integrin β1 | Sc-9970 | Yes | Dako (Carpinteria,
CA, USA) |
| E-cadherin | MAB-0589 | Yes | Maxin (Fujian,
China) |
| Focal adhesion
kinase | bs-1340R | Yes | Biosan (Beijing,
China) |
| Talin | sc-81805 | Yes | Santa Cruz (Santa
Cruz, CA, USA) |
| Vinculin | sc-59803 | Yes | Santa Cruz (Santa
Cruz, CA, USA) |
Immunohistochemical staining
The protocol for immunohistochemical staining has
been previously published (17).
All immunochemical staining section images were digitized using a
TCA-9.0C CMOS microscope camera (Tucsen Imaging Technology) and
Image-Pro plus software (Media Cybernetics, Silver Spring, MD,
USA). The statistical analysis of the integrated optical density
(IOD) was performed using Minitab and GraphPad software.
Evaluation of immunostaining
All slides stained using immunohistochemistry were
double read by two independent observers and scored as negative
(−), weak (+), moderate (++) or strong (+++).
FFPE tissue blocks transformed to
ultra-thin sections
Using the H&E staining results to determine the
location of interest in the FFPE tissue blocks, each was trimmed to
approximately 2×2×2 mm3. The small block was packed with
lens tissue, and deparaffinized in xylene for 40 h. The block was
then passed through a graded acetone series and rinsed in 0.1 M
phosphate buffered saline (PBS) buffer 2X 10 min. The block was
placed in 1% OsO4 at 4°C for 1 h, washed in 0.1 M PBS
(pH 7.4) buffer overnight, rinsed in ddH2O, dehydrated
in a graded ethanol series and polymerized in embedding solution.
The small block was cut into semi-thin sections using an LKB-V
ultramicrotome until the point of interest was reached and then
ultra-thin sections were cut. These sections were double stained
with uranyl acetate and lead citrate and examined with a
transmission electron microscope (TEM) (JEM-1200EX II, JEOL, Tokyo,
Japan).
Results
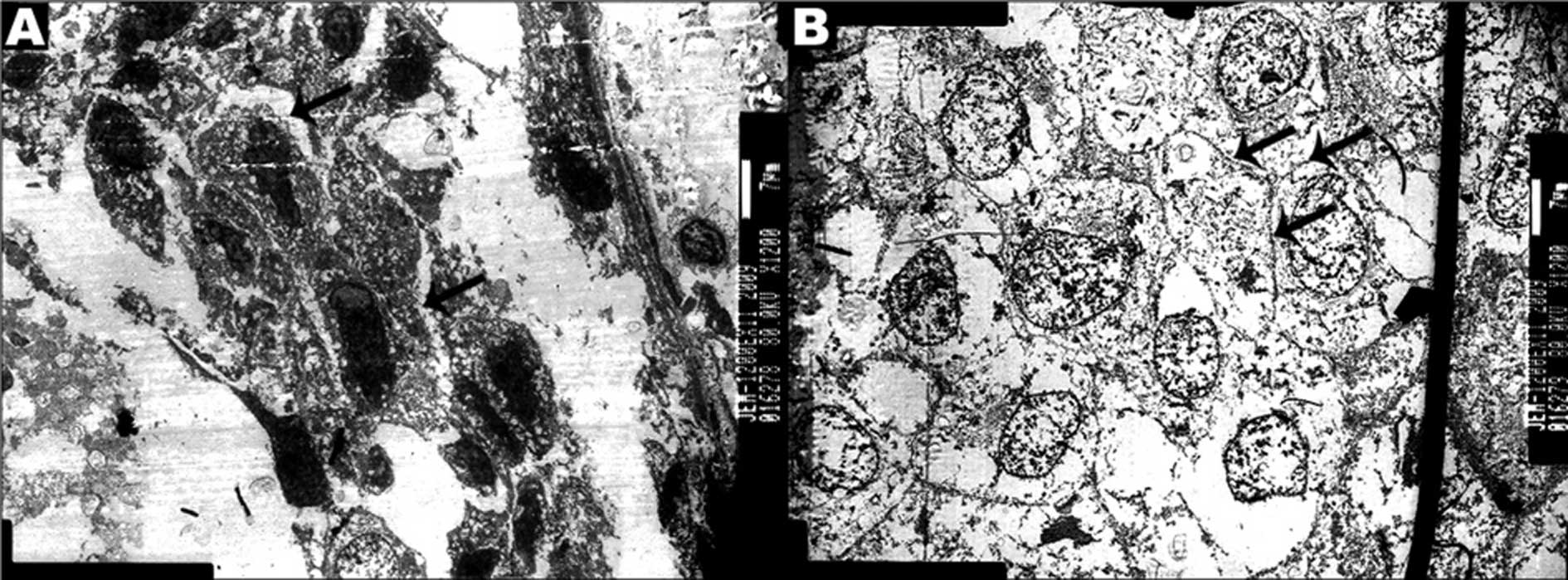
Ultrastructural characteristics
Two FFPE blocks of ER-positive cases with FMCLD
lesions were transformed to ultra-thin sections. Four FMCLD regions
on the tissue blocks were carved out and also cut into ultrathin
sections. The results of TEM showed that the tumor cells overlying
FMCLDs had darkly stained nuclei and cytoplasm, with elongated
nuclei and stellate cell bodies. The cells were separated from each
other by thin gaps, 5–30 μm wide (Fig.
1A). Compared to these cells, the adjacent tumor cells within
the associated duct were rounded, with weakly stained nuclei and
cytoplasm, with high electronic density at the regions of membrane
contact. This demonstrated that the membrane junction was tight
between in situ tumor cells (Fig. 1B).
Expression of vinculin, talin, integrin
β1, FAK and E-cadherin
Double immunohistochemical staining was performed on
all 17 ER-positive cases for α-SMA and ER to locate FMCLD lesions.
Seven cases harbored FMCLD lesions and 61 FMCLD lesions were
identified. Immunohistochemical staining for vinculin, talin,
integrin β1, FAK and E-cadherin was performed on these seven cases.
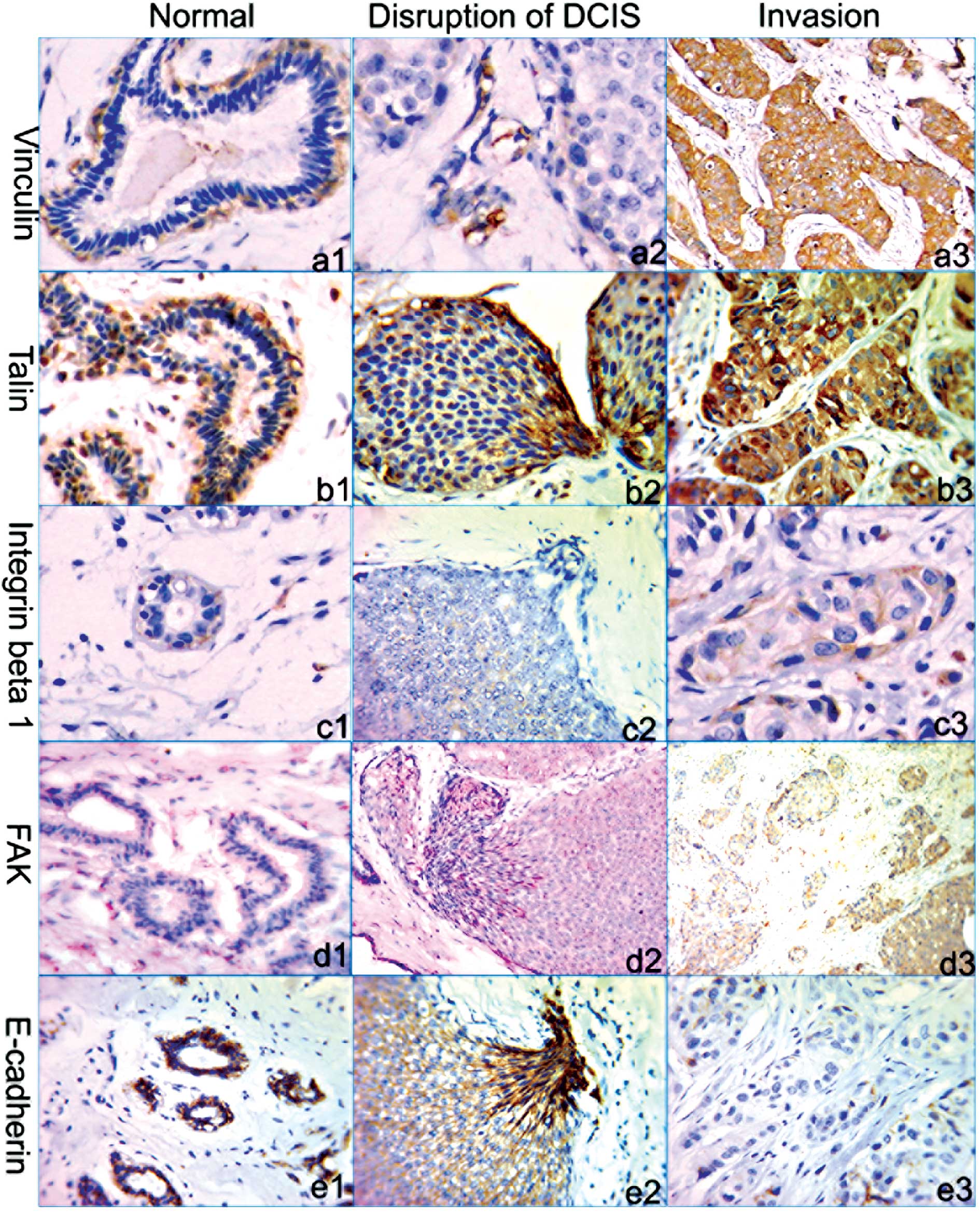
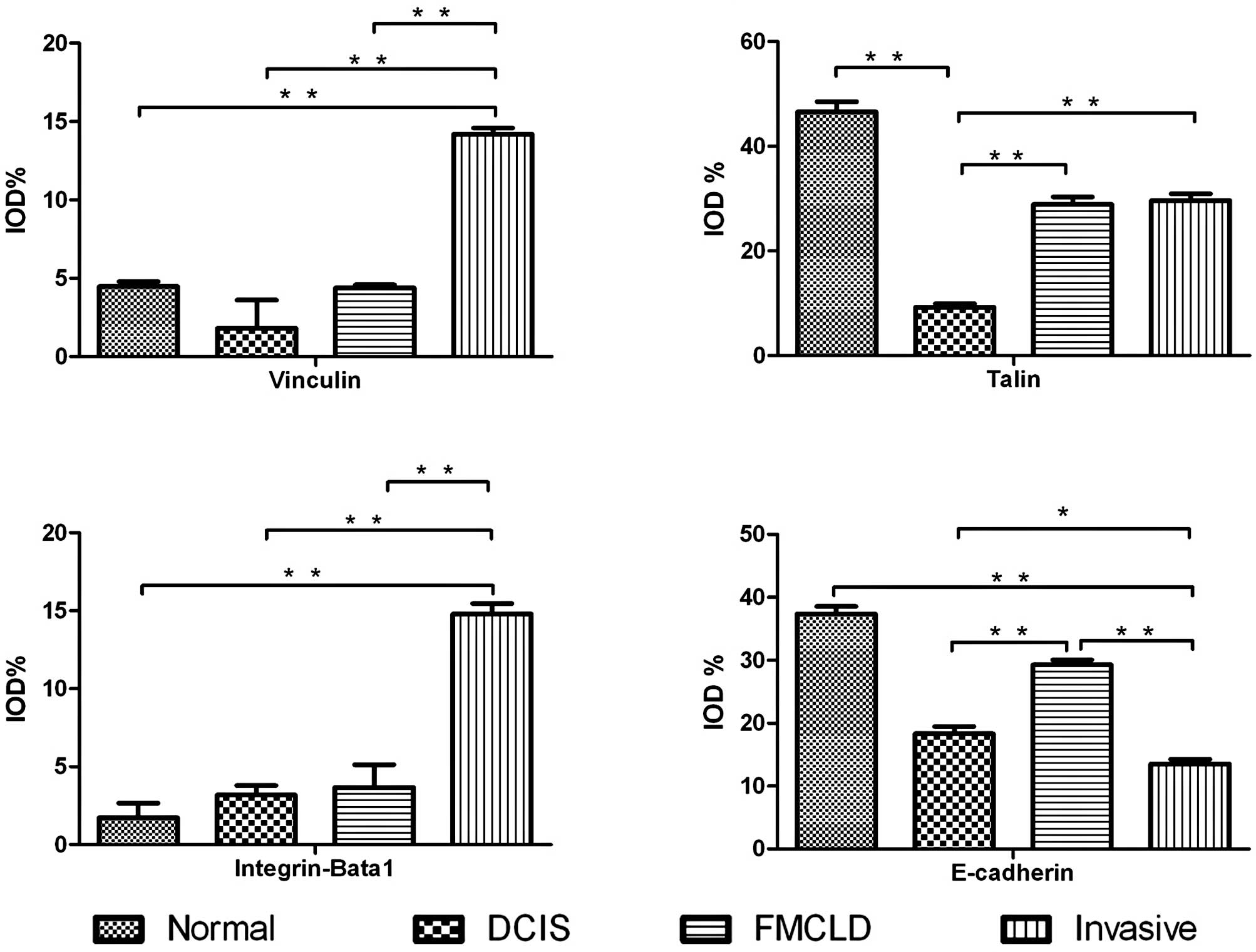
The staining results showed that vinculin was strongly expressed
(+++) in the cytoplasm of ME cells of all normal mammary ducts,
while not expressed in luminal cells. The same result was also
observed for α-SMA staining. Of 61 ducts with FMCLD lesions in DCIS
cases, vinculin was moderately (++) or weakly (+) expressed in ME
cells, and not expressed in luminal tumor cells. The tumor cells
overlying FMCLDs of 11 ducts (18%) had very weak vinculin
expression (+). Compared to the expression in normal ducts and DCIS
lesions, in invasive lesions vinculin expression was elevated (++;
Figs. 2a1, a2, a3 and 3A).
In normal ducts, strong talin expression (+++) was
detected in the cytoplasm of both ME cells and parts of glandular
epithelial cells. In DCIS lesions with FMCLDs, the cells overlying
the disruptions were strongly talin-positive (+++), in sharp
contrast to adjacent counterparts within the same ducts. Talin was
also strongly expressed (+++) in invasive lesions (Figs. 2b1, b2, b3 and 3B).
Integrin β1 was detected in the cytoplasm of ME and
luminal epithelial cells of normal ducts. In DCIS lesions, integrin
β1 was detected only in a small number of cells, and its staining
was weak (+). No distinct difference in integrin β1 expression was
observed between the tumor cells overlying FMCLDs and their
adjacent counterparts within the same duct. In invasive lesions, a
relatively high expression (+) of integrin β1 was detected, while
only a portion of cells were stained (Figs. 2c1, c2, c3 and 3C).
FAK staining was weak (+) in normal ducts and a
higher level of expression (++) was detected in DCIS lesions of all
cases. In the tumor cell clusters overlying FMCLDs, FAK was a bit
more highly expressed (+++) than in the adjacent tumor cells within
the same duct. FAK expression was weak (+) in invasive lesions and
lower than in DCIS (Fig. 2d1, d2 and
d3).
Strong E-cadherin expression (+++) was detected in
the plasma membranes of both ME and epithelial cells in normal
ducts. Altered expression was detected in the cells overlying
FMCLDs, where E-cadherin was strongly expressed (+++) in both the
plasma membrane and cytoplasm, but only expressed in the membranes
of adjacent tumor cells within the same duct. In invasive lesions,
E-cadherin expression was only detected in a small number of tumor
cells and the staining was very weak (+; Figs. 2e1, e2, e3 and 3D).
Discussion
Previous studies have shown that a subset of cell
clusters overlying FMCLDs of DCIS present many features similar to
malignant tumors. Our present study further revealed that the cell
clusters overlying FMCLDs were morphologically and
immunohistochemically different from adjacent tumor cells within
the corresponding duct: i) the cells emerging from FMCLDs had lost
tight contacts with their neighbors and they had gained
characteristics associated with cell motility; ii) the expression
of talin and FAK in these cells were higher than that in adjacent
cells, while vinculin and integrin β1 immunostaining were
infrequently detected; iii) by contrast, the expression of these
molecules in the cells overlying FMCLDs was different from that in
the epithelial cells of normal ducts and invasive tumor cells.
These phenotypic characteristics are typically associated with the
malignancy of tumor cells.
To migrate, the cell body must modify its shape and
firmness to move through the surrounding tissue structures. Cells
with motile capability generally present elongated nuclei and
stellate cell bodies. Under TEM, we found that the cells overlying
FMCLDs had polarized and elongated nuclei, and cell bodies with
many protrusions. The cells had lost their tight junctions and
there were gaps between them. These results imply that the tumor
cell could fall off from the tumor cell cluster. All of these
phenotypes are associated with cell motility, which have been
described in many previous studies on cell motility and migration
(18–20).
Cancer cell motility involves integrin signaling,
focal adhesion formation and actomyosin-dependent contractility
(21). Most breast tumor cells use
chain migration or collective migration mechanisms (22–24).
The molecular interactions that underlie changes in shape and
regulate migration are mainly related to focal adhesion dynamics.
There are several adhesion systems in normal mammary glands to
maintain cell integrity. The main adhesion molecules are cadherins
and integrins. To date, only a few studies have described the
pathological characteristics of focal adhesion molecule expression
in breast carcinoma in vivo. Glukhova et al (25) examined the expression of adhesion
molecules in breast cancer, and reported that the expression of
talin, vinculin and integrin was decreased in invasive breast
tumors, compared to normal mammary ducts. Our study further
confirmed their results. Also we demonstrated that the expression
of talin, vinculin and integrin was higher in invasive lesions
compared to that in DCIS. We also found that the expression of
these molecules in cells overlying FMCLDs was different from that
of luminal epithelial cells in the corresponding duct (Fig. 3). Therefore, altered expression in
these cells may be a beginning event of tumor invasion.
Generally, E-cadherin maintains mammary gland
epithelial cell junctions, whereas integrins are mainly distributed
in basal layer cells to interact with the extracellular matrix.
Previous studies provide evidence that many adhesion molecules,
such as E-cadherin and integrins, are down-regulated in breast
carcinoma and this down-regulation is associated with poor
prognosis (26–28). However, interestingly, cells
maintain a certain aggregation in invasive breast tumors. Our
finding demonstrated that the expression levels of adhesion
molecules, such as E-cadherin, vinculin and talin, were lower in
DCIS ducts and invasive lesions than in normal ducts, while the
expression levels of adhesion molecules in invasive lesions were
higher than those in DCIS ducts. The likely explanation is that the
loss of cell-cell junctions is a pre-requisite for cells to fall
off the tumor core, which may allow them to spread into the
stroma.
Although the tight junctions were reduced in the
cells over-lying FMCLDs, the cells also retained a degree of
adhesion, which may contribute to their maintaining a certain
integrity and ability to interact with the extracellular matrix,
otherwise they would not be able to adapt to the stromal
environment or migrate within it. When the ME cell layer is
disrupted, tumor cells at the FMCLD are exposed to stroma and
interact with extracellular matrix. This interaction perhaps
induces the tumor cell to express elevated levels of adhesion
molecules, and so the expression of talin, vinculin and FAK in the
cells overlying the FMCLD is higher than that of the tumor cells
within the corresponding duct.
In the progression from DCIS to invasive tumor, we
found that the altered expression of talin, vinculin, integrin β1
and FAK was not coordinated. The expression level of talin was
markedly higher in the cells overlying the FMCLD than that in their
counterparts of the same duct, while integrin and vinculin were
weakly expressed (Fig. 3). Many
previous studies confirm talin is required for cells to form new
adhesions on fibronectin during cell spreading and migration
(29–31). Since talin plays an important role
in forming focal adhesions, a high talin expression in cells
overlying FMCLDs implies that talin is of prime importance in
initiating the invasion of the tumor cell through the stroma.
However, details of the signaling pathways that regulate talin
activity are only just beginning to emerge, and the structural
basis for activation of the many ligand-binding sites in talin is
not yet fully understood. The role of talin in the early stages of
tumor invasion needs to be addressed.
Vinculin plays a crucial role in linking focal
adhesions to actin-cytoskeleton. Generally, its expression is
restricted in ME cells of the normal mammary duct. It is lowly
expressed in the ME of DCIS and not expressed during the disruption
of the ductal wall. Our results demonstrated that vinculin was very
weakly or not expressed in the cells over-lying FMCLDs, while
highly expressed in invasive lesions. Compared to talin, vinculin
expression was much weaker. Vinculin activation may lag behind that
of talin in the early stages of invasive tumor development. Recent
studies found that vinculin regulates cell-surface E-cadherin
expression by binding to β-catenin (32–34).
Our result confirmed this point; vinculin expression was increased
in invasive tumors and that of E-cadherin gradually decreased as
cells transformed from luminal cells of normal ducts to invasive
tumor cells. However, altered E-cadherin expression in the cells
reflects the complex molecular changes in the early stage of tumor
invasion. By contrast, the expression of vinculin gradually
increased as cells moved from overlying FMCLDs to invasive tumor
cells in the stroma. This means that as E-cadherin-mediated
cell-cell adhesion is reduced, it is replaced by integrin-mediated
cell-cell or cell-matrix adhesion and motility.
FAK is involved in integrin-mediated signaling which
has a profound impact on cell proliferation, survival and
migration. Previous studies (35–37)
suggest that FAK overexpression is significantly associated with
HER2 overexpression and Akt phosphorylation. Akt may be a
downstream target of FAK-mediated signaling. Since Akt activation
is associated with a worse prognosis in breast cancer, and HER2
overexpression is associated with tumor migration, FAK
overexpression may play an important role in promoting tumor
progression. Similar to talin, elevated FAK expression in DCIS and
the cells overlying FMCLDs imply their importance in the early
stage of invasive tumor development. However, the invasive lesion
exhibited lower FAK immunostaining than DCIS. The reason remains
unknown and further studies are required to address this issue.
Many reports have attempted to discover the
mechanism by which DCIS develops into invasive tumor; the
histological characteristics of DCIS and invasive tumors have been
widely examined. However, the tumor cells overlying FMCLDs of DCIS
have been rarely studied, perhaps because: i) as the disruption of
the ME cell layer is usually very small, it is difficult to
identify in clinical diagnostic histology by H&E staining and
can only be detected by immunohistochemistry; ii) past studies have
primarily focused on pure DCIS and invasive lesions; and iii) the
frequency of these lesions in the clinical setting is low
(∼15%).
The development from breast DCIS to invasive tumor
is a complex process. Our findings elucidated both the expression
characteristics of molecular markers that are correlated with cell
adhesion and motility, and the ultrastructural characteristics of
the cells overlying FMCLDs. These findings may provide more
detailed information about the origin of invasive tumor cells and
facilitate early diagnosis of breast cancer.
Since the frequency of these lesions in the clinic
is relatively low, there is still much work to do to investigate
the characteristics of tumor cells overlying FMCLDs.
In summary, the present study provided additional
morphological, ultrastructural and immunohistochemical data
confirming that the cells overlying FMCLDs likely represent the
specific precursor of invasive breast lesions. Our findings may
facilitate the identification of specific targets for further
molecular profiling in order to characterize this important cell
population.
Acknowledgements
This study was supported in part by
grant 2006CB910505 from the Ministry of Chinese Science and
Technology Department to Dr Xi-Chen Zhang. The authors thank
Medjaden Bioscience Ltd. for assisting in the preparation of this
manuscript.
References
|
1.
|
Beckmann MW, Niederacher D, Schnurch HG,
Gusterson BA and Bender HG: Multistep carcinogenesis of breast
cancer and tumour heterogeneity. J Mol Med. 75:429–439. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Schmitt FC: Multistep progression from an
oestrogen-dependent growth towards an autonomous growth in breast
carcinogenesis. Eur J Cancer. 31A:2049–2052. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Clarke R, Brunner N, Katzenellenbogen BS,
et al: Progression of human breast cancer cells from
hormone-dependent to hormone-independent growth both in vitro and
in vivo. Proc Natl Acad Sci USA. 86:3649–3653. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Tsubura A, Shikata N, Inui T, et al:
Immunohistochemical localization of myoepithelial cells and
basement membrane in normal, benign and malignant human breast
lesions. Virchows Arch A Pathol Anat Histopathol. 413:133–139.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Jolicoeur F, Seemayer TA, Gabbiani G, et
al: Multifocal, nascent, and invasive myoepithelial carcinoma
(malignant myoepithelioma) of the breast: an immunohistochemical
and ultrastructural study. Int J Surg Pathol. 10:281–291. 2002.
View Article : Google Scholar
|
|
6.
|
Slade MJ, Coope RC, Gomm JJ and Coombes
RC: The human mammary gland basement membrane is integral to the
polarity of luminal epithelial cells. Exp Cell Res. 247:267–278.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Miosge N: The ultrastructural composition
of basement membranes in vivo. Histol Histopathol. 16:1239–1248.
2001.PubMed/NCBI
|
|
8.
|
Nerlich A: Morphology of basement membrane
and associated matrix proteins in normal and pathological tissues.
Veroff Pathol. 145:1–139. 1995.(In German).
|
|
9.
|
Man YG, Tai L, Barner R, et al: Cell
clusters overlying focally disrupted mammary myoepithelial cell
layers and adjacent cells within the same duct display different
immunohistochemical and genetic features: implications for tumor
progression and invasion. Breast Cancer Res. 5:R231–R241. 2003.
View Article : Google Scholar
|
|
10.
|
Yousefi M, Mattu R, Gao C and Man YG:
Mammary ducts with and without focal myoepithelial cell layer
disruptions show a different frequency of white blood cell
infiltration and growth pattern: implications for tumor progression
and invasion. Appl Immunohistochem Mol Morphol. 13:30–37. 2005.
View Article : Google Scholar
|
|
11.
|
Man YG, Zhang Y, Shen T, et al: cDNA
expression profiling reveals elevated gene expression in cell
clusters overlying focally disrupted myoepithelial cell layers:
implications for breast tumor invasion. Breast Cancer Res Treat.
89:199–208. 2005. View Article : Google Scholar
|
|
12.
|
Man YG, Zhao CQ and Wang J: Breast tumor
cell clusters and their budding derivatives show different
immunohistochemical profiles during stromal invasion: implications
for hormonal and drug therapies. Cancer Ther. 4:193–204. 2006.
|
|
13.
|
Zhang X, Hashemi SS, Yousefi M, et al:
Aberrant c-erbB2 expression in cell clusters overlying focally
disrupted breast myoepithelial cell layers: a trigger or sign for
emergence of more aggressive cell clones? Int J Biol Sci.
4:259–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Banyard J and Zetter BR: The role of cell
motility in prostate cancer. Cancer Metastasis Rev. 17:449–458.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yam JW, Tse EY and Ng IO: Role and
significance of focal adhesion proteins in hepatocellular
carcinoma. J Gastroenterol Hepatol. 24:520–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Albiges-Rizo C, Destaing O, Fourcade B,
Planus E and Block MR: Actin machinery and mechanosensitivity in
invadopodia, podosomes and focal adhesions. J Cell Sci.
122:3037–3049. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Man YG and Tavassoli FA: A simple epitope
retrieval method without the use of microwave oven or enzyme
digestion. Appl Immunohistochem. 4:139–141. 1996.
|
|
18.
|
Friedl P and Brocker EB: The biology of
cell locomotion within three-dimensional extracellular matrix. Cell
Mol Life Sci. 57:41–64. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Mseka T, Coughlin M and Cramer LP: Graded
actin filament polarity is the organization of oriented actomyosin
II filament bundles required for fibroblast polarization. Cell
Motil Cytoskeleton. 66:743–753. 2009. View
Article : Google Scholar
|
|
20.
|
Popow-Wozniak A, Nowak D and
Malicka-Blaszkiewicz M: [Types of tumor cells movement]. Postepy
Biochem. 55:113–120. 2009.
|
|
21.
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Pitts WC, Rojas VA, Gaffey MJ, et al:
Carcinomas with metaplasia and sarcomas of the breast. Am J Clin
Pathol. 95:623–632. 1991.PubMed/NCBI
|
|
23.
|
Klinowska TC, Soriano JV, Edwards GM, et
al: Laminin and beta1 integrins are crucial for normal mammary
gland development in the mouse. Dev Biol. 215:13–32. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Simian M, Hirai Y, Navre M, Werb Z,
Lochter A and Bissell MJ: The interplay of matrix
metalloproteinases, morphogens and growth factors is necessary for
branching of mammary epithelial cells. Development. 128:3117–3131.
2001.PubMed/NCBI
|
|
25.
|
Glukhova M, Koteliansky V, Sastre X and
Thiery JP: Adhesion systems in normal breast and in invasive breast
carcinoma. Am J Pathol. 146:706–716. 1995.PubMed/NCBI
|
|
26.
|
Coopman PJ, Thomas DM, Gehlsen KR and
Mueller SC: Integrin alpha 3 beta 1 participates in the
phagocytosis of extracellular matrix molecules by human breast
cancer cells. Mol Biol Cell. 7:1789–1804. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Teuliere J, Faraldo MM, Deugnier MA, et
al: Targeted activation of beta-catenin signaling in basal mammary
epithelial cells affects mammary development and leads to
hyperplasia. Development. 132:267–277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Mierke CT, Kollmannsberger P, Zitterbart
DP, et al: Vinculin facilitates cell invasion into
three-dimensional collagen matrices. J Biol Chem. 285:13121–13130.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Tanentzapf G, Martin-Bermudo MD, Hicks MS
and Brown NH: Multiple factors contribute to integrin-talin
interactions in vivo. J Cell Sci. 119:1632–1644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Critchley DR and Gingras AR: Talin at a
glance. J Cell Sci. 121:1345–1347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Beckerle MC and Yeh RK: Talin: role at
sites of cell-substratum adhesion. Cell Motil Cytoskeleton.
16:7–13. 1990. View Article : Google Scholar
|
|
32.
|
Berx G and van Roy F: The
E-cadherin/catenin complex: an important gatekeeper in breast
cancer tumorigenesis and malignant progression. Breast Cancer Res.
3:289–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Le Duc Q, Shi Q, Blonk I, et al: Vinculin
potentiates E-cadherin mechanosensing and is recruited to
actin-anchored sites within adherens junctions in a myosin
II-dependent manner. J Cell Biol. 189:1107–1115. 2010.PubMed/NCBI
|
|
34.
|
Sawada K, Mitra AK, Radjabi AR, et al:
Loss of E-cadherin promotes ovarian cancer metastasis via alpha
5-integrin, which is a therapeutic target. Cancer Res.
68:2329–2339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Schmitz KJ, Grabellus F, Callies R, et al:
High expression of focal adhesion kinase (p125FAK) in node-negative
breast cancer is related to overexpression of HER-2/neu and
activated Akt kinase but does not predict outcome. Breast Cancer
Res. 7:R194–R203. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Ren XD, Kiosses WB, Sieg DJ, Otey CA,
Schlaepfer DD and Schwartz MA: Focal adhesion kinase suppresses Rho
activity to promote focal adhesion turnover. J Cell Sci.
113:3673–3678. 2000.PubMed/NCBI
|
|
37.
|
Lark AL, Livasy CA, Dressler L, et al:
High focal adhesion kinase expression in invasive breast carcinomas
is associated with an aggressive phenotype. Mod Pathol.
18:1289–1294. 2005. View Article : Google Scholar : PubMed/NCBI
|