Introduction
In a T-cell acute lymphoblastic leukemia xenograft
model, systemic administration of γ-secretase inhibitor was found
to result in significant regression of tumors directly via
apoptosis, and indirectly via disturbance of tumor angiogenesis
through the inhibition of non-cell-autonomous Notch signaling
(1). Bone marrow-derived vascular
precursor cells pre-treated with the γ-secretase inhibitor DAPT
failed to induce angiogenesis at the wound site and did not promote
wound healing in vivo (2).
Thus, the γ-secretase inhibitor may be applied as a pharmacological
regulator of angiogenesis. However, the mechanism of angiogenesis
regulation by γ-secretase inhibitors has not been clearly
understood.
Vascular endothelial growth factor receptor (VEGFR)
is the dominant angiogenic factor, and VEGFR inhibitors are useful
in treating cancer via anti-angiogenesis (3). Moreover, nitric oxide (NO),
synthesized by endothelial nitric oxide synthase (eNOS), is the
most effective endothelium-derived relaxing factor which leads to
vasodilation and better microvascular perfusion (4,5).
Thus, we used DAPT {N-[N-(3,5-difluorophenacetyl)-L-alanyl]-
S-phenylglycine t-butyl ester}, a γ-secretase inhibitor, to
determine the role of this γ-secretase inhibitor on VEGFR and eNOS
regulation in in vitro models.
Materials and methods
Cell culture and reagents
The mouse microvascular endothelial H5V cell line
was obtained from the Cell Bank of the Shanghai Institute of
Biological Sciences, Chinese Academy of Sciences. H5V cells were
maintained in DMEM (Gibco) supplemented with 10% fetal bovine serum
(FBS; PAA, Pasching, Austria). The cells were incubated at 37°C in
a 5% CO2-humidified atmosphere. DAPT and VEGFR-2 kinase
inhibitors were purchased from Sigma Aldrich and Merck,
respectively. DAPT was dissolved in DMSO (Sigma Aldrich) to
concentrations of 25×103, 50×103,
75×103 and 100×103 μmol/l.
DAPT treatment and VEGFR expression
H5V cells (1×106) were cultured with DAPT
(final concentration 100 μmol/l) or the equivalent amount of DMSO
as a negative control. Total protein or RNA was harvested 48 h
later. Protein and mRNA expression of VEGFR-1, VEGFR-2 and VEGFR-3
was measured by Western blotting and real-time PCR,
respectively.
DAPT treatment and eNOS expression
For the assessment of the role of VEGFR-2 on eNOS
regulation under DAPT treatment, 1×106 H5V cells were
cultured with DAPT (100 μmol/l), DAPT (100 μmol/l) + VEGFR-2 kinase
inhibitor (200 μmol/ ml), or DMSO (equivalent amount) for 48 h. To
assess the effects of different concentrations of DAPT,
1×106 H5V cells were cultured with DAPT (25, 50, 75 and
100 μmol/l) for 48 h, and the equivalent amount of DMSO was used as
a negative control. To assess the effect of different treatment
times, 1×106 H5V cells were cultured with 100 μmol/l
DAPT or DMSO (equivalent amount) for 0, 6, 12, 24 and 48 h. Protein
and mRNA expression of eNOS was measured by Western blotting and
real-time PCR, respectively.
Western blotting
H5V cells were dissolved and boiled in Laemmli
buffer for 10 min. The samples were then separated by SDS-PAGE
using 10% gel and transferred to polyvinylidene fluoride membranes
(Bio-Rad) at 80 mA for 2 h. After blocking with Tris
(hydroxymethyl) aminomethane-buffered solution containing Tween-20
(TBST), the membranes were incubated with the primary antibody in
TBST at 37°C for 2 h. Secondary antibodies in TBST were added and
incubated for 2 h at room temperature. Bound secondary antibodies
were detected using an ECL detection system (Amersham Pharmacia
Biotech). β-actin (Santa Cruz) was used as a loading control. The
primary antibodies used were as follows: monoclonal VEGFR-1
antibody (Santa Cruz), polyclonal VEGFR-2 antibody (Cell
Signaling), monoclonal VEGFR-3 antibody (eBioscience) and
monoclonal eNOS antibody (Abcam).
RNA extraction and real-time PCR
analysis
Total RNA was extracted from H5V cells using the
RNeasy Mini kit (Qiagen, Valencia, CA, USA), and 1 μg of total RNA
was converted to cDNA by using the ExScript RT reagent kit (Takara,
Otsu Shiga, Japan). Real-time PCR was carried out using the SYBR
Green PCR Master Mix (ABI) and i-Cycler (Bio-Rad) according to the
manufacturer’s instructions. Target gene expression levels in each
sample were subsequently normalized by the mRNA level of β-actin
mRNA in the same mRNA sample. The expression levels of each gene
were expressed as normalized fold expression. Results were reported
as the means ± SD of triplicate experiments from three independent
samples per group. Primers and probes included: VEGFR-1 forward
primer 5′-ggcccgggatatttataagaac-3′; VEGFR-1 reverse primer
5′-ccatccattttaggggaagtc-3′; VEGFR-2 forward primer
5′-cagtggtactggcagctagaag-3′; VEGFR-2 reverse primer 5′-aca
agcatacgggcttgttt-3′; VEGFR-3 forward primer 5′-gaatgag
agccccggaac-3′; VEGFR-3 reverse primer 5′-ggtctccagaccagc aactc-3′;
eNOS forward primer 5′-ccagtgccctgcttcatc-3′; eNOS reverse primer
5′-gcagggcaagttaggatcag-3′.
Statistical analysis
For all experiments triplicate measurements were
obtained, and the results are shown as the means ± SD. Statistical
analysis of data was performed using the two-tailed Student’s
t-test. P<0.01 and P<0.05 were considered statistically
significant.
Results
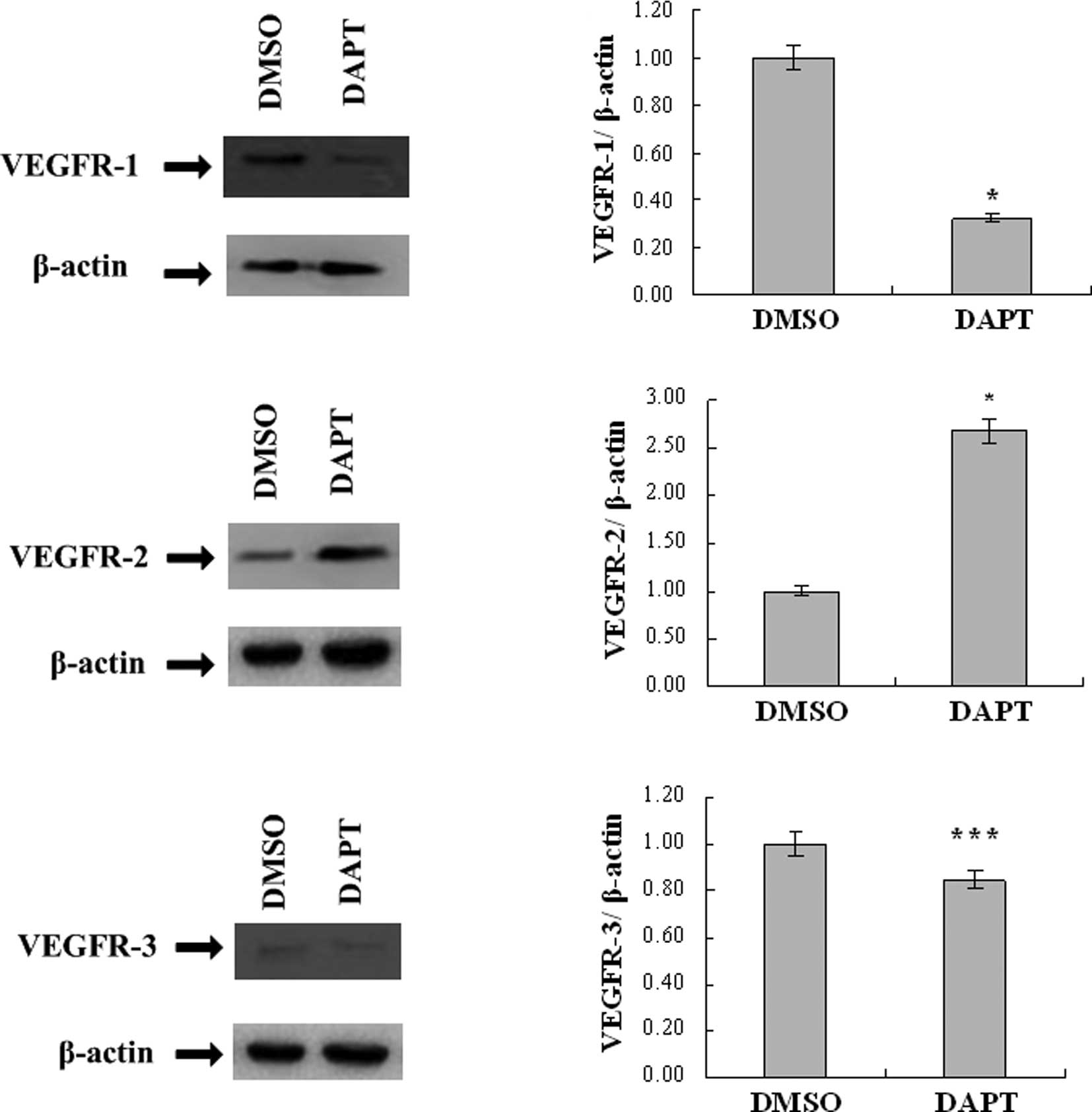
DAPT down-regulates VEGFR-1
expression
To assess whether the γ-secretase inhibitor DAPT
affects VEGFR-1 expression, H5V cells were cultured with 100 μmol/l
DAPT, or the equivalent amount of DMSO as a negative control.
Western blot analysis revealed that the level of VEGFR-1 protein
expression was decreased after 48 h. Upon real-time PCR analysis,
the mRNA level of VEGFR-1 was found to be down-regulated to 32.2%
compared to the DMSO-treated H5V cells (Fig. 1A and B).
DAPT up-regulates VEGFR-2 expression
VEGFR-2 is the primary VEGF signaling receptor, and
its expression is absolutely essential for vascular development and
angiogenesis (6). Therefore, an
in vitro model was used to determine VEGFR-2 expression
under 100 μmol/l DAPT treatment. After 48 h, VEGFR-2 protein
expression was increased, as revealed by Western blotting, and the
mRNA level was up-regulated by 2.68-fold compared to the control
groups (Fig. 1C and D).
DAPT has no significant effect on VEGFR-3
expression
Although previous research showed that Notch
signaling, the target of the γ-secretase inhibitor, altered VEGF
responsiveness in murine endothelial cells by direct regulation of
VEGFR-3 expression (7), our result
failed to show any significant difference between the DAPT and DMSO
groups in VEGFR-3 protein and mRNA expression (Fig. 1E and F).
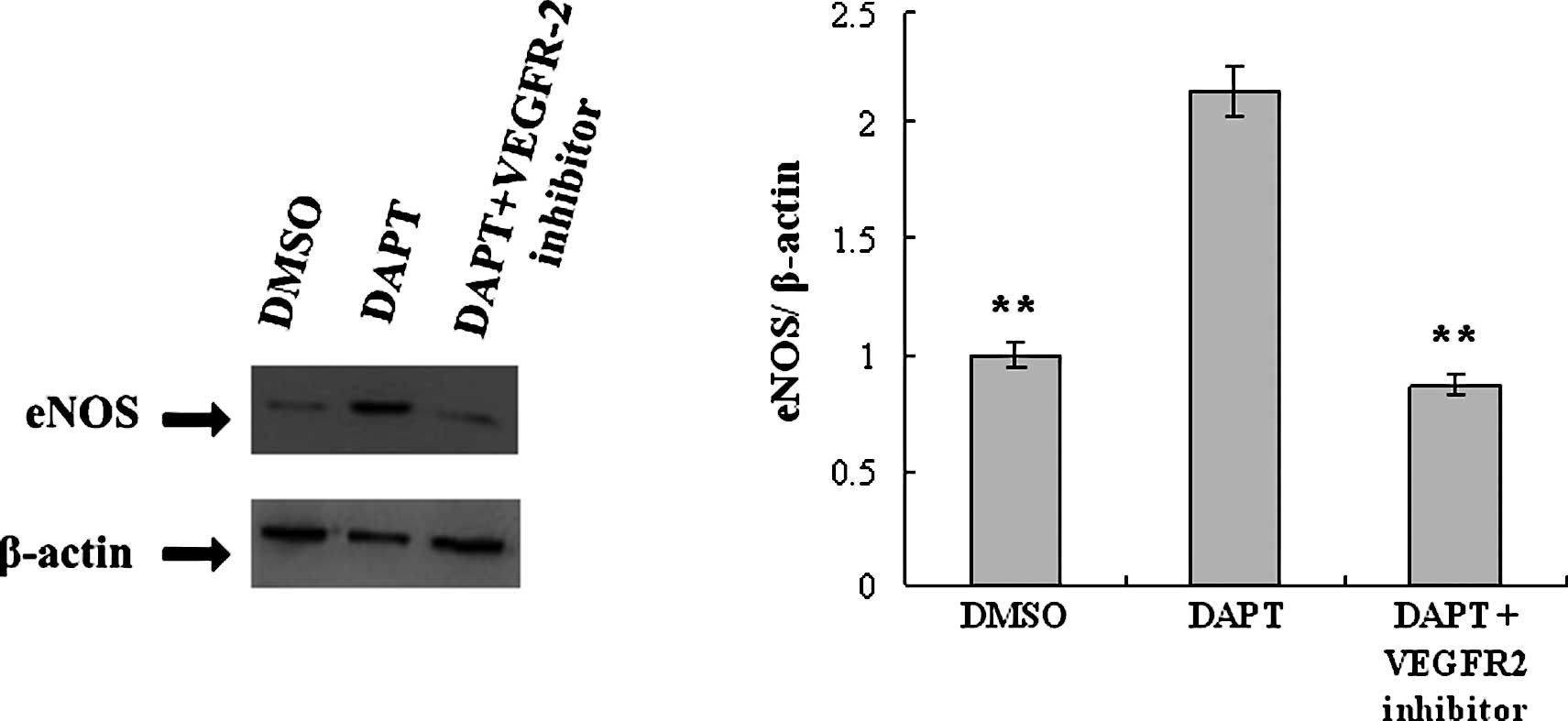
DAPT induces up-regulation of eNOS via
VEGFR-2
The eNOS expression level of H5V cells treated with
DAPT was measured by Western blotting and real-time PCR, and the
effect of VEGFR-2 was evaluated. After 48 h of DAPT treatment, eNOS
expression was up-regulated at both the protein and mRNA level.
Upon real-time PCR analysis, the level of mRNA expression of eNOS
in the DAPT group was found to be increased by 2.13-fold compared
to the control. This effect was neutralized when H5V cells were
pre-treated with the VEGFR-2 kinase inhibitor (Fig. 2).
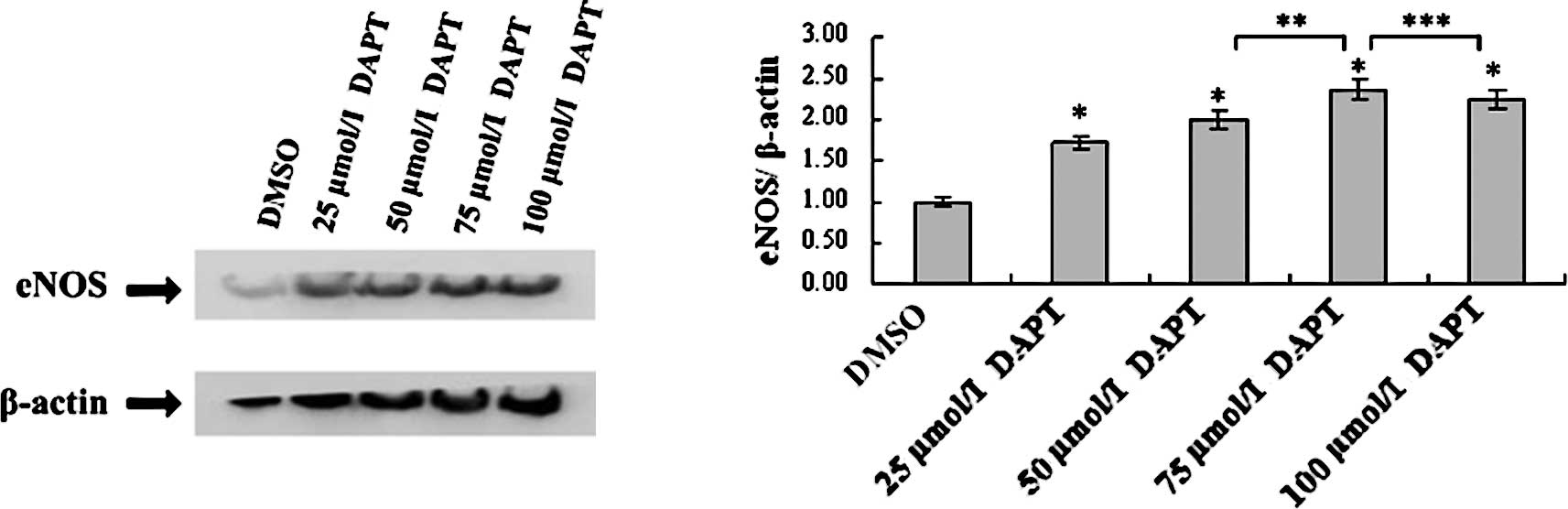
DAPT up-regulates eNOS in a
concentration-dependent manner
The eNOS protein level of the 75 μmol/l DAPT group
was higher than that of the control, 25 and 50 μmol/l DAPT groups,
but demonstrated no significant different with the 100 μmol/l DAPT
group. The mRNA level of eNOS was up-regulated by DAPT in a
concentration-dependent manner, which increased by 1.73-fold in H5V
cells treated with 25 μmol/l DAPT, by 2-fold in H5V cells treated
with 50 μmol/l DAPT, by 2.37-fold in H5V cells treated with 75
μmol/l DAPT and by 2.24-fold in H5V cells treated with 100 μmol/l
DAPT compared to the expression level of DMSO-treated H5V cells
(Fig. 3).
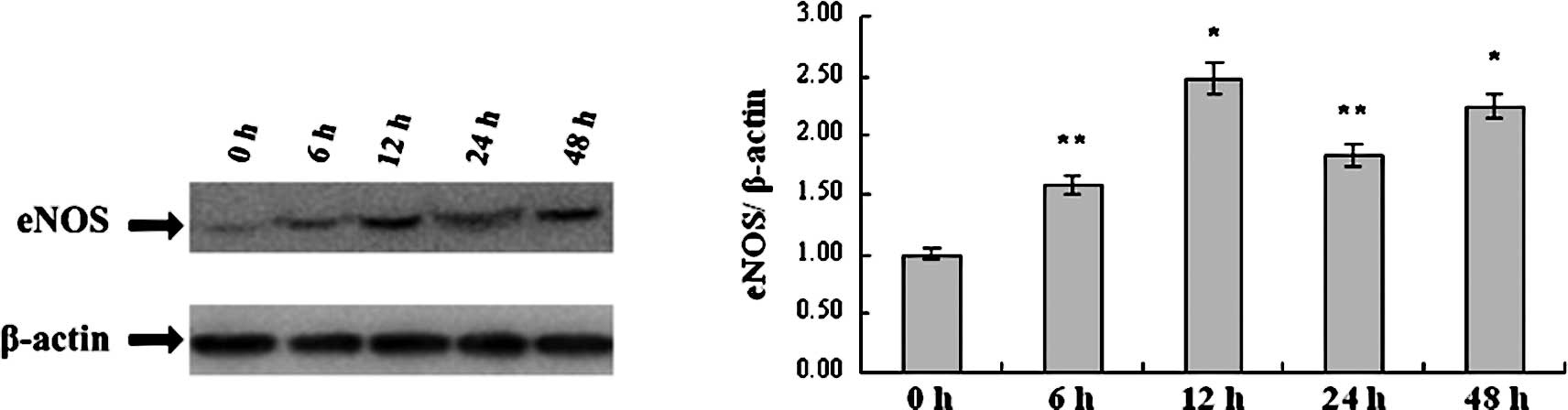
DAPT up-regulates eNOS in a
time-dependent manner
Up-regulation of eNOS induced by DAPT treatment was
found to be time-dependent. After treatment with 100 μmol/l of
DAPT, the level of eNOS protein began to increase at 6 h and
achieved peak levels at 12 and 48 h, separately. As determined by
real-time PCR analysis, mRNA expression increased by 1.58-fold at 6
h, by 2.48-fold at 12 h and by 2.25-fold at 48 h, compared to the
eNOS mRNA level of the control H5V cells at 0 h. The alternating
pattern of eNOS mRNA was similar to the pattern displayed by the
eNOS protein as showed in the Western blot analysis (Fig. 4).
Discussion
Previous studies have shown that γ-secretase
inhibitors may find application as pharmacological regulators of
angiogenesis. In a T-cell acute lymphoblastic leukemia xenograft
model, γ-secretase inhibitor indirectly suppressed tumor growth via
disturbance of tumor angiogenesis (1). In another wound-healing model, bone
marrow-derived vascular precursor cells pre-treated with the
γ-secretase inhibitor DAPT failed to induce angiogenesis at the
wound site in vivo (2).
However, the mechanism of how γ-secretase inhibitors regulate
angiogenesis has not been clearly understood. Given the important
role of VEGFR and eNOS in angiogenesis, in vitro models were
used to define the role of γ-secretase inhibitor DAPT on VEGFR and
eNOS regulation.
In our study, after DAPT treatment for 48 h, the
expression level of VEGFR-1 was lower compared to the control
groups, and VEGFR-2 was up-regulated compared to the control groups
at both the protein and mRNA level. However, VEGFR-3 expression
displayed no significant difference between DAPT-treated H5V cells
and the control. To our knowledge, this is the first study to
demonstrate that the γ-secretase inhibitor DAPT regulates VEGF
signaling via alteration of the expression of VEGFR-1 and VEGFR-2.
The mechanism will be investigated in subsequent research, and the
results may aid in future γ-secretase inhibitor therapy
strategies.
The effects of the γ-secretase inhibitor DAPT on
eNOS expression were investigated. VEGF-A has been described to
increase eNOS mRNA and protein expression in endothelial cells via
VEGFR-2, but not VEGFR-1 in vivo, and the
phosphatidylinositol 3-kinase (PI3-K) signaling pathway plays a
crucial role in this process (8,9).
Blockage of VEGFR-2 via a monoclonal antibody was found to cause a
marked reduction in the expression of eNOS and iNOS, which
decreased NO generation and led to robust hypertension (10). Here, we found that the eNOS
expression level was up-regulated in the DAPT-treated groups, at
both the protein and mRNA level. This effect was neutralized by the
VEGFR-2 kinase inhibitor. These findings indicate that DAPT
regulates eNOS via VEGFR-2. Furthermore, our research showed that
DAPT up-regulated eNOS in a concentration- and time-dependent
manner. Given that eNOS leads to vasodilation and better
microvascular perfusion via NO production, it is rational to infer
that enhancement of eNOS expression may offer some compensation in
the microvascular after γ-secretase inhibitor therapy, which may be
beneficial to normal tissues, but detrimental to cancer treatment.
Further research by us will focus on this issue.
In summary, we initially demonstrated the
relationship between VEGFR and eNOS under γ-secretase inhibitor
treatment. The γ-secretase inhibitor down-regulated VEGFR-1, but
up-regulated VEGFR-2, and the up-regulation of VEGFR-2 resulted in
an increase in eNOS expression. Our findings suggest that
administration of a γ-secretase inhibitor should be combined with
disruption of eNOS or VEGF signaling to improve the anti-angiogenic
therapeutic efficacy.
Acknowledgements
The authors would like to thank all
members of our laboratory for the helpful discussions and comments
on the manuscript. They also thank Dr Guo-kun Wang, of the
Department of Cardiology, Changhai Hospital, Shanghai, China, and
Dr Qiong Wang, of the Department of Medical Information, Changhai
Hospital, Shanghai, China, for the technical assistance.
References
|
1.
|
Masuda S, Kumano K, Suzuki T, et al: Dual
antitumor mechanisms of Notch signaling inhibitor in a T-cell acute
lymphoblastic leukemia xenograft model. Cancer Sci. 100:2444–2450.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Caiado F, Real C, Carvalho T and Dias S:
Notch pathway modulation on bone marrow-derived vascular precursor
cells regulates their angiogenic and wound healing potential. PLoS
One. 3:e37522008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Cook KM and Figg WD: Angiogenesis
inhibitors: current strategies and future prospects. CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Michel T and Vanhoutte PM: Cellular
signaling and NO production. Pflugers Arch. 459:807–816. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Toda N, Ayajiki K and Okamura T:
Interaction of endothelial nitric oxide and angiotensin in the
circulation. Pharmacol Rev. 59:54–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hirashima M: Regulation of endothelial
cell differentiation and arterial specification by VEGF and Notch
signaling. Anat Sci Int. 84:95–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Shawber CJ, Funahashi Y, Francisco E, et
al: Notch alters VEGF responsiveness in human and murine
endothelial cells by direct regulation of VEGFR-3 expression. J
Clin Invest. 117:3369–3382. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kroll J and Waltenberger J: VEGF-A induces
expression of eNOS and iNOS in endothelial cells via VEGF
receptor-2 (KDR). Biochem Biophys Res Commun. 252:743–746. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wang Y, Nagase S and Koyama A: Stimulatory
effect of IGF-I and VEGF on eNOS message, protein expression, eNOS
phosphorylation and nitric oxide production in rat glomeruli, and
the involvement of PI3-K signaling pathway. Nitric Oxide. 10:25–35.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Facemire CS, Nixon AB, Griffiths R,
Hurwitz H and Coffman TM: Vascular endothelial growth factor
receptor 2 controls blood pressure by regulating nitric oxide
synthase expression. Hypertension. 54:652–658. 2009. View Article : Google Scholar : PubMed/NCBI
|