Introduction
Lung cancer is the leading cause of cancer-related
death in the world (1). The
incidence of adenocarcinoma, one of the major histological subtypes
of non-small cell lung cancer (NSCLC), is increasing (2). The prognosis is dismal as the 5-year
survival is only approximately 50%, even in patients who achieve
complete surgical resection (3).
This suggests that occult metastases are present at the time of
surgical intervention. As a consequence, adjuvant chemotherapy is
required (4). However, the 5-year
survival rate of patients with resected stage IB NSCLC is 74%
without adjuvant chemotherapy, suggesting that not all patients
require chemotherapy after a complete resection (5). Therefore, it is necessary to identify
patients who may benefit the most from post-operative adjuvant
chemotherapy to, not only precisely select the patients who require
additional treatment, but also to prevent the occurrence of adverse
events in patients who do not require treatment (4). Therefore, it is important to evaluate
the biological and molecular characteristics of lung adenocarcinoma
to identify the factors related to recurrence following surgery.
However, there are currently no useful markers that predict
clinical recurrence.
Insulin-like growth factor receptor-1 (IGFR1) is a
transmembrane heterotetrameric protein implicated in promoting
oncogenic transformation, growth and survival of cancer cells
(6). The binding of insulin-like
growth factor (IGF) to the extracellular domain of IGFR1 activates
the tyrosine kinase activity of IGFR1 and triggers a cascade of
reactions involving signal transduction pathways (7). The overexpression of IGFR1 has been
shown to correlate with a poor prognosis in NSCLC patients
(8). However, the precise reason
for the poor prognosis remains unclear. Therefore, we hypothesized
that IGFR1 may be a useful indicator of tumor recurrence in
patients following complete resection. This is the first molecular
analysis of the IGFR1 status and tumor recurrence related to the
disease-free survival (DFS) of patients with lung adenocarcinoma,
and association of EGFR-related molecules.
Materials and methods
Patients, clinical features and
follow-up
Tumor samples were obtained from 296 patients with
primary lung adenocarcinoma who had undergone a surgical resection
between 2003 and 2007 at the Second Department of Surgery. Nine of
these patients were stage IV and 25 underwent an incomplete
resection. The tumor samples from 80 patients were too small to
evaluate by immunohistochemical (IHC) staining. As a result, 114
patients were excluded from further analysis and 182 tumor
specimens were evaluated. All the patients were Japanese, including
100 males and 82 females in this series, with a mean age of 68.5
years (range 23–88). No patients had received either chemotherapy
or radiotherapy prior to the resection. There were 75 never
smokers, 50 former and 57 current smokers. Former smokers were
defined as those who quit smoking at least 3 years before the time
of surgery. The tumor stage was classified according to the TNM
Classification for Lung Cancer (7th edition) (9). According to the pathological stage,
105 patients had tumors of stage IA, 39 of IB, 13 of IIA, 6 of IIB,
16 of IIIA and 3 of stage IIIB.
The patients were followed up every month during the
first postoperative year and at approximately 2- to 4-month
intervals thereafter. The evaluations included a physical
examination, chest roentgenography, an analysis of blood chemistry
and measurements of tumor markers, such as CEA, SCC and CYFRA.
Chest and abdominal computed tomography, brain magnetic resonance
imaging and a bone scintiscan were performed every 6 months for 3
years after surgery. Additional examinations were performed when
any symptoms or signs of recurrence were detected. Twenty-seven
(14.8%) patients received adjuvant chemotherapy, 18 received
carboplatin plus paclitaxel, 7 received carboplatin plus
gemcitabine and 2 received tegafur-uracil. A follow-up was
conducted in all patients, and the median follow-up period was 68.7
months. One hundred and forty-three patients were alive and free of
cancer at the last follow-up, while 11 patients had died of other
causes without evidence of cancer, 12 patients were alive with
recurrent cancer and 16 patients had died of cancer.
Immunohistochemical staining and
evaluation for IGFR1
The institutional review board approved the study
protocol, and informed consent for the use of the tumor specimens
was obtained either from the patients or from the patients' legal
guardians. IHC staining was conducted using serial sections from
the same paraffin-embedded blocks according to previously described
methods (10,11). All specimens were stained with
H&E for the histological diagnosis. Briefly, the sections were
placed in 0.01 mol/l citrate buffer (pH 6.0) and autoclaved at
121°C for 10 min. They were treated with 3%
H2O2 for 5 min to block the endogenous
peroxidase activity. The primary antibody used was a mouse
monoclonal antibody against human IGFR1 (3C8B1; Abcam, Cambridge,
MA, USA) (12), diluted 1:500 in
PBS and incubated for 18 h at 4°C. Thereafter, IHC staining was
performed by the labeled polymer method (Histofine Simple Stain
MAX-PO kit; Nichirei, Tokyo, Japan) according to the manufacturer's
instructions (13,14). The positive and negative controls
were processed using primary lung squamous cell carcinoma specimens
expressing IGFR1 and by the exclusion of the primary antibody,
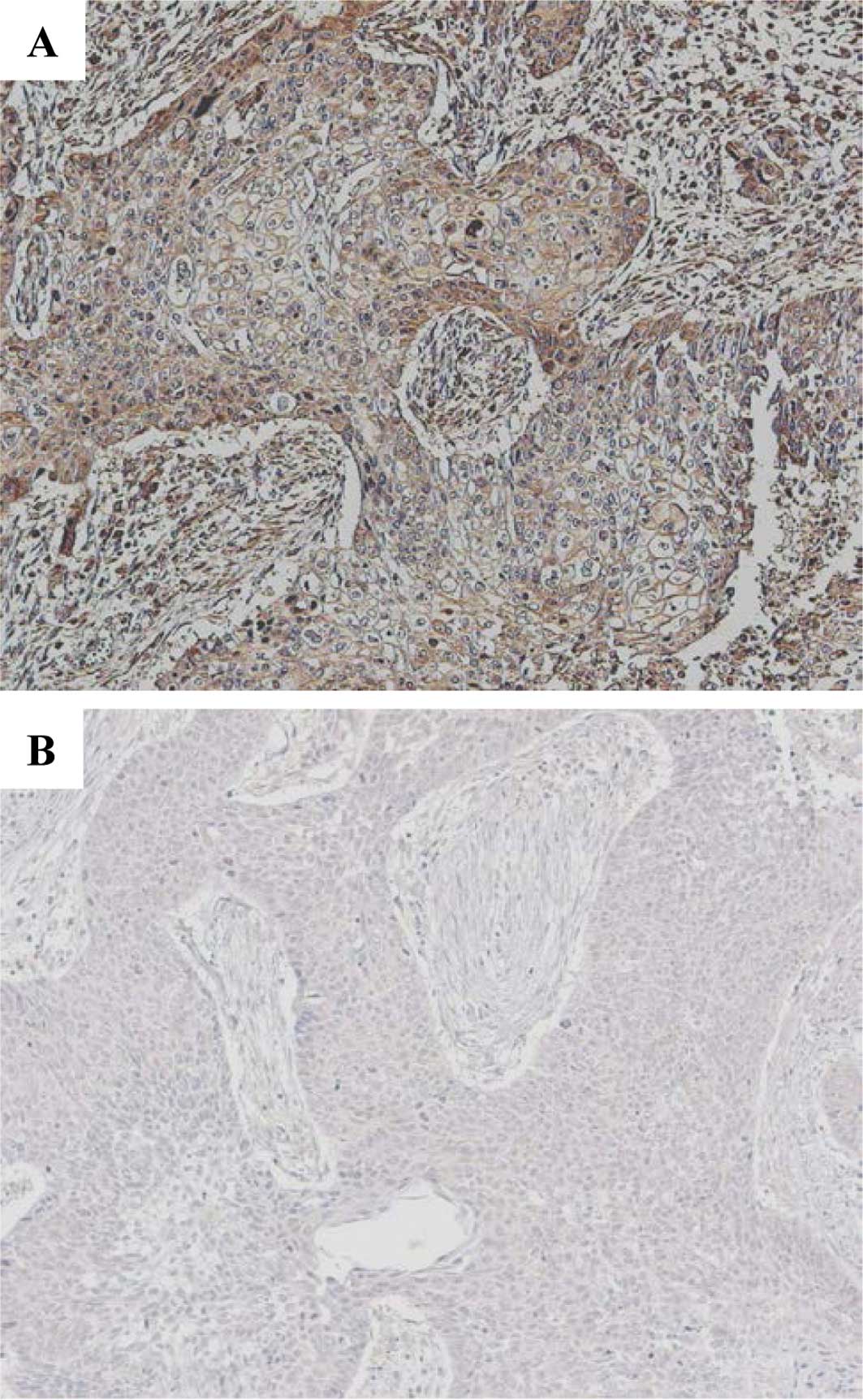
respectively. IHC was considered to be positive only when a
distinct cell membrane staining was evident (Fig. 1A). An average of 1,500 cells were
evaluated per section utilizing a semi-quantitative grading system
based on four stages: 0, no staining; 1+, staining in 1–10% of the
cells; 2+, staining in 11–25% of the cells; 3+, staining in >25%
of the cells. A cutoff value of 10% positive cells (stages of 2+
and 3+) was used in order to avoid inclusion of scattered
positivity of the same intensity found in the normal bronchial
tissue (15). The slides were
independently examined by two of the investigators (M.N. and H.S.)
who were blinded to the clinicopathological data. Any discrepancy
between the two investigators was resolved by their simultaneous
examination using a double-headed microscope. The correlation of
IGFR1 status and the genetic factors included below were also
analyzed.
Detection and quantification of
EGFR-related signaling molecules
The genomic DNA was extracted from each tumor by
previously described methods (16). The EGFR and K-ras mutations were
investigated by PCR-based analyses (13). The status of phosphorylation of MET
and HGF was examined by IHC staining (13). The MET gene copies were determined
by real-time PCR assays (13).
Statistical analyses
Statistical associations were determined by the
χ2 test or Fisher's exact test. A multivariate logistic
regression was used to evaluate independent associations. DFS and
95% confidence intervals (95% CI) were evaluated by the
Kaplan-Meier method comparing the different groups by log-rank
test. The Cox proportional hazards model was applied to the
multivariate survival analysis. The odds ratio (OR) was calculated
for each variable. The statistical difference was considered to be
significant at p<0.05. The data were analyzed with the Abacus
Concepts, Survival Tools for Stat View software package (Abacus
Concepts, Inc., Berkeley, CA, USA).
Results
Detection of IGFR1 expression and
clinicopathological characteristics
Positive expression of IGFR1 was identified in 43
(23.6%) of the 182 patients. There was no significant association
between IGFR1 expression and the clinical factors (Table I).
 | Table I.Relationship between the IGF1R
expression and clinicopathological characteristics. |
Table I.
Relationship between the IGF1R
expression and clinicopathological characteristics.
| Variables | No. of patients | IGF1R expression
|
|---|
| Positive n (%) | Negative n |
|---|
| Total patients | 182 | 43 (23.6) | 139 |
| Gender | | | |
| Male | 100 | 25 (25.0) | 75 |
| Female | 82 | 18 (22.0) | 64 |
| Age (years) | | | |
| <68 | 74 | 18 (24.3) | 56 |
| ≥68 | 108 | 25(23.1) | 83 |
| Pathologic stage | | | |
| IA | 105 | 24 (22.9) | 81 |
| IB–III | 77 | 19 (24.7) | 58 |
| T status | | | |
| T1a | 76 | 19 (25.0) | 57 |
| T1b–4 | 106 | 24 (22.6) | 82 |
| N status | | | |
| Negative | 151 | 33 (21.9) | 118 |
| Positive | 31 | 10 (32.3) | 21 |
| Smoking history | | | |
| Never | 75 | 18 (24.0) | 57b |
| Former | 50 | 11 (22.0) | 39 |
| Current | 57 | 14 (24.6) | 43 |
| Tumor gradea | | | |
| G1 | 94 | 20 (21.3) | 74c |
| G2 | 59 | 16 (27.1) | 43 |
| G3 | 15 | 3 (20.0) | 12 |
| CEAc | | | |
| <2.5 | 123 | 28 (22.8) | 95 |
| ≥2.5 | 57 | 15 (26.3) | 42 |
| SCCa | | | |
| <1.5 | 140 | 34 (24.3) | 106 |
| ≥1.5 | 28 | 5 (17.9) | 23 |
| CYFRAa | | | |
| <2.0 | 104 | 24 (23.1) | 80 |
| ≥2.0 | 74 | 19 (25.7) | 55 |
Relationship between IGFR1 expression and
recurrence
The majority of the first sites of tumor recurrence
were hematogenous metastases. Twenty-five and 8 cases had
hematogenous (10, lung; 9, brain; 5, bone; 1, adrenal metastasis)
and loco-regional (4, lymph node metastasis; 3, pleural
dissemination) recurrences, respectively (Table II). The positive expression of
IGFR1 was identified in 12 (42.9%) of 28 patients and 31 (20.1%) of
154 patients with and without recurrence, respectively (p=0.009;
Table III). The univariate and
multivariate logistic regression models indicated that expression
of IGFR1 was an independent predictor for recurrence, as were young
age and N status (Tables IV and
V).
 | Table II.Recurrent sites of tumors. |
Table II.
Recurrent sites of tumors.
| Site | No.a |
|---|
| Hematogenous
(n=25)b | Lung | 10 |
| Brain | 9 |
| Bone | 5 |
| Adrenal | 1 |
| Locoregional
(n=8)b | Lymph node | 5 |
| Pleural
dissemination | 3 |
 | Table III.Relationship between IGF1R expression
and recurrence. |
Table III.
Relationship between IGF1R expression
and recurrence.
| Variables | IGF1R expression
|
|---|
| Positive n (%) | Negative |
|---|
| Cases with
recurrence | 12 (42.9) | 16 |
| Cases without
recurrence | 31 (20.1) | 123 |
 | Table IV.Univariate analysis of the factors
contributing to recurrence. |
Table IV.
Univariate analysis of the factors
contributing to recurrence.
| Variables | Odds ratio | 95% confidence
interval | p-value |
|---|
| Gender: male | 1.322 | 0.581–3.008 | 0.506 |
| Age: <68
years | 2.630 | 1.151–6.007 | 0.022 |
| Smoking history:
former + current | 1.582 | 0.673–3.717 | 0.292 |
| T status: 1b–4 | 3.056 | 1.174–7.955 | 0.022 |
| N status:
positive | 9.952 | 4.025–24.609 | <0.001 |
| Tumor grade:
G2–3 | 6.149 | 2.171–17.415 | <0.001 |
| IGF1R expression:
positive | 2.976 | 1.277–6.934 | 0.012 |
 | Table V.Multivariate analysis of the factors
contributing to recurrence. |
Table V.
Multivariate analysis of the factors
contributing to recurrence.
| Variables | Odds ratio | 95% confidence
interval | p-value |
|---|
| Gender: male | 1.444 | 0.291–7.160 | 0.653 |
| Age: <68
years | 3.981 | 1.461–10.851 | 0.007 |
| Smoking history:
former + current | 1.076 | 0.210–5.556 | 0.927 |
| T status: 1b–4 | 2.630 | 0.870–7.949 | 0.087 |
| N status:
positive | 10.319 | 3.623–29.389 | <0.001 |
| IGF1R expression:
positive | 3.153 | 1.150–8.648 | 0.026 |
Influence of IGFR1 expression and
clinicopathological factors on DFS
The detectable relative risk was estimated to be 2.0
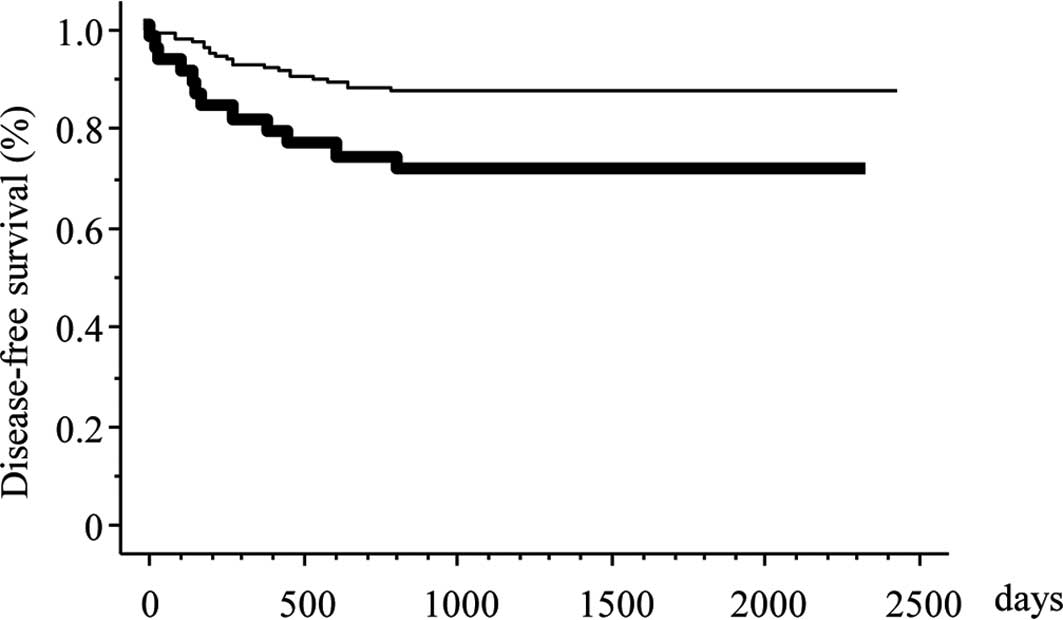
with 90% statistical power. The 5-year DFS rate in patients with
negative and positive IGFR1 expression was 87.6 and 70.7%,
respectively (p=0.007). Positive IGFR1 expression was associated
with a poorer DFS according to the univariate survival analysis
(Fig. 2) (p=0.001; Table VI). A multivariate survival
analysis also demonstrated that positive IGFR1 expression was
independently associated with an increased risk for a poor DFS
(p=0.020; Table VII).
 | Table VI.Univariate analysis using a
proportional hazards model for disease-free survival. |
Table VI.
Univariate analysis using a
proportional hazards model for disease-free survival.
| Variables | Characteristics
| 95% confidence
interval | Hazard ratio | p-value |
|---|
| Unfavorable | Favorable |
|---|
| Gender | Male | Female | 0.613–2.793 | 1.309 | 0.488 |
| Age (years) | <68 | ≥68 | 1.082–4.934 | 2.311 | 0.031 |
| Smoking
history | Former +
Current | Never | 0.709–3.472 | 1.567 | 0.267 |
| T status | 1b–4 | 1a | 1.180–7.184 | 2.912 | 0.020 |
| N status | Positive | Negative | 3.704–16.667 | 7.874 | <0.001 |
| Tumor grade | G2–3 | G1 | 2.104–15.123 | 5.642 | <0.001 |
| IGF1R
expression | Positive | Negative | 1.269–5.682 | 2.681 | 0.001 |
 | Table VII.Multivariate analysis using a
proportional hazards model for disease-free survival. |
Table VII.
Multivariate analysis using a
proportional hazards model for disease-free survival.
| Variables | Characteristics
| 95% confidence
interval | Hazard ratio | p-value |
|---|
| Unfavorable | Favorable |
|---|
| Gender | Male | Female | 0.317–3.650 | 1.076 | 0.906 |
| Age (years) | <68 | ≥68 | 1.222–5.686 | 2.636 | 0.014 |
| Smoking
history | Former +
Current | Never | 0.318–4.132 | 1.147 | 0.835 |
| T status | 1b–4 | 1a | 0.955–6.095 | 2.412 | 0.063 |
| N status | Positive | Negative | 2.959–14.286 | 6.494 | <0.001 |
| IGF1R
expression | Positive | Negative | 1.157–5.435 | 2.506 | 0.020 |
Relationship between IGFR1 and molecular
markers
EGFR and K-ras mutations were identified in 63
(34.6%) and 17 (9.3%) patients in these series, respectively. P-MET
1234/1235 and HGF were identified in 12 (6.6%) and 104 (57.1%)
patients, respectively. MET amplification was identified only in 8
patients (4.4%). There was no significant association of positive
IGFR1 expression with the EGFR mutation, an overexpression of p-MET
1234/1235 and HGF, and MET amplification. There were significantly
more tumors with IGFR1 expression among those with the K-ras
mutation when compared with the wild type group (p=0.017; Table VIII).
 | Table VIII.Association among molecular
markers. |
Table VIII.
Association among molecular
markers.
| Variables | No. of
patients | IGF1R expression
|
|---|
| Positive n (%) | Negative n |
|---|
| Total patients | 182 | 43 (23.6) | 139 |
| EGFR mutation | | | |
| Mutated | 63 | 17 (27.0) | 46 |
| Wild-type | 119 | 26 (21.8) | 93 |
| K-ras mutation | | | |
| Mutated | 17 | 8 (47.1) | 9 |
| Wild-type | 165 | 35 (21.2) | 130 |
| p-MET | | | |
| Positive | 12 | 4 (33.3) | 8 |
| Negative | 170 | 39 (22.9) | 131 |
| MET
amplification | | | |
| Positive | 8 | 0 (0.00) | 8 |
| Negative | 174 | 43 (24.7) | 131 |
| HGF expression | | | |
| Positive | 104 | 25 (24.0) | 79 |
| Negative | 78 | 18 (23.1) | 60 |
Discussion
The present study revealed two significant findings.
First, an increased expression of IGFR1 was significantly
correlated with postoperative recurrence. Furthermore, positive
IGFR1 expression was associated with a poorer DFS, thus suggesting
a more aggressive tumor behavior. This finding suggests that IGFR1
expression is a suitable biomarker with which to identify those
candidates who may benefit most from adjuvant chemotherapy in
adenocarcinoma following a complete resection. Notably, metastatic
NSCLC patients treated with gefitinib with high levels of IGF1R
expression survived longer than such patients lacking expression of
the protein (17). Collectively,
this trend for IGF expression, similar to Her2 status, is both a
poor prognostic marker in untreated patients and a favorable
predictive marker for treated patients, suggesting IGRF1 as a good
molecular target. In fact, the clinical benefit of an anti-IGF1R
antibody has been demonstrated in a phase II clinical study
(18). The prognostic impact of
IGFR1 remains controversial. Merrick et al showed that high
IGFR-1 expression indicated a poor prognosis in a cohort of
surgically treated NSCLC patients (8), which was consistent with the present
data. On the other hand, others reported that IGFR-1 protein
expression alone was not significantly associated with survival
(15,19). The discrepancy between these
findings may be due to the number of patients analyzed,
homogeneity, such as a different pathological stage, histology and
the method used for IHC.
Secondly, a significant correlation was observed
between positive expression of IGFR1 and K-ras mutation. These
results suggest that a correlation exists between the expression
status of IGFR1 and EGFR signaling, including the K-ras pathway
(20,21). Shen et al reported that the
combination of both K-ras and IGFR1 antisense oligodeoxynucleotide
cooperatively inhibited the growth of pancreatic cancer cell lines
in vitro, and induced their apoptosis in vivo
(22). Furthermore, combined IGF-1
and K-ras analyses have been shown to be beneficial for the better
selection of colorectal cancer patients that may respond to therapy
(23). Therefore, a new strategy
to co-target both IGFR-1 and K-ras may be required to control lung
cancers expressing IGFR1 with the K-ras mutation.
In conclusion, the present results revealed that the
incidence of IGFR1 overexpression was significantly higher in
recurrent cases than in non-recurrent ones. Furthermore, IGFR1
overexpression was also associated with poorer DFS. The present
results therefore indicate that IGFR1 expression may be a useful
marker for predicting postoperative recurrence in patients with
lung adenocarcinoma following surgery.
Abbreviations:
|
IGFR1,
|
insulin-like growth factor
receptor-1;
|
|
EGFR,
|
epidermal growth factor receptor;
|
|
HGF,
|
hepatocyte growth factor;
|
|
DFS,
|
disease free survival;
|
|
NSCLC,
|
non-small cell lung cancer;
|
|
IGF,
|
insulin-like growth factor;
|
|
IHC,
|
immunohistochemical;
|
|
95% CI,
|
95% confidence interval;
|
|
OR,
|
odds ratio
|
Acknowledgements
The authors thank Misako Fukumoto and
Yukiko Koyanagi for the valuable technical assistance. This study
was supported, in part, by Grants-in-Aid for Scientific Research
from the Ministry of Education, Culture, Sports, Science and
Technology (MEXT), Japan.
References
|
1.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Janssen-Heijnen ML and Coebergh JW: Trends
in incidence and prognosis of the histological subtypes of lung
cancer in North America, Australia, New Zealand and Europe. Lung
Cancer. 31:123–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Goya T, Asamura H, Yoshimura H, Kato H,
Shimokata K, Tsuchiya R, Sohara Y, Miya T and Miyaoka E: The
Japanese Joint Committee of Lung Cancer Registry Prognosis of 6644
resected non-small cell lung cancers in Japan: a Japanese lung
cancer registry study. Lung Cancer. 50:227–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pignon JP, Tribodet H, Scagliotti GV, et
al: Lung adjuvant cisplatin evaluation: a pooled analysis by the
LACE Collaborative Group. J Clin Oncol. 26:3552–3559. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kato H, Ichinose Y and Ohta M, Hata E,
Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N and
Ohta M: Japan Lung Cancer Research Group on Postsurgical Adjuvant
Chemotherapy. A randomized trial of adjuvant chemotherapy with
uracil-tegafur for adenocarcinoma of the lung. N Engl J Med.
350:1713–1721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Khandwala HM, McCutcheon IE, Flyvbjerg A
and Friend KE: The effects of insulin-like growth factors on
tumorigenesis and neoplastic growth. Endocr Rev. 21:215–244. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Merrick DT, Dziadziuszko R, Szostakiewicz
B, Szymanowska A, Rzyman W, Jassem E, Jassem J, Franklin WA, Bunn
PA and Hirsch FR: High insulin-like growth factor 1 receptor (IGF
1R) expression is associated with poor survival in surgically
treated non-small cell lung cancer (NSCLC) patients. J Clin Oncol.
25:75502007.
|
|
9.
|
Vallières E, Shepherd FA, Crowley J, van
Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw
P: International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions. The
IASLC Lung Cancer Staging Project: proposals regarding the
relevance of TNM in the pathologic staging of small cell lung
cancer in the forthcoming (seventh) edition of the TNM
classification for lung cancer. J Thorac Oncol. 4:1049–1059.
2009.
|
|
10.
|
Onitsuka T, Uramoto H, Nose N, Takenoyama
M, Hanagiri T, Sugio K and Yasumoto K: Acquired resistance to
gefitinib: the contribution of mechanisms other than the T790M,
MET, and HGF status. Lung Cancer. 68:198–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yamashita T, Uramoto H, Onitsuka T, Ono K,
Baba T, So T, So T, Takenoyama M, Hanagiri T, Oyama T and Yasumoto
K: Association between lymphangiogenesis-/micrometastasis- and
adhesion-related molecules in resected stage I NSCLC. Lung Cancer.
70:320–328. 2010. View Article : Google Scholar
|
|
12.
|
Chang MH, Lee J, Han J, Park YH, Ahn JS,
Park K and Ahn MJ: Prognostic role of insulin-like growth factor
receptor-1 expression in small cell lung cancer. APMIS.
117:861–869. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Onitsuka T, Uramoto H, Ono K, Takenoyama
M, Hanagiri T, Oyama T, Izumi H, Kohno K and Yasumoto K:
Comprehensive molecular analyses of lung adenocarcinoma with regard
to the epidermal growth factor receptor, K-ras, MET, and hepatocyte
growth factor status. J Thorac Oncol. 5:591–596. 2010.PubMed/NCBI
|
|
14.
|
Shimokawa H, Uramoto H, Onitsuka T, Iwata
T, Nakagawa M, Ono K and Hanagiri T: TS expression predicts
postoperative recurrence in adenocarcinoma of the lung. Lung
Cancer. Oct. 21–2010, (E-pub ahead of print).
|
|
15.
|
Ludovini V, Bellezza G, Pistola L, et al:
High coexpression of both insulin-like growth factor receptor-1
(IGFR-1) and epidermal growth factor receptor (EGFR) is associated
with shorter disease-free survival in resected non-small-cell lung
cancer patients. Ann Oncol. 20:842–849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Uramoto H, Sugio K, Oyama T, Ono K, Sugaya
M, Yoshimatsu T, Hanagiri T, Morita M and Yasumoto K: Epidermal
growth factor receptor mutations are associated with gefitinib
sensitivity in non-small cell lung cancer in Japanese. Lung Cancer.
51:71–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Cappuzzo F, Toschi L, Tallini G, et al:
Insulin-like growth factor receptor 1 (IGFR-1) is significantly
associated with longer survival in non-small-cell lung cancer
patients treated with gefitinib. Ann Oncol. 17:1120–1127. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Karp DD, Paz-Ares LG, Novello S, et al:
Phase II study of the anti-insulin-like growth factor type 1
receptor antibody CP-751,871 in combination with paclitaxel and
carboplatin in previously untreated, locally advanced, or
metastatic non-small-cell lung cancer. J Clin Oncol. 27:2516–2522.
2009. View Article : Google Scholar
|
|
19.
|
Cappuzzo F, Tallini G, Finocchiaro G, et
al: Insulin-like growth factor receptor 1 (IGF1R) expression and
survival in surgically resected non-small-cell lung cancer (NSCLC)
patients. Ann Oncol. 21:562–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lee AV, Cui X and Oesterreich S:
Cross-talk among estrogen receptor, epidermal growth factor, and
insulin-like growth factor signaling in breast cancer. Clin Cancer
Res. 7:4429–4435. 2001.PubMed/NCBI
|
|
21.
|
Gilmore AP, Valentijn AJ, Wang P, Ranger
AM, Bundred N, O'Hare MJ, Wakeling A, Korsmeyer SJ and Streuli CH:
Activation of BAD by therapeutic inhibition of epidermal growth
factor receptor and transactivation by insulin-like growth factor
receptor. J Biol Chem. 277:27643–27650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Shen YM, Yang XC, Yang C and Shen JK:
Enhanced therapeutic effects for human pancreatic cancer by
application K-ras and IGF-IR antisense oligodeoxynucleotides. World
J Gastroenterol. 14:5176–5185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Scartozzi M, Mandolesi A, Giampieri R, et
al: Insulin-like growth factor 1 expression correlates with
clinical outcome in K-RAS wild-type colorectal cancer patients
treated with cetuximab and irinotecan. Int J Cancer. 127:1941–1947.
2010. View Article : Google Scholar
|