Introduction
The role of blood vessels in tumor progression and
metastasis has been reported in various malignancies, including
oral and cervical cancers (1–3).
However, the relationship between blood vessels and lymph node
metastasis in malignant tumors is largely unknown. Angiogenesis
plays a crucial role in hematogenous and lymphatic metastases for
which studies have suggested that lesions that entered a higher
angiogenic state have an increased probability of metastasis
(3,4). However, certain tumors with marked
angiogenesis surprisingly had no evidence of metastasis and were
associated with a good prognosis (5,6).
Thus, an unclear relationship exists among angiogenesis, metastasis
and prognosis.
Clinically, lymph node metastasis in oral and
cervical cancers occurs in 20–30% of cases and is considered a
major factor in determining prognosis. Lymphatic spread is more
important than other routes. Via this route malignant cells
preferentially metastasize in lymph nodes in the cervical region
(7). Also, lymphatic vessels are
the preferential routes for metastatic spread of most carcinomas
that arise in the cervix (8).
Distant lymph node metastasis of cancer cells is often due to the
involvement of lymphatic vessels (4,5).
Despite the occurrence of lymphatic metastasis in those tumors, few
studies have focused on the distribution and characteristics of
lymphatic vessels in cancers (9,10).
Moreover, it remains questionable whether tumor cells induce
lymphangiogenesis.
Several lymphatic-specific antibodies have been
developed (11). Kahn and Marks
developed an antibody called D2-40, reported to be effective in
detecting lymphatic vessels in formalin-fixed paraffin sections
(12). The antibody does not
require any special treatment for antigen retrieval and so is
considered to be useful in lymphatic research.
The aim of this study was to clarify the
distribution of lymphatic tissues in oral and cervical squamous
cell carcinoma (SCC) using immunohistochemistry. The study also
elucidated the characteristics of lymphatic vessels involved in
lymphangiogenesis.
Materials and methods
Case selection and tissue
preparation
A total of 20 cases of oral SCC was acquired from
the Department of Oral Pathology, Okayama University Hospital. Ten
cases had lymph node metastasis and 10 cases were non-lymph node
metastatic. Another 20 cases of cervical SCC from patients treated
at Taipei Medical University were also included. Ten cases had
lymph node metastasis and 10 were non-lymph node metastatic. Five
cases of each normal oral and cervical mucosal tissue were used as
controls. The ethics committee of the university approved the study
protocol. Conventional method for tissue preparation was performed
where tissues were fixed in 10% neutral buffered formalin solution
and embedded in paraffin. Serial sections (4-μm) were prepared from
paraffin blocks, stained with H&E and examined under a light
microscope.
Immunohistochemistry
Table I shows the
primary antibodies and their corresponding dilution. D2-40 is an
antibody that detects lymphatic vessels and CD31, CD34, CD105 are
antibodies against blood vessels. Keratin was used to identify SCC
cells.
 | Table I.Primary antibodies used. |
Table I.
Primary antibodies used.
| Antibody | Manufacturer | Dilution |
|---|
| D2-40 | Dako | 1:50 |
| CD31 | Dako | 1:100 |
| CD34 | Nichirei | RTU |
| CD105 | Dako | 1:50 |
| Keratin | Dako | 1:400 |
After deparaffinization, sections were immersed in
0.03% hydrogen peroxide in methanol for 30 min. For CD31 and CD105,
antigen retrieval was carried out by immersing the slides in 10 mM
sodium citrate buffer (pH 6.0) and were high-pressure heat treated
at 121°C for 15 min. Immunohistochemical staining was performed
using the Vectastain ABC kit (Vector Laboratories, CA, USA). The
antigen reaction was revealed using 3,3′-diaminobenzidine (DAB;
Sigma-Aldrich, Tokyo, Japan) chromogenic substrate, counterstained
with Mayer’s hematoxylin and examined under a light microscope.
To identify cancer cell invasion in lymphatic
vessels, double staining was performed using keratin and D2-40
antibodies. For keratin, immunohistochemistry was performed using
the Vectastain ABC kit (rabbit IgG; Vector Laboratories) followed
by DAB chromogenic substrate. For D2-40, immunohistochemistry was
carried out using the Vectastain ABC kit (mouse IgG; Vector
Laboratories) and antigenic sites were revealed using
3-amino-9-ethylcarbazole (AEC; Dako, CA, USA). Sections were
examined under a light microscope.
Lymphatic vessel density measurement and
statistical analysis
Lymphatic vessel density (LVD) was measured by
counting the number of lymphatic vessels stained with D2-40 on the
superficial layer of normal mucosa as well as in the SCCs. Each
sample was observed under a x20 objective (∼0.54 mm2),
and five locations were chosen. Three different observers counted
the lymphatic vessels and then the average was computed. LVD was
calculated per unit area (1 mm2).
Results
Distribution of lymphatic vessels in
normal oral and cervical mucosa
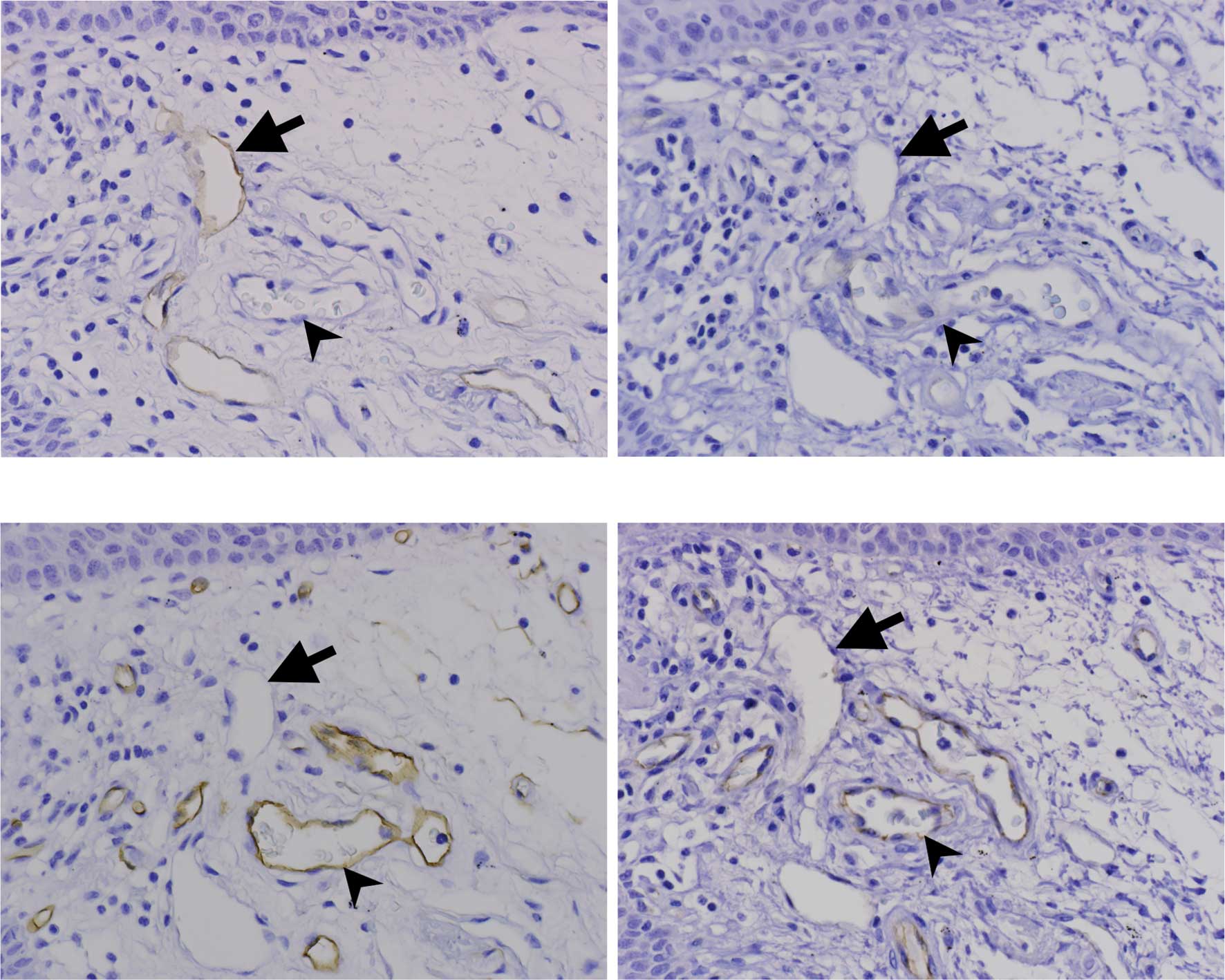
Lymphatic vessels in the oral and cervical mucosa
stained with D2-40 in three consecutive tissue sections were
examined as well as the surrounding blood vessels. In both oral and
cervical mucosa, the blood vessels (arrowheads) were negative to
D2-40 and CD105 (Fig. 1A and B)
and positive to CD34 and CD31 (Fig. 1C
and D). On the other hand, lymphatic vessels (arrows) were
positive to D2-40 (Fig. 1A) and
negative to CD105, CD34 and CD31 (Fig.
1B–D). Lymphatic vessels in the oral mucosa were distributed in
the papillary and reticular layer (Fig. 1A). Lymphatic vessels in cervical
mucosa were distributed in the reticular dermis and muscle layer
(data not shown).
Localization and characterization of
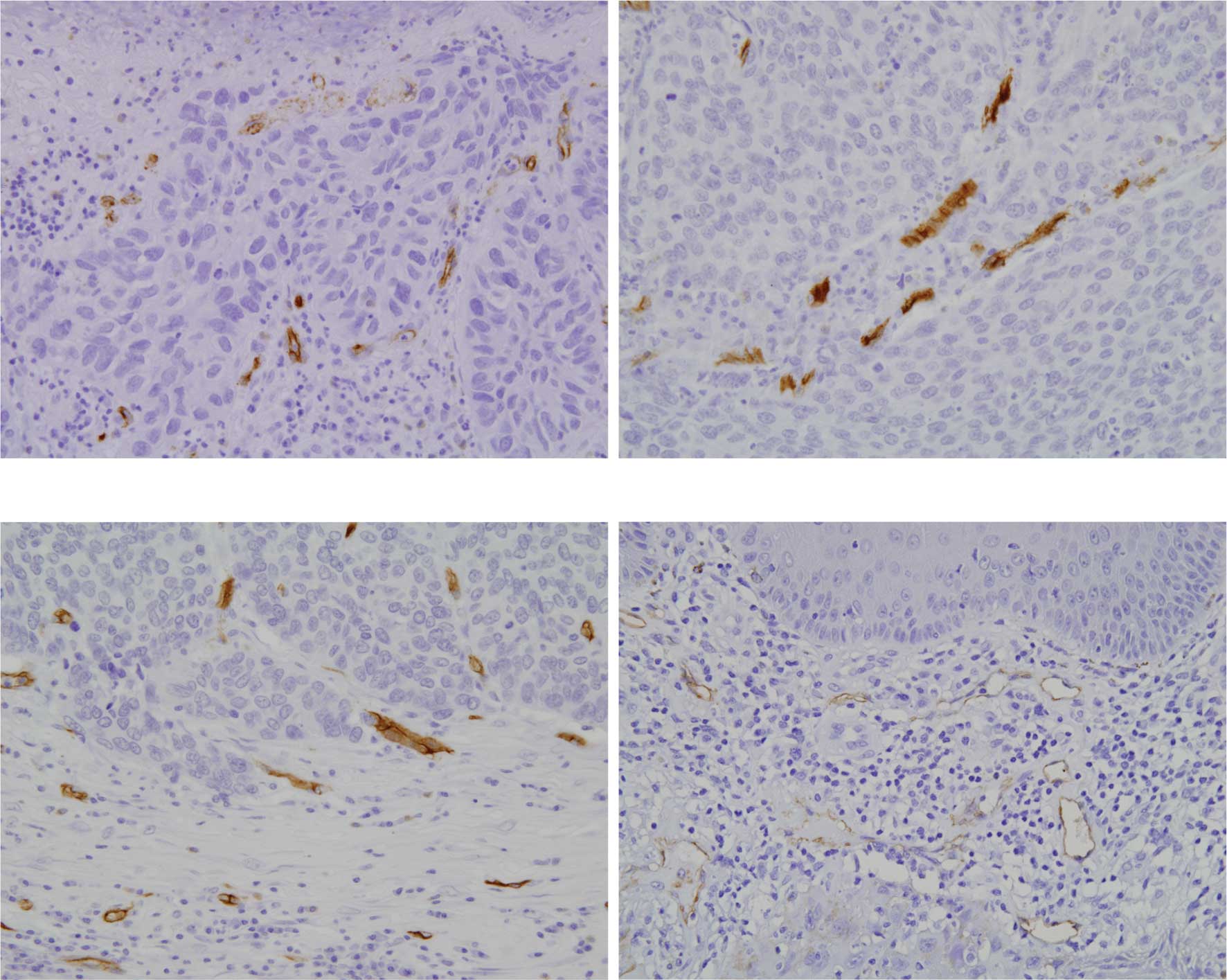
lymphatic vessels in oral SCC
Both metastatic and non-metastatic oral SCCs
exhibited invasion of cancer cells in the connective tissue with
proliferation of cancer nests. Blood vessels positive to CD34 were
evenly distributed in the superficial cancer nests and invasive
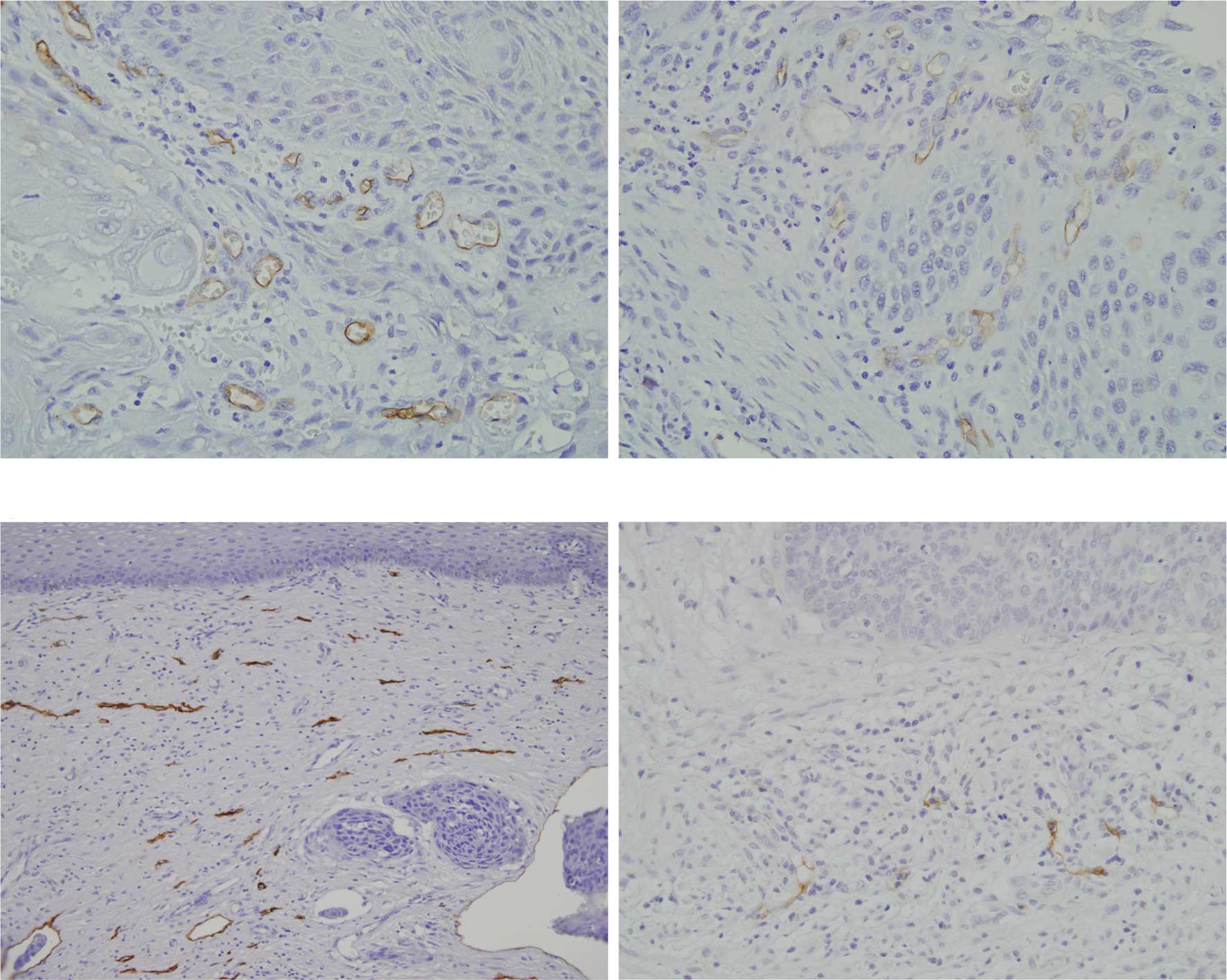
fronts (Fig. 2A–C). Lymphatic
vessels positive to D2-40 were only localized in the superficial
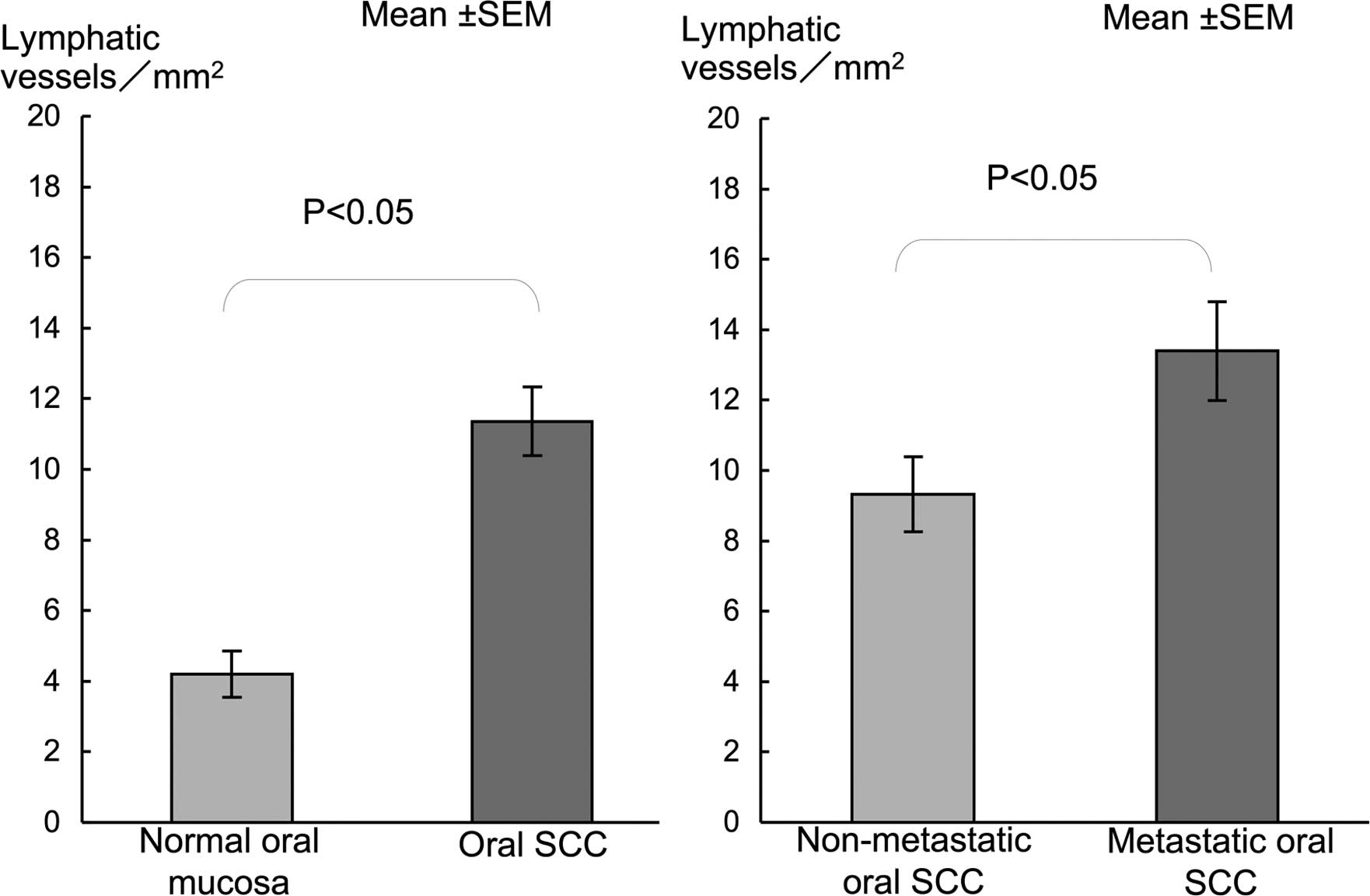
cancer nests (Fig. D). LVD in oral SCC was significantly higher
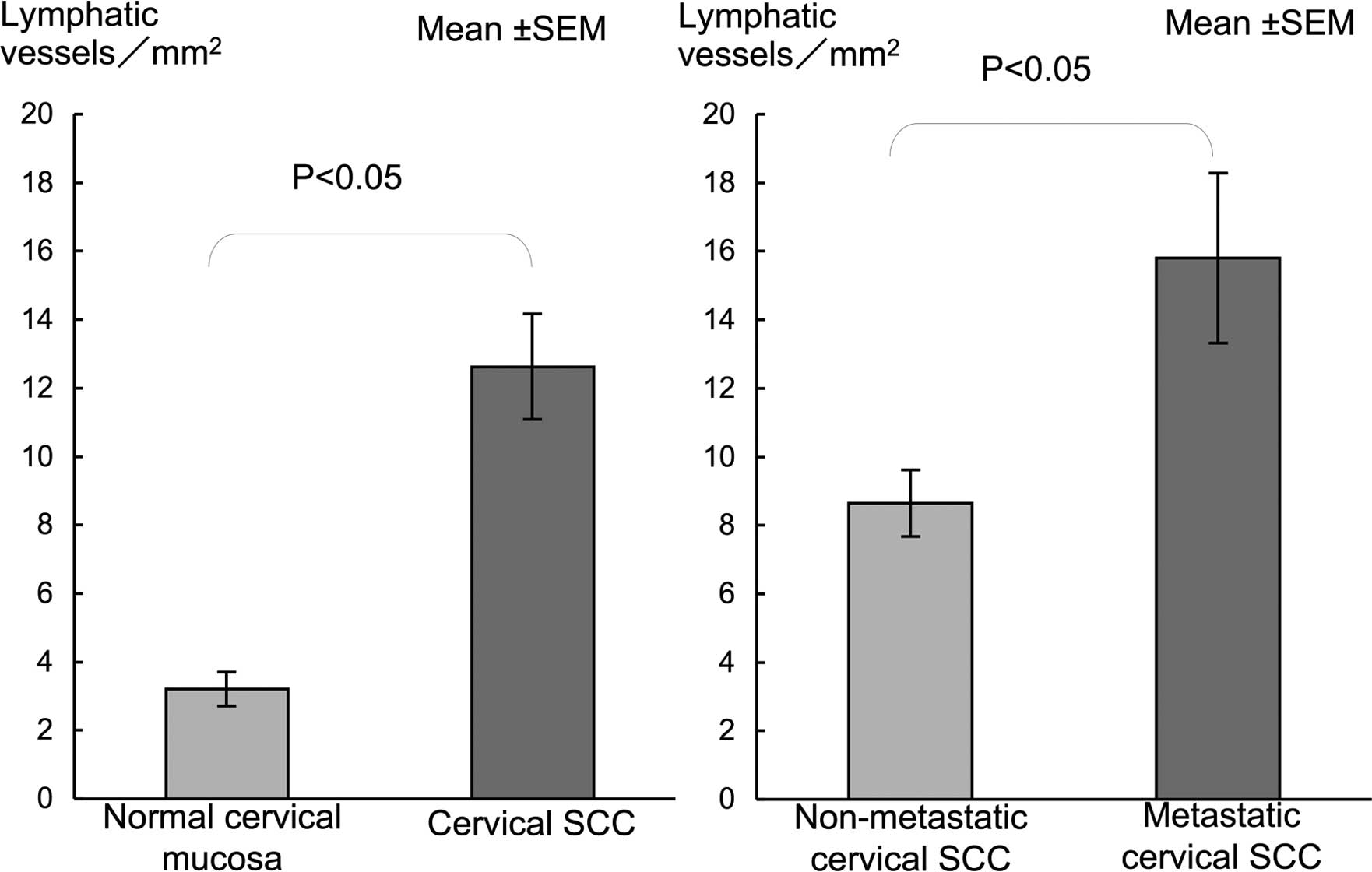
than that in the normal mucosa (Fig.
3). LVD in metastatic SCC was significantly higher compared to
that in the non-metastatic SCC (Fig.
3).
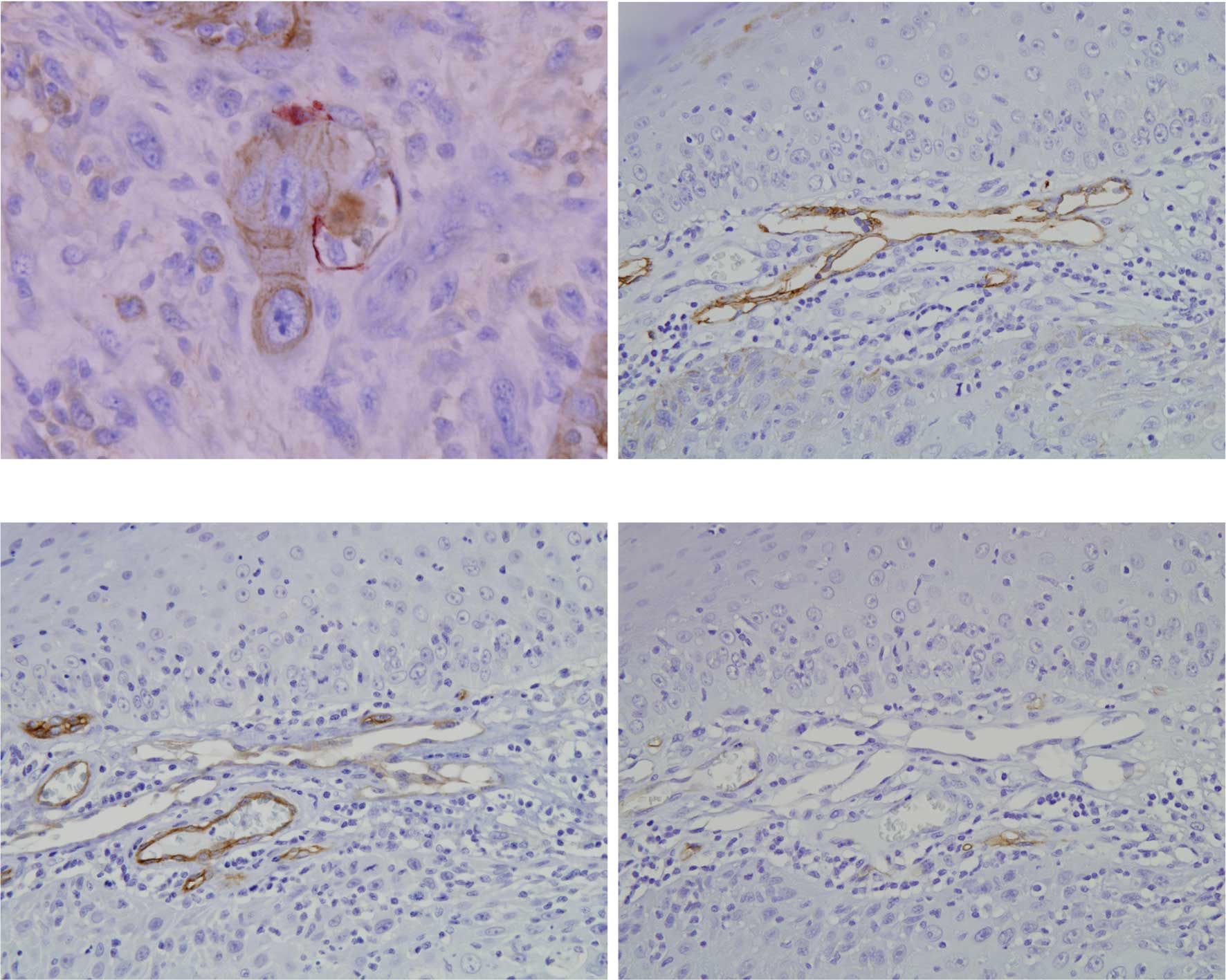
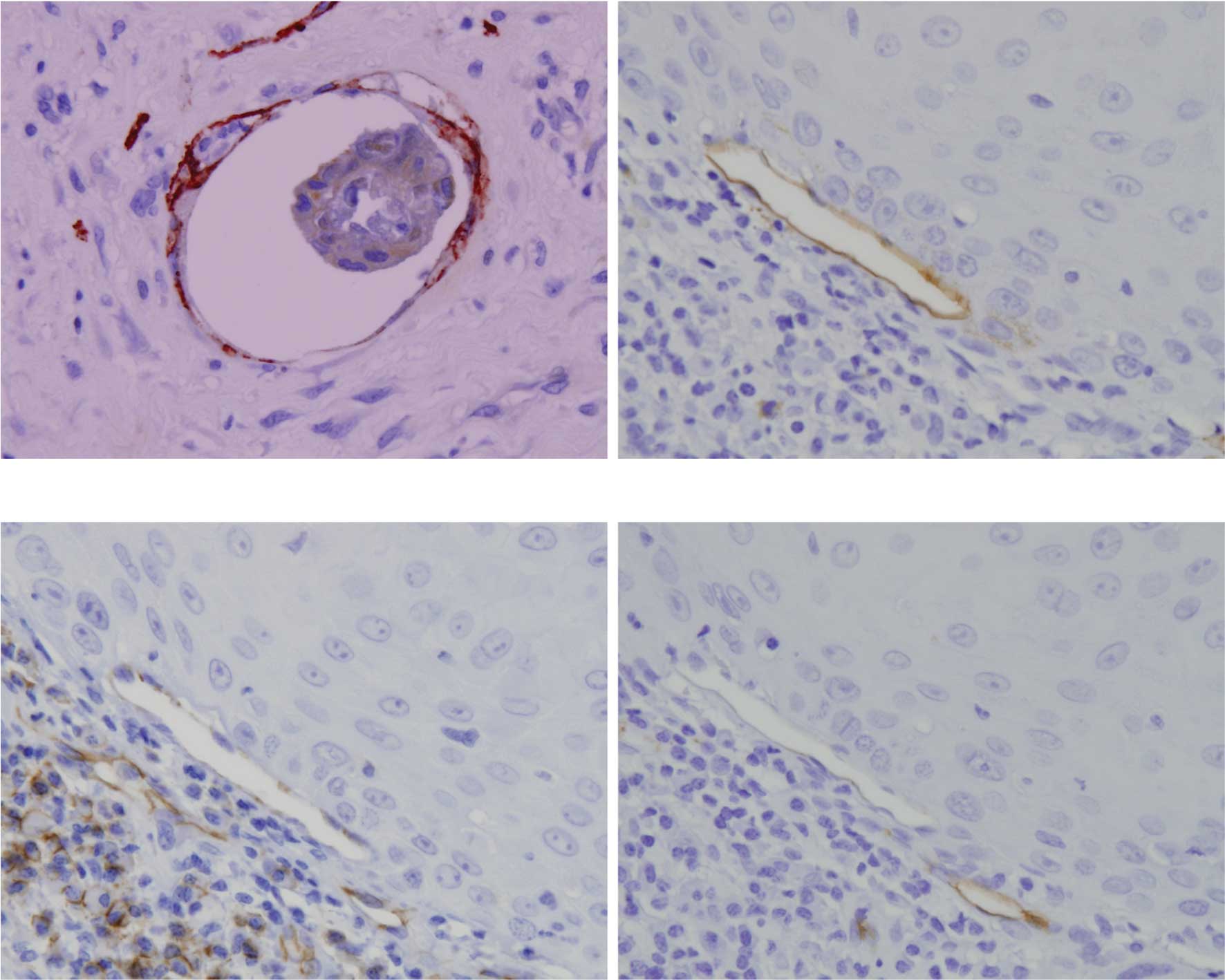
Double staining with D2-40 and keratin showed cancer
cell infiltration in the lymphatic vessels (Fig. 4A) in a few non-metastatic and
metastatic SCC. Cancer cell infiltration in lymphatic vessels was
observed in 3 out of 10 metastatic SCC. Cancer cells were also
observed around blood vessels. Notably, certain lymphatic vessels
surrounding cancer nests were positive to D2-40 (Fig. 4B), CD31 (data not shown) and CD105
(Fig. 4C), but negative to CD34
(Fig. 4D).
Localization and characterization of
lymphatic vessels in cervical SCC
Both non-metastatic and metastatic cervical SCC
formed small nests, which invaded underneath the mucous membrane.
In each serial section, blood vessels positive to CD34 were
observed in the superficial and deep cancer nests showing the same
degree of vascularity (Fig. 5A and
B). Lymphatic vessels positive to D2-40 were localized in the
superficial cancer nests (Fig.
5C). Scanty lymphatic vessels were noted in the central region
and in the proliferating cancer nests (Fig. 5D). LVD in cervical SCC showed a
significantly higher LVD than in normal mucosa (Fig. 6). A significantly higher LVD was
noted in the metastatic compared to the non-metastatic SCC cases
(Fig. 6).
Double staining with D2-40 and keratin in both
non-metastatic and metastatic SCC clearly showed infiltration of
cancer cells in the lymphatic vessels (Fig. 7A). Cancer cell infiltration in
lymph vessels was noted in 5 out of 10 metastatic cervical SCCs,
which was almost the same with oral SCC. On the other hand, no
cancer cells were observed in the blood vessels. Similar to oral
SCC, lymphatic vessels around the cancer nests were positive to
D2-40 (Fig. 7B), CD31 (Fig. 7C) and CD105 (data not shown), but
negative to CD34 (Fig. 7D).
Discussion
Carcinomas preferentially metastasize via the lymph
nodes. Clinical and pathological studies suggest that in many
carcinomas, transport of tumor cells through the lymphatics is a
common pathway of primary dissemination via afferent lymphatic
vessels following the routes of natural drainage (8). The lymphatic system has many
advantages compared to blood circulation thereby making it the
preferred route of metastasizing tumor cells (13). Among these, lymph promotes cell
viability since it is nearly identical to interstitial fluid
without the serum toxins present in the blood. Furthermore,
lymphatic vessels have low shear stress and mechanical deformation
than blood vessels (14,15). In addition, lymphatic vessels have
thin walls lined by a layer of endothelial cells and discontinuous
basement membrane. These features make lymphatic vessels optimally
suited for entry and transport of cells (16). Hence, penetration and survival of
metastasizing tumor cells is highly facilitated via lymphatic
vessels.
In this study, lymphatic vessels expressing D2-40
were located at superficial cancer nests. LVD in the tumors were
significantly higher than that in the normal mucosa. Moreover, LVD
was significantly higher in cases with metastasis to the lymph
nodes compared to the non-metastatic cases. These results suggest
that tumor cells may have induced lymphangiogenesis during the
initial or early stage in order to promote their initial
dissemination. This may be the reason why oral SCC in particular
involves the lymph nodes even at an early stage. As previously
mentioned, lymphatic vessels provide a pathway conducive for the
survival and dissemination of cancer cells.
Characterization of proliferating lymphatic vessels
in SCC has not yet been elucidated. Lymphatic vessels surrounding
cancer nests were positive to D2-40, CD31 and CD105, suggesting
that the proliferating vessels associated with tumors have
different biological characteristics than normal lymphatic vessels.
The lumen of lymphatic capillaries is composed of a single layer of
endothelial cells lining a thin part that overlaps each other and
is known to have a discontinuous basement membrane unlike the
immature capillary. The two theories on lymphangiogenesis include
the centrifugal and centripetal theories (17). In the centrifugal theory, lymph
sacs sprouting from venous endothelial cells occur early in life,
spread to surrounding tissues and organs followed by budding
endothelial cells forming around local lymphatic vessels. In the
centripetal theory, lymphatic vessels originate by the fusion of
flattened mesenchymal spaces into a primitive lymphatic network,
which spreads integrally and then establishes a connection to the
venous system. Both theories do not resolve the fundamental enigma
of whether lymphatic differentiation originates from a primitive
lymph sac from a vein or from a mesenchymal tissue space (18). Recent research has focused on the
relationship between the differentiation of hematopoietic stem
cells and endothelial cells (17).
Lymphangiogenesis associated with tumor invasion is believed to
arise by the growth of existing and budding from main lymphatic
vessels (17). Studies have shown
that lymphatic vessels during the embryonic stage are positive to
CD31, and CD105 expression was noted during angiogenesis. New
lymphatic vessels which proliferate around SCC are thought to
revert back to a less differentiated form believed to have
primitive characteristics of endothelial cells (19,20),
suggesting that lymphangiogenesis in cancer tissue supports the
centrifugal theory.
The present study clarified the localization and
characteristics of lymphatic vessels in oral and cervical SCCs.
Lymphatic vessels in both SCCs were distributed mainly in the
superficial region beneath the epithelium, and LVD was
significantly higher in the SCCs than that in the normal mucosa.
LVD in cases with lymph node metastasis was significantly higher
compared to that in the non-metastatic SCC. Cancer cell
infiltration in the lymphatic vessels was clearly observed
suggesting the existence of lymph node involvement. The new
lymphatic endothelial cells that proliferated around the cancer
nests had primitive endothelial cell characteristics thought to be
associated with early lymphatic development and initial
dissemination of cancer cells.
References
|
1.
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:15–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Abulafia O, Triest WE and Sherer DM:
Angiogenesis in malignancies of the female genital tract. Gynecol
Oncol. 72:220–231. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Nagatsuka H, Hibi K, Gunduz M, et al:
Various immunostaining patterns of CD31, CD34 and endoglin and
their relationship with lymph node metastasis in oral squamous cell
carcinomas. J Oral Pathol Med. 34:70–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 342:1–8. 1991. View Article : Google Scholar
|
|
5.
|
Ranieri G, Labriola A, Achille G, et al:
Microvessel density, mast cell density and thymidine phosphorylase
expression in oral squamous cell carcinoma. Int J Oncol.
21:1317–1323. 2002.PubMed/NCBI
|
|
6.
|
Hannen EJ, van der Laak JA, Manni JJ, et
al: Computer assisted analysis of the microvasculature in
metastasized and nonmetastasized squamous cell carcinomas of the
tongue. Head Neck. 24:643–650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zhang Z, Helman JI and Li LJ:
Lymphangiogenesis, lymphatic endothelial cells and lymphatic
metastasis in head and neck cancer – a review of mechanisms. Int J
Oral Sci. 2:5–14. 2010.
|
|
8.
|
Van Trappen PO and Pepper MS:
Lymphangiogenesis in human gynaecological cancers. Angiogenesis.
8:137–145. 2005.
|
|
9.
|
Agarwal B, Saxena R, Morimiya A, Mehrotra
S and Badve S: Lymphangiogenesis does not occur in breast cancer.
Am J Surg Pathol. 29:1449–1455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Xuan M, Fang YR, Wato M, Hata S and Tanaka
A: Immuno-histochemical co-localization of lymphatics and blood
vessels in oral squamous cell carcinomas. J Oral Pathol Med.
34:334–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Marks A, Sutherland DR, Bailey D, et al:
Characterization and distribution of an oncofetal antigen (M2A
antigen) expressed on testicular germ cell tumours. Br J Cancer.
80:569–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kahn HJ and Marks A: A new monoclonal
antibody, D2-40, for detection of lymphatic invasion in primary
tumors. Lab Invest. 82:1255–1257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Pepper MS, Tille JC, Nisato R and Skobe M:
Lymphangiogenesis and tumor metastasis. Cell Tissue Res.
314:167–177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Weiss L and Schmid-Schönbein GW:
Biomechanical interactions of cancer cells with the
microvasculature during metastasis. Cell Biophys. 14:187–215. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Witte MH, Way DL, Witte CL and Bernas M:
Lymphangiogenesis: mechanisms, significance and clinical
implications. EXS. 79:65–112. 1997.PubMed/NCBI
|
|
17.
|
Kato S: Science of lymphangiogenesis. The
Cell. 37:178–179. 2005.
|
|
18.
|
Kotani M: The lymphatics and
lymphoreticular tissues in relation to the action of sex hormones.
Arch Histol Cytol. 53:1–76. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wang JM, Kumar S, Pye D, van Agthoven AJ,
Krupinski J and Hunter RD: A monoclonal antibody detects
heterogeneity in vascular endothelium of tumours and normal
tissues. Int J Cancer. 54:363–370. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Podgrabinska S, Braun P, Velasco P, Kloos
B, Pepper MS and Skobe M: Molecular characterization of lymphatic
endothelial cells. Proc Natl Acad Sci USA. 99:16069–16074. 2002.
View Article : Google Scholar : PubMed/NCBI
|