Introduction
Despite a major decline in the incidence and
mortality of gastric cancer (GC) over the past several decades, GC
remains the fourth most common cancer and the second leading cause
of cancer-related death in the world (1). The incidence of GC is particularly
high in East Asia, Eastern Europe and parts of Central and South
America (2). Male-to-female
incidence ratios generally are in the 1.5–2.5 range, with higher
ratios for intestinal than diffuse cancers and higher-risk
populations (3). GC is a major
health problem worldwide due to its high incidence, poor prognosis
and limited treatment options (4).
GC is a multifactorial disease with environmental and lifestyle
factors as major contributors (5).
Epidemiological investigations have identified many risk factors
for GC, including age, male gender, Helicobacter pylori
(H. pylori) bacteria infection, smoking, obesity, radiation,
diet and hereditary factors (4–6). The
most important etiological factors implicated in gastric
carcinogenesis are H. pylori infection, and dietary and
hereditary factors (7–9). Individual variations in cancer risk
have been associated with specific variant alleles of different
genes (polymorphisms) that are present in a significant proportion
of the normal population (10).
Polymorphisms in a wide variety of genes may modify the effect of
environmental exposures, and these gene-environmental interactions
could explain the high variation in the GC incidence observed
worldwide (11,12). However, the interaction between
environmental factors and genetic susceptibility has not yet been
adequately addressed.
Tumor angiogenesis is a vital process for the
progression of a neoplasm from a small localized tumor to an
enlarged tumor with the ability to metastasize (13). Vascular endothelial growth factor
(VEGF) as a vascular permeability factor is the major mediator of
physiologic and pathologic angiogenesis (14). Evidence from in vitro and
in vivo experiments has shown that increased VEGF expression
is associated with tumor growth and metastasis, whereas the
inhibition of VEGF signaling results in suppression of both tumor
angiogenesis and tumor growth (15). The VEGF gene is assigned to
chromosome 6p12-p21 and consists of eight exons separated by seven
introns that exhibit alternative splicing to form a family of
proteins (16). In mammals, the
VEGF family consists of seven secreted glycoproteins that are
designated VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, placental growth
factor (PIGF) and VEGF-F (17).
VEGF and its receptors play an important role in the development of
the vascular system, via angiogenesis mechanisms, as well as in the
formation of the lymphatic vascular system (18). In the stomach, GC frequently
displays high levels of VEGF expression which has been correlated
with vascular involvement, and lymph node and liver metastasis
(19,20). Moreover, high levels of VEGF
expression have also been observed in gastric premalignant lesions,
such as chronic atrophic gastritis and intestinal metaplasia,
suggesting that alterations in VEGF expression may also contribute
to the process of gastric carcinogenesis via angiogenesis (21).
Several polymorphisms in the VEGF gene involved in
the development of GC have been described in the literature
(22). Among these, VEGF +936C/T
(rs3025039) in the 3′-untranslated region is one of the most common
polymorphisms. Numerous studies have evaluated the association
between the VEGF +936C/T polymorphism and GC risk. However, the
results have been inconsistent. The aim of this meta-analysis was
to investigate the association of VEGF polymorphisms with GC risk
by conducting a meta-analysis from all eligible case-control
studies published to date.
Materials and methods
Literature search
An electronic search of the Pubmed, Embase and CBM
was performed to retrieve studies linking the VEGF +936C/T
polymorphism and GC risk available by December 2010 without
language restrictions, using the following query: [‘Vascular
Endothelial Growth Factor A’ or ‘Vascular Endothelial Growth Factor
B’ or ‘Vascular Endothelial Growth Factor C’ or ‘Vascular
Endothelial Growth Factor D’ or ‘Vascular Endothelial Growth
Factor, Endocrine-Gland-Derived’ or ‘Vascular Endothelial Growth
Factors’ (Mesh)] and [‘Polymorphism, Single Nucleotide’ or
‘Polymorphism, Restriction Fragment Length’ or ‘Polymorphism,
Single-Stranded Conformational’ or ’Genomic Structural Variation’
or ‘Polymorphism, Genetic’ (Mesh)] and [‘Gastric Cancer’ or
‘Gastric Neoplasm’ or ‘Stomach Neoplasms’ (Mesh)]. The reference
lists of major textbooks, reviews and included articles were
identified through manual searches to find other potentially
eligible studies. Studies reported by the same authors, yet
published in different journals, were checked for possible
overlapping participant groups. When pertinent data were not
included or data that were presented were unclear, the authors were
directly contacted.
Inclusion criteria and exclusion
criteria
To be eligible for inclusion in this meta-analysis,
the following criteria were established: i) case-control studies
that addressed GC cases and healthy or benign disease controls; ii)
studies that evaluated the association between the VEGF +936C/T
polymorphism and GC risk; iii) studies that included sufficient
genotype data for extraction. Studies excluded were: i) not
case-control studies that evaluated the association between the
VEGF +936C/T polymorphism and GC risk; ii) case reports, letters,
reviews and editorial articles; iii) studies based on incomplete
raw data and no usable data reported; iv) studies containing
duplicate data; v) family-based design.
Data extraction
Using a standardized form, data from the published
studies were extracted independently by two reviewers (X.H. Dong
and G.J. Jin) to populate the necessary information. From each of
the included articles, the following information was extracted:
first author, year of publication, country, language, ethnicity,
study design, diagnostic criteria, source of cases and controls,
number of cases and controls, sample, detection methods,
polymorphisms and evidence of Hardy-Weinberg equilibrium (HWE) in
controls. For conflicting evaluations, an agreement was reached
following discussion.
Quality assessment of the included
studies
The quality of the studies was also independently
assessed by two reviewers (X.H. Dong and G.J. Jin) based on the
STROBE quality score systems (23). Thirty items relevant to the quality
appraisal were used for assessment in this meta-analysis, with
scores ranging from 0 to 30. Any discrepancies between the two
reviewers were resolved by discussion and consultation with a third
reviewer (H. Shang).
Statistical analysis
The meta-analysis was carried out using the Review
Manager version 5.0.25 (provided by The Cochrane Collaboration) and
STATA package version 9.2 (Stata Corporation, College Station, TX,
USA). The strength of the associations between the VEGF +936C/T
polymorphism and GC risk was estimated using odds ratio (OR) and
95% confidence interval (95% CI). The following contrasts for the
VEGF +936C/T polymorphism were evaluated: comparison of the variant
allele with ancestral allele (T vs. C); comparison of the variant
homozygote combined with the heterozygote vs. ancestral homozygote
(T/T+C/T vs. C/C); comparison of the variant homozygote combined
with the heterozygote vs. ancestral homozygote (T/T vs. C/C+C/T);
comparison of the variant homozygote vs. ancestral homozygote (T/T
vs. C/C); comparison of the variant homozygote vs. heterozygote
(T/T vs. C/T). Between-study heterogeneities were estimated using
Cochran’s Q test (24,25). We also quantified the effect of
heterogeneity using the I2 test. I2 ranges
between 0 and 100% and represents the proportion of inter-study
variability that can be attributed to heterogeneity rather than
chance. I2 values of 25, 50 and 75% were defined as low,
moderate and high estimates, respectively. When a significant Q
test (P<0.10) or I2<50% indicated heterogeneity
across studies, the random effects model was used for
meta-analysis, or else the fixed effects model was used (26). Before the effect estimation of
associations between the VEGF +936C/T polymorphism and GC risk, we
tested whether genotype frequencies of the controls were in HWE
using the χ2 test. Subgroup analysis based on ethnicity
was used to explore and to explain the diversity among the results
of different studies. Sensitivity analysis was mainly performed by
sequential omission of individual studies. Publication bias was
investigated by Begg’s funnel plot, and funnel plot asymetry was
assessed by Egger’s linear regeression test (27). Statistical significance was
considered when the P-value of Egger’s test was ≤0.10. All P-values
were two-sided. To ensure the reliability and the accuracy of the
results, two reviewers (L.P. Zhou and H. Luan) populated the data
in the statistical software programs independently and obtained the
same results.
Results
Characteristics of the included
studies
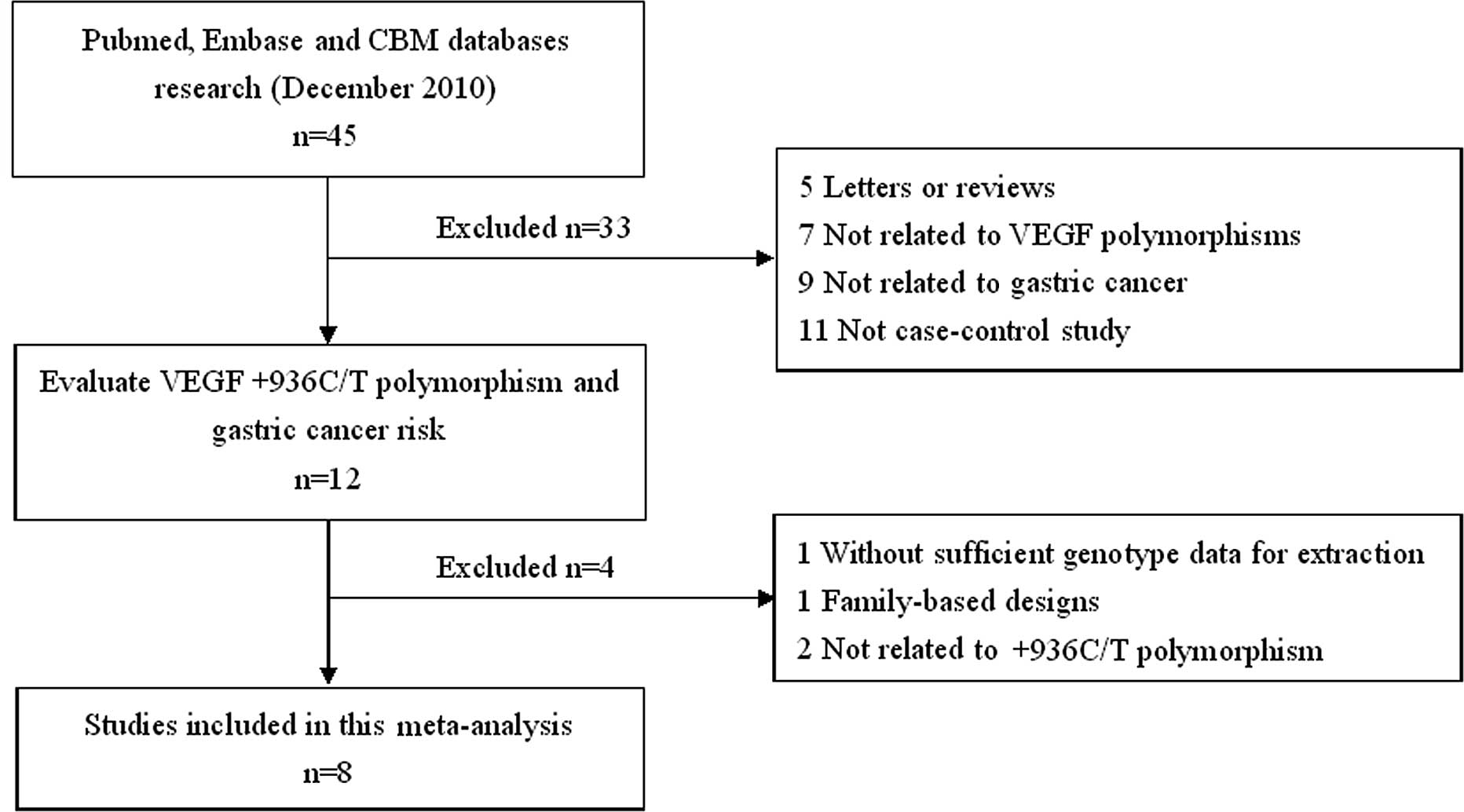
The search strategy retrieved 45 potentially
relevant studies. According to the inclusion criteria, only 8
studies (28–35) with full-text were included in this
meta-analysis and 37 studies were excluded. The flow chart of the
study selection is summarized in Fig.
1. These 8 case-control studies selected included a total of
2,131 GC cases and 2,670 healthy or benign disease controls. All
were case-control studies which evaluated the association between
VEGF +936C/T polymorphism and GC risk. The year of publication of
the included studies ranged from 2006 to 2010. All included
articles were written in English except one in Chinese (35). The source of controls was mainly
based on healthy population. Diverse genotyping methods mainly used
polymerase chain reaction-restriction fragment length polymorphism
(PCR-RFLP). The baseline characteristics and methodological quality
of all included studies are summarized in Table I. The genotype distribution and
risk allele frequency are summarized in Table II.
 | Table I.Baseline characteristics of the
studies included in the meta-analysis. |
Table I.
Baseline characteristics of the
studies included in the meta-analysis.
| First author
(ref.) | Year | Country | Ethnicity | Source of
controls | Source of cases | Detection method | No. of subjects
| Gender (male/female)
| Age, in years
(mean±SD)
|
|---|
| Cases | Controls | Cases | Controls | Cases | Controls |
|---|
| Tzanakis, et
al (28) | 2006 | Greece | Caucasian | Population-based | Hospital-based | PCR-RFLP | 100 | 100 | 58/42 | - | 65.0±12.3 | - |
| Chae, et al
(29) | 2006 | Korea | Asian | Population-based | Hospital-based | PCR-RFLP | 413 | 413 | 313/100 | 333/80 | 60.2±10.3 | 60.2±9.0 |
| Bae, et al
(30) | 2008 | Korea | Asian | Population-based | Hospital-based | PCR-RFLP | 154 | 229 | 91/63 | 112/117 | 58.1±12.7 | 59.6±11.8 |
| Ke, et al
(31) | 2008 | China | Asian | Population-based | Population-based | PCR-RFLP | 540 | 561 | 371/169 | 384/177 | 60.4±9.5 | 59.5±10.2 |
| Al-Moundhri, et
al (32) | 2009 | Oman | Caucasian |
Population-based | Hospital-based | PCR-RFLP | 130 | 130 | 75/55 | - | 56.8±12.2 | 41.3±11.6 |
| Guan, et al
(33) | 2009 | USA | Caucasian |
Population-based | Hospital-based | PCR-RFLP | 171 | 353 | 56/115 | 104/249 | 59.7±12.6 | 57.6±11.2 |
| Tahara, et
al (34) | 2009 | Japan | Asian | Hospital-based | Hospital-based | PCR-RFLP | 385 | 459 | 271/114 | 267/192 | 65.5±11.0 | 61.5±13.0 |
| Xia, et al
(35) | 2010 | China | Asian |
Population-based | Hospital-based | - | 238 | 425 | 191/47 | 336/89 | 61.0±9.4 | 60.6±8.4 |
 | Table II.Genotype distribution and risk allele
frequency of all studies included. |
Table II.
Genotype distribution and risk allele
frequency of all studies included.
| First author
(Ref.) | Cases
| Controls
| HWE test (P-value)
|
|---|
| Total | CC | CT | TT | Total allele | C | T | AF | Total | CC | CT | TT | Total allele | C | T | AF | |
|---|
| Tzanakis, et
al (28) | 100 | 41 | 33 | 26 | 200 | 115 | 85 | 0.425 | 100 | 51 | 27 | 22 | 200 | 129 | 71 | 0.355 | 0.000 |
| Chae, et al
(29) | 413 | 283 | 122 | 8 | 826 | 688 | 138 | 0.167 | 413 | 252 | 149 | 12 | 826 | 653 | 173 | 0.209 | 0.069 |
| Bae, et al
(30) | 154 | 89 | 58 | 7 | 308 | 236 | 72 | 0.234 | 229 | 169 | 57 | 3 | 458 | 395 | 63 | 0.138 | 0.458 |
| Ke, et al
(31) | 540 | 373 | 152 | 15 | 1,080 | 898 | 182 | 0.169 | 561 | 386 | 164 | 11 | 1,122 | 936 | 186 | 0.166 | 0.177 |
| Al-Moundhri, et
al (32) | 130 | 109 | 2 | 19 | 260 | 220 | 40 | 0.154 | 130 | 110 | 0 | 20 | 260 | 220 | 40 | 0.154 | 0.000 |
| Guan, et al
(33) | 171 | 127 | 41 | 3 | 342 | 295 | 47 | 0.137 | 353 | 276 | 70 | 7 | 706 | 622 | 84 | 0.119 | 0.309 |
| Tahara, et
al (34) | 385 | 256 | 118 | 11 | 770 | 630 | 140 | 0.182 | 459 | 300 | 140 | 19 | 918 | 740 | 178 | 0.194 | 0.603 |
| Xia, et al
(35) | 238 | 155 | 63 | 10 | 476 | 373 | 83 | 0.174 | 425 | 276 | 131 | 6 | 850 | 683 | 143 | 0.168 | 0.021 |
Main meta-analysis results
A summary of the meta-analysis findings of the
association between the VEGF +936C/T polymorphism and GC risk is
provided in Table III.
Meta-analysis results identified no significant association between
the VEGF +936C/T polymorphism and GC risk in all comparisons of T
allele vs. C allele (OR=1.08, 95% CI 0.90–1.30, P=0.42), CT+TT vs.
CC (OR=1.08, 95% CI 0.87–1.34, P=0.49), TT vs. CC+CT (OR=1.14, 95%
CI 0.85–1.53, P=0.37), TT vs. CC (OR=1.18, 95% CI 0.87–1.59,
P=0.28) and TT vs. CT (OR=1.11, 95% CI 0.79–1.56, P=0.56). In the
subgroup analysis based on ethnicity, the subjects of all included
studies were divided into Caucasian and Asian populations. Results
of subgroup analysis confirmed that there was also no association
between the VEGF +936C/T polymorphism and GC risk in both Caucasian
and Asian populations. Sensitivity analysis was performed by
sequential omission of individual studies. The significance of the
pooled OR in all individual analyses and subgroup analyses was not
influenced excessively by omitting any single study. In addition,
we also performed sensitivity analysis by omission of studies
without HWE, but the results were also not influenced.
 | Table III.Meta-analysis of the association
between the VEGF +936C/T polymorphism and gastric cancer risk. |
Table III.
Meta-analysis of the association
between the VEGF +936C/T polymorphism and gastric cancer risk.
| Comparisons | OR | 95% CI | P-value | Heterogeneity
| Effects model |
|---|
| I2 | P-value |
|---|
| T vs. C | 1.08 | 0.90–1.30 | 0.42 | 64% | 0.007 | Random |
| Caucasian | 1.18 | 0.93–1.51 | 0.17 | 0% | 0.650 | |
| Asian | 1.05 | 0.82–1.34 | 0.72 | 76% | 0.002 | |
| T/T+C/T vs.
C/C | 1.08 | 0.87–1.34 | 0.49 | 63% | 0.008 | Random |
| Caucasian | 1.27 | 0.94–1.72 | 0.12 | 0% | 0.730 | |
| Asian | 1.02 | 0.77–1.35 | 0.88 | 75% | 0.003 | |
| T/T vs.
C/C+C/T | 1.14 | 0.85–1.53 | 0.37 | 32% | 0.170 | Fixed |
| Caucasian | 1.07 | 0.68–1.66 | 0.78 | 0% | 0.810 | |
| Asian | 1.21 | 0.82–1.79 | 0.34 | 59% | 0.050 | |
| T/T vs. C/C | 1.18 | 0.87–1.59 | 0.28 | 41% | 0.100 | Fixed |
| Caucasian | 1.15 | 0.73–1.82 | 0.55 | 0% | 0.660 | |
| Asian | 1.20 | 0.81–1.77 | 0.37 | 64% | 0.030 | |
| T/T vs. C/T | 1.11 | 0.79–1.56 | 0.56 | 27% | 0.220 | Fixed |
| Caucasian | 0.82 | 0.43–1.57 | 0.55 | 0% | 0.590 | |
| Asian | 1.25 | 0.83–1.87 | 0.28 | 47% | 0.110 | |
Publication bias
Publication bias of the literature was assessed
using the Begg’s funnel plot and Egger’s linear regression test.
Egger’s linear regression test was used to measure the asymmetry of
the funnel plot. The results of Egger’s linear regression test are
shown in Table IV. Results showed
that there was no publication bias (all P>0.05).
 | Table IV.Evaluation of publication bias by
Egger’s linear regression test. |
Table IV.
Evaluation of publication bias by
Egger’s linear regression test.
| Comparison | Coefficient | Standard error | t | P> |t| | 95% CI |
|---|
| T vs. C | 3.69 | 2.07 | 1.78 | 0.13 | −1.38–8.76 |
| T/T+C/T vs.
C/C | 3.12 | 1.77 | 1.76 | 0.13 | −1.22–7.46 |
| T/T vs.
C/C+C/T | 1.87 | 1.71 | 1.09 | 0.32 | −2.32–6.06 |
| T/T vs. C/C | 2.11 | 1.91 | 1.11 | 0.31 | −2.57–6.80 |
| T/T vs. C/T | −0.21 | 1.37 | −0.16 | 0.88 | −3.58–3.15 |
Discussion
Evidence suggests that VEGF plays an important role
in the carcinogenesis pathway, such as in the inhibition of
apoptosis, tumor growth, angiogenesis, invasion and metastasis
(36). The specific function of
VEGF in the formation of prostaglandins makes it a strong candidate
for increasing the susceptibility to common cancers, such as GC,
colorectal, lung, breast, prostate cancer and other solid tumors
(37–40). As is known, genetic polymorphisms
altering the level of protein expressed are anticipated to have a
substantial influence on disease activity (41). Several polymorphisms in VEGF have
been previously reported, although some of these polymorphisms are
not functionally significant and not associated with a
susceptibility to GC.
Our meta-analysis quantitatively assessed the
association between the VEGF +936C/T polymorphism and GC risk.
Finally, 8 case-control studies were included and comprised a total
of 2,131 GC cases and 2,670 healthy or benign disease controls. The
main meta-analysis results showed that there was no association
between the VEGF +936C/T polymorphism and GC risk in all
comparisons of T allele vs. C allele, CT+TT vs. CC, TT vs. CC+CT,
TT vs. CC and TT vs. CT, suggesting that the VEGF +936C/T
polymorphism is not a risk factor for GC. Similarly, in the
subgroup analysis by ethnicity, no association was found between
the VEGF +936C/T polymorphism and GC risk in all comparisons in
both Caucasian and Asian populations. Between-study heterogeneity
was found in the comparisons of the T allele vs. C allele and CT+TT
vs. CC; the random effects model was used. No heterogeneity was
found in other comparisons; the fixed effects model was used. No
evidence of publication bias was noted in this meta-analysis for
the VEGF +936C/T polymorphism.
There were some limitations in our meta-analysis.
Firstly, due to incomplete raw data or publication limitations,
several relevant studies could not be included. Secondly, we were
not able to address the sources of heterogeneity existing among
studies for most polymorphisms. However, we could not perform
subgroup stratifications analysis for the limited number of
published studies. Thirdly, the lack of genotype frequency
information provided by some published studies did not allow the
estimation of the best genetic model of inheritance to follow.
Although we actively contacted the authors, they did not provide a
comprehensive set of data. In addition, the small sample size
available was not ideal for detecting small genetic effects.
Finally, our systematic review was based on unadjusted data, as the
genotype information stratified for the main confounding variables
was not available in the original studies and also the confounding
factors addressed across the different studies were variable.
In conclusion, our meta-analysis of 8 case-control
studies demonstrated that there was lack of association between the
VEGF +936C/T polymorphism and GC risk. Therefore, the necessity to
conduct large studies with an adequate methodological quality,
properly controlling for possible confounds in order to obtain
valid results should be emphasized.
Acknowledgements
This study was supported by a grant
from the Science and Technology Research Project of the Higher
Education Department of Liaoning Province (no. L2010695).
References
|
1.
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar
|
|
2.
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.
|
|
3.
|
Roder DM: The epidemiology of gastric
cancer. Gastric Cancer. 5(Suppl 1): 5–11. 2002. View Article : Google Scholar
|
|
4.
|
Compare D, Rocco A and Nardone G: Risk
factors in gastric cancer. Eur Rev Med Pharmacol Sci. 14:302–308.
2010.
|
|
5.
|
Krejs GJ: Gastric cancer: epidemiology and
risk factors. Dig Dis. 28:600–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
7.
|
Uemura N, Okamoto S, Yamamoto S, et al:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar
|
|
8.
|
Liu C and Russell RM: Nutrition and
gastric cancer risk: an update. Nutr Rev. 66:237–249. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Oliveira C, Seruca R and Carneiro F:
Hereditary gastric cancer. Best Prac Res Clin Gastroenterol.
23:147–157. 2009. View Article : Google Scholar
|
|
10.
|
González CA, Sala N and Capellá G: Genetic
susceptibility and gastric cancer risk. Int J Cancer. 100:249–260.
2002.
|
|
11.
|
Perera FP and Weinstein IB: Molecular
epidemiology: recent advances and future directions.
Carcinogenesis. 21:517–524. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ames BN: Cancer prevention and diet: help
from single nucleotide polymorphisms. Proc Natl Acad Sci USA.
96:12216–12218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
McMahon G: VEGF receptor signaling in
tumor angiogenesis. Oncologist. 5(Suppl 1): 3–10. 2000. View Article : Google Scholar
|
|
14.
|
Gaur P, Bose D, Samuel S and Ellis LM:
Targeting tumor angiogenesis. Semin Oncol. 36(Suppl 1): 12–19.
2009. View Article : Google Scholar
|
|
15.
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Vincenti V, Cassano C, Rocchi M and
Persico G: Assignment of the vascular endothelial growth factor
gene to human chromosome 6p21.3. Circulation. 93:1493–1495. 1996.
View Article : Google Scholar
|
|
17.
|
Otrock ZK, Makarem JA and Shamseddine AI:
Vascular endothelial growth factor family of ligands and receptors:
review. Blood Cells Mol Dis. 38:258–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zhang Z, Neiva KG, Lingen MW, et al:
VEGF-dependent tumor angiogenesis requires inverse and reciprocal
regulation of VEGFR1 and VEGFR2. Cell Death Differ. 17:499–512.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ichikura T, Tomimatsu S, Ohkura E, et al:
Prognostic significance of the expression of vascular endothelial
growth factor (VEGF) and VEGF-C in gastric carcinoma. J Surg Oncol.
78:132–137. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lieto E, Ferraraccio F, Orditura M, et al:
Expression of vascular endothelial growth factor (VEGF) and
epidermal growth factor receptor (EGFR) is an independent
prognostic indicator of worse outcome in gastric cancer patients.
Ann Surg Oncol. 15:69–79. 2008. View Article : Google Scholar
|
|
21.
|
Smith MG, Hold GL, Tahara E, et al:
Cellular and molecular aspects of gastric cancer. World J
Gastroenterol. 12:2979–2990. 2006.
|
|
22.
|
Watson CJ, Webb NJ, Bottomley MJ, et al:
Identification of polymorphisms within the vascular endothelial
growth factor (VEGF) gene: correlation with variation in VEGF
protein production. Cytokine. 12:1232–1235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Vandenbroucke JP, von Elm E, Altman DG, et
al: Strengthening the reporting of observational studies in
epidemiology (STROBE): explanation and elaboration. Epidemiology.
18:805–835. 2007. View Article : Google Scholar
|
|
24.
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in meta-analysis of genome searches. Genet
Epidemiol. 28:123–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1158. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Viechtbauer W: Confidence intervals for
the amount of heterogeneity in meta-analysis. Stat Med. 26:37–52.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Peters JL, Sutton AJ, Jones DR, et al:
Comparison of two methods to detect publication bias in
meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tzanakis N, Gazouli M, Rallis G, et al:
Vascular endothelial growth factor polymorphisms in gastric cancer
development, prognosis, and survival. J Surg Oncol. 94:624–630.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chae YS, Kim JG, Sohn SK, et al:
Investigation of vascular endothelial growth factor gene
polymorphisms and its association with clinicopathologic
characteristics in gastric cancer. Oncology. 71:266–272. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Bae SJ, Ahn DH, Hong SP, et al:
Gender-specific association between polymorphism of vascular
endothelial growth factor (VEGF 936C>T) gene and patients with
stomach cancer. Yonsei Med J. 49:783–791. 2008.
|
|
31.
|
Ke Q, Liang J, Wang LN, et al: Potentially
functional polymorphisms of the vascular endothelial growth factor
gene and risk of gastric cancer. Mol Carcinog. 47:647–651. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Al-Moundhri MS, Al-Nabhani M, Burney IA,
et al: Gastric cancer risk predisposition and prognostic
significance of vascular endothelial growth factor (VEGF) gene
polymorphisms: a case-control study in an Omani population. Mol
Carcinog. 48:1170–1176. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Guan X, Zhao H, Niu J, et al: The VEGF
-634G>C promoter polymorphism is associated with risk of gastric
cancer. BMC Gastroenterol. 9:772009.
|
|
34.
|
Tahara T, Shibata T, Nakamura M, et al:
Effect of polymorphisms in the 3′ untranslated region (3′-UTR) of
vascular endothelial growth factor gene on gastric cancer and
peptic ulcer diseases in Japan. Mol Carcinog. 48:1030–1037.
2009.
|
|
35.
|
Xia HZ, Wu Q, Liu Y, et al: Association
ofVEGF rs3025039 and rs3025021 with VEGF and COX-2 expression in
gastric cancer tissues. J Clin Exp Pathol. 26:139–143. 2010.
|
|
36.
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Uthoff SM, Duchrow M, Schmidt MH, et al:
VEGF isoforms and mutations in human colorectal cancer. Int J
Cancer. 101:32–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Yuan A, Yu CJ, Chen WJ, et al: Correlation
of total VEGF mRNA and protein expression with histologic type,
tumor angiogenesis, patient survival and timing of relapse in
non-small-cell lung cancer. Int J Cancer. 89:475–483. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Krippl P, Langsenlehner U, Renner W, et
al: A common 936 C/T gene polymorphism of vascular endothelial
growth factor is associated with decreased breast cancer risk. Int
J Cancer. 106:468–471. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Lin CC, Wu HC, Tsai FJ, et al: Vascular
endothelial growth factor gene -460 C/T polymorphism is a biomarker
for prostate cancer. Urology. 62:374–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Taylor JG, Choi EH, Foster CB, et al:
Using genetic variation to study human disease. Trends Mol Med.
7:507–512. 2001. View Article : Google Scholar : PubMed/NCBI
|