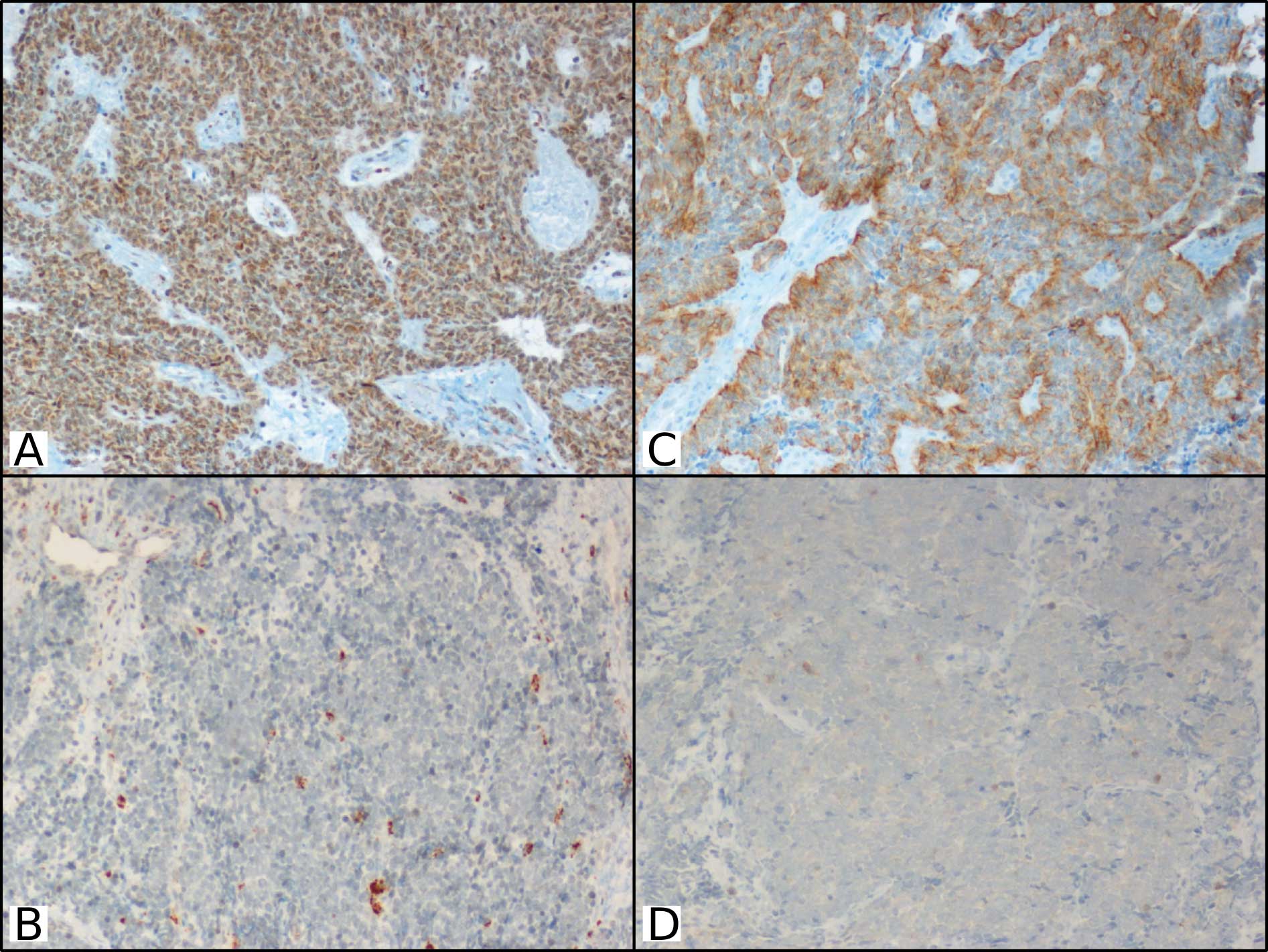
Introduction
Bronchopulmonary neuroendocrine tumors (BP-NETs)
comprise approximately 20% of all lung cancers and represent a
distinct spectrum of tumors arising from the neuroendocrine cells
of the BP-epithelium that share ultrastructural, morphologic and
immunohistochemical characteristics (1). BP-NETs are separated into four
subgroups of increasing aggressiveness: low-grade typical carcinoid
(TC) with <2 mitoses/2 mm2 and lacking necrosis;
intermediate-grade atypical carcinoid (AC) with 2–10 mitoses/2
mm2 and/or foci of necrosis; high-grade small-cell lung
carcinoma (SCLC) and high-grade large-cell neuroendocrine carcinoma
(LCNEC), characterized by abundant mitotic activity with >11
mitoses/2 mm2 and prominent necrosis (1,2).
Neuroendocrine tumorlets (NTs) display the same architectural and
cytologic features as TCs; however, NTs are microscopic in size: an
arbitrary size of 4 mm or less has been suggested as the
classification of NTs, and they are often associated with chronic
lung disease (3,4).
Despite common classification, these neuroendocrine
tumors differ regarding the natural course of disease and treatment
strategies. Although TCs are generally regarded as low-grade
carcinomas, approximately 10–23% of cases metastasize to the
regional lymph nodes at presentation, with 5-year overall survival
rates ranging from 82 to 100% (5,6). By
contrast, 40–50% of ACs metastasize to the regional lymph nodes at
presentation, with 5-year overall survival rates ranging from 25 to
78% (2,7). The highly malignant LCNECs and SCLCs
are generally widespread at diagnosis with a poor overall
prognosis, despite aggressive treatment with extensive surgical
resection, multi-agent chemotherapy and radiotherapy (8).
The molecular profile of BP-NETs has been
extensively investigated with the aim of identifying features for
diagnosis, prognosis and even therapy for this particular category
of lung tumors. The gradual increase of certain molecular
abnormalities along the spectrum of neuroendocrine lung tumors
strongly supports the grading concept of typical carcinoid as low
grade, atypical carcinoid as intermediate grade and large cell
neuroendocrine and SCLCs as high-grade neuroendocrine tumors.
However, while BP-NETs share certain molecular abnormalities,
several differences have been observed. For example, SCLCs and
LCNECs display high rates of p53 mutations, while TCs and ACs are
characterized by mutations in the menin gene (1,9,10).
The mammalian target of rapamycin (mTOR) is a
serine/ threonine kinase that is ubiquitously expressed in
mammalian cells (11). mTOR is
activated downstream of multiple distinct growth factor receptors
that have been implicated in lung cancer biology. Additionally, it
is of crucial importance in the regulation of cell growth,
proliferation and survival (12–14).
mTOR acts as a point of convergence of several different signaling
pathways, including the phosphatidylinositol 3-kinase (PI3K)/AKT
pathway, which responds to growth factors and nutritional status.
Both AKT and mTOR are activated through phosphorylation of specific
amino acid residues (15). The
AKT/mTOR signaling pathway is aberrantly activated in many tumor
types (13,14,16,17).
In particular, this pathway seems to play a role in tumor cell
growth and proliferation in human pulmonary carcinoid cells
(18,19), as well in small-cell lung cancer
cells (12,20). Because of these functions, mTOR has
been considered an attractive target for anticancer agents. In
vitro studies have established the potential of rapamycin to
inhibit cellular transformation, and several types of tumors that
exhibit the activation of the AKT/mTOR pathway, including SCLCs,
are hypersensitive to rapamycin in vitro (12,21).
However, overexpression or activation of AKT and
mTOR in the spectrum of BP-NETs and/or their associations with
clinicopathological characteristics remain unclear. Therefore, the
present study aimed to examine the expression of the phosphorylated
forms of AKT and mTOR in a large series of neuroendocrine lung
lesions, including tumorlets, TCs, ACs, LCNECs and surgically
resected SCLCs. Additionally, the direct correlations between the
expression of these genes and clinicopathological parameters were
investigated.
Materials and methods
Patients and lung tissue specimens
Neuroendocrine lung tumor specimens (n=210) were
obtained from patients (116 males and 94 females) who consecutively
underwent surgical resection at the Department of Cardio-Thoracic
Surgery of the University of Pisa from January 2000 to July 2009.
No patient had received chemotherapy or radiotherapy prior to
surgery. All material from the neuroendocrine lesions was obtained
from the primary lung tumors. No cytological or bioptical material
was included in the present study. Clinical information, including
patient gender, age, tumor size and lymph node metastasis, was
reviewed for each patient with SCLCs, LCNECs, TCs and ACs.
All tumor samples were formalin-fixed and
paraffin-embedded for microscopic examination. The most
representative paraffin block for each tumor was selected for
analysis. Histological diagnosis and pathological features were
reviewed by two pathologists (G. Alì and G. Fontanini) according to
the WHO 2004 histologic criteria (1). Neuroendocrine differentiation was
detected by positive immunohistochemical staining for chromogranin
A, synaptophysin or CD56.
Disagreements concerning histologic diagnosis were
discussed, and following which a mutual agreement was reached.
Pathological staging was performed according to the TNM
classification (22).
Immunohistochemistry
Immunohistochemical analyses were performed on 3-μm
tissue sections using specific antibodies. Immunoreaction was
displayed using the avidin-biotin-peroxidase complex (ABC) method.
Peroxidase activity was visualized with diaminobenzidine.
Counterstaining was performed with hematoxylin. Immunostaining was
performed using a Benchmark immunostainer (Ventana, Tucson, AZ,
USA). In all cases, the immunohistochemical evaluations were
independently performed by two pathologists (G. Alì and G.
Fontanini) who were blinded to the clinicopathological
characteristics of the patients. In all discordant cases, mutual
agreement was reached.
For immunohistochemical staining, sections were
incubated with a rabbit anti-human phospho-AKT (Ser473) polyclonal
antibody (Abcam, Cambridge, UK) at a dilution of 1:50 and with a
rabbit anti-human p-mTOR (Ser2448) (clone 49F9; Cell Signaling
Technology, Inc., Danvers, MA, USA) antibody used at a dilution of
1:100.
Normal bronchial epithelial cells were used as
internal positive controls for p-AKT and p-mTOR staining. Negative
controls were conducted by omitting the primary antibodies.
p-AKT expression was assessedd in both the cytoplasm
and the nucleus of the neuroendocrine tumors. p-mTOR expression was
assessed in both the cytoplasm and plasmatic membrane.
Immunohistochemical expression of both p-AKT and
p-mTOR was evaluated as the percentage of tumor cells displaying
immunoreactivity. At least 1,000 cancer cells (100 cells in 10
HPFs) were counted for each section (Fig. 1). The median value of p-AKT and
p-mTOR for each type of BP-NET was used as a cutoff value to
distinguish tumors with low p-AKT and p-mTOR expression levels from
those with high expression levels. The staining intensity was
analyzed by distinguishing four categories: negative (0), weak
staining (+), intermediate staining (++) and strong staining
(+++).
Statistical analysis
All statistical analyses were conducted using
Statistica software. A Chi-square test was used to analyze the
associations between the different variables. The a priori
level of significance was set at a p-value of <0.05.
Results
Clinicopathological characteristics
BP-NETs were reevaluated and reclassified according
to the WHO classification of tumors 2004 criteria (1). The most common histological type was
SCLC (40.5%; 85 cases), followed by TC (35.7%; 75 cases), AC
(12.4%, 26 cases), LCNEC (8.1%; 17 cases) and tumorlet (3.3%, 7
cases). The TNM classification was applied to carcinoids, SCLCs and
LCNECs (22). Other
histopathological and clinical characteristics of the patients are
summarized in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Clinicopathological
characteristics | Tumorlet n=7 | TC n=75 | AC n=26 | LCNEC n=17 | SCLC n=85 |
|---|
| Age, years | | | | | |
| Median
(range) | 68 (53–77) | 61 (24–82) | 65 (23–82) | 67.5 (45–84) | 68 (45–83) |
| Gender | | | | | |
| Male | 3 (42.9%) | 32 (42.7%) | 6 (23.1%) | 13 (76.5%) | 62 (72.9%) |
| Female | 4 (57.1%) | 43 (57.3%) | 20 (76.9%) | 4 (23.5%) | 23 (27.1%) |
| Tumor size (T) | | n=74 | n=26 | n=16 | n=70 |
| T1 (T1a-T1b) | | 50 (67.6%) | 11 (42.3%) | 4 (25.0%) | 21 (30.0%) |
| T2 (T2a-T2b) | | 19 (25.7%) | 12 (46.2%) | 7 (43.8%) | 33 (47.1%) |
| T3–T4 | | 5 (6.7%) | 3 (11.5%) | 5 (31.2%) | 16 (22.9%) |
| Lymph node
metastasis (N) | | n=65 | n=22 | n=13 | n=58 |
| Absent, N0 | | 62 (95.4%) | 15 (68.2%) | 11 (84.6%) | 34 (58.6%) |
| Present,
N1+N2 | | 3 (4.6%) | 7 (31.8%) | 2 (15.4%) | 24 (41.4%) |
p-AKT expression according to
histology
p-AKT expression was analyzed as a dichotomous
variable in all tumor samples, using the median value as the cutoff
to distinguish tumors with low expression from tumors with high
expression. High p-AKT expression was observed in 6 cases (85.7%)
of tumorlets, whereas only 1 case (14.3%) displayed low expression
(median 80; range 20–90). Regarding staining intensity, 3 tumorlets
exhibited weak immunoreactivity, 2 exhibited intermediate staining
and 2 displayed strong staining (Table
II). p-AKT expression was high in 45 cases of TCs (60%) (median
60; range 0–90). The staining intensity was weak in 34 cases,
intermediate in 23 cases and strong in 10 cases (Table II). p-AKT expression was also high
in the majority of ACs. In fact, 73.1% (19/26) of the cases
displayed high p-AKT expression (median 70; range 0–90). In terms
of staining intensity, 9 cases exhibited weak staining, 11
exhibited intermediate staining and 3 exhibited strong staining
(Table II).
 | Table II.p-AKT and p-mTOR expression in tumor
tissue. |
Table II.
p-AKT and p-mTOR expression in tumor
tissue.
| Tumorlet n=7 | TC n=75 | AC n=26 | LCNEC n=17 | SCLC n=85 |
|---|
| p-AKT expression
(p-AKT %) | | | | | |
| Low (<
median) | 1 (<80) | 30 (<60) | 7 (<70) | 13 (<10) | 54 (<40) |
| High (≥
median) | 6 (≥80) | 45 (≥60) | 19 (≥70) | 4 (≥10) | 31 (≥40) |
| p-AKT (p-AKT
+) | | | | | |
| 0 | 0 | 8 | 3 | 3 | 13 |
| 1 | 3 | 34 | 9 | 11 | 42 |
| 2 | 2 | 23 | 11 | 3 | 19 |
| 3 | 2 | 10 | 3 | 0 | 11 |
| p-mTOR expression
(p-mTOR %) | | | | | |
| Low (<
median) | 2 (<50) | 27 (<25) | 10 (<30) | 11 (<10) | 59 (<5) |
| High (≥
median) | 5 (≥50) | 48 (≥25) | 16 (≥30) | 6 (≥10) | 26 (≥5) |
| p-mTOR (p-mTOR
+) | | | | | |
| 0 | 1 | 19 | 6 | 6 | 36 |
| 1 | 2 | 27 | 12 | 9 | 35 |
| 2 | 4 | 26 | 5 | 1 | 13 |
| 3 | 0 | 3 | 3 | 1 | 1 |
Conversely, in high-grade tumors (SCLCs and LCNECs),
lower p-AKT expression was observed compared to low-grade lesions,
such as ACs, TCs and tumorlets (p=0.0001) (Table III). Low p-AKT expression was
observed in 54 of the 85 cases (63.5%) of SCLCs (median 40; range
0–90) and in 13 out of 17 cases (76.5%) of LCNECs (median 10; range
0–90). The staining intensity in SCLCs was weak in 42 cases,
intermediate in 19 cases and strong in 11 cases, whereas the
intensity in LCNECs was weak in 11 cases and intermediate in 3
cases (Table II).
 | Table III.Correlations between p-AKT and p-mTOR
expression and histology. |
Table III.
Correlations between p-AKT and p-mTOR
expression and histology.
| Lesion type | p-AKT expression
(no. of patients)
| p-mTOR expression
(no. of patients)
|
|---|
| Low | High | p-value | Low | High | p-value |
|---|
| Tumorlet | 1 | 6 | 0.0001a | 2 | 5 | 0.0002b |
| TC | 30 | 45 | | 27 | 48 | |
| AC | 7 | 19 | | 10 | 16 | |
| LCNEC | 13 | 4 | | 11 | 6 | |
| SCLC | 54 | 31 | | 59 | 26 | |
p-mTOR expression according to
histology
p-mTOR expression was also analyzed as a dichotomous
variable, using the median value as a cutoff to distinguish two
categories: tumors with high m-TOR expression and those with low or
no m-TOR expression. High p-mTOR expression was observed in the
majority of well-differentiated tumors: 71.4% (5/7) of tumorlets
(median 50; range 0–90), 64% (48/75) of TCs (median 25; range 0–90)
and 61.5% (16/26) of ACs (median 30; range 0–80) (Table II). Regarding staining intensity,
weak intensity was observed in 1 case of tumorlets, 27 cases of TC
and 12 cases of AC, whereas intermediate intensity was observed in
4 cases of tumorlets, 26 cases of TC and 5 cases of AC. Strong
intensity was noted in 3 cases of TC and 3 of AC (Table II).
By contrast, p-mTOR expression was significantly
lower in high-grade BP-NETs, such as LCNECs and SCLCs (p=0.0002)
(Table III). High p-mTOR
expression was observed in only 35.3% (6/17) of the LCNECs (median
10; range 0–70) and in 30.6% (26/85) of the SCLCs (median 5; range
0–90) (Table II). Immunoreactivity
in LCNECs was weak in 9 cases, intermediate in 1 case and strong in
1 case. In SCLCs, staining was weak in 35 cases, intermediate in 13
cases and strong in only 1 case (Table
II).
Associations between p-AKT and p-mTOR
expression and clinicopathological characteristics in BP-NETs
No significant association was found between p-AKT
expression and other clinicopathological parameters, including age,
gender, tumor size and lymph node status, in the BP-NETs (data not
shown).
A significant association was observed between
p-mTOR expression and tumor size (T) in high-grade tumors, SCLCs
(p=0.04) and LCNECs (p=0.03); patients with T3–T4 tumors exhibited
significantly lower levels of p-mTOR expression compared to those
with T1 (T1a-T1b) or T2 (T2a-T2b) tumors (Table IV). Conversely, no significant
association between p-mTOR expression and tumor size was observed
in low- and intermediate-grade tumors, such as TCs and ACs.
Additionally, no association was identified between m-TOR
expression and any other clinicopathological characteristic (age,
gender, lymph node status) in the spectrum of BP-NETs (Table IV).
 | Table IV.Association of p-mTOR expression with
clinicopathological parameters. |
Table IV.
Association of p-mTOR expression with
clinicopathological parameters.
| Clinicopathological
characteristics | Tumorlet
(p-value) | TC (p-value) | AC (p-value) | LCNEC
(p-value) | SCLC (p-value) |
|---|
| Age | 0.32 | 0.61 | 0.33 | 0.31 | 0.41 |
| Gender | 0.14 | 0.45 | 0.76 | 0.66 | 0.98 |
| Tumor size (T) | NE | 0.94 | 0.34 | 0.03a | 0.04a |
| Lymph node
metastasis (N) | NE | 0.98 | 0.66 | 0.22 | 0.19 |
Discussion
SCLCs, LCNECs and pulmonary carcinoids are classic
neuroendocrine tumors that reflect all of the characteristics of
neuroendocrine cells. WHO classification divides BP-NETs into four
categories: low-grade TCs, intermediate-grade ACs, high-grade SCLCs
and high-grade LCNECs (1). While
these tumors share certain clinical, molecular and genetic
abnormalities, they also exhibit differences (1,9,10).
mTOR is a serine/threonine kinase that is
ubiquitously expressed in mammalian cells (11), and which regulates cell growth and
proliferation. mTOR is also one of the main downstream effectors in
the PI3K/AKT pathway that is critically involved in the mediation
of cell survival (12–14). This pathway is aberrantly activated
in several different tumor models (13,14,16,17).
Due to these functions, mTOR has been regarded as an attractive
target of anticancer agents; the functions of mTOR are blocked by
rapamycin, as well as by other mTOR inhibitors, such as everolimus
and temsirolimus (23).
Various studies have evaluated the effect of the
inhibition of the PI3K/AKT/mTOR pathway in experimental models of
human pulmonary carcinoid and SCLC cells. In particular, the
treatment of lung carcinoid cells with inhibitors of the PI3K/
AKT/mTOR pathway significantly reduced cellular growth and
neuroendocrine marker expression in vitro (18,19).
In SCLC cells, the inhibition of PI3K/AKT signaling resulted in the
inhibition of cellular growth, promotion of apoptosis and enhanced
sensitivity of cancer cells to chemotherapy (20,24).
Moreover, Marinov et al (12) reported that targeting mTOR with the
clinically approved inhibitor RAD001 (everolimus) significantly
reduced the cell growth of SCLCs and increased their sensitivity to
the antitumor effects of commonly used chemotherapeutic agents.
However, recent preliminary data from a phase II clinical trial
evaluating the effects of the rapamycin derivate temsirolimus in
SCLC patients following chemotherapy failed to exhibit any
beneficial effect (25).
Nevertheless, the efficacy of mTOR inhibitors in the
treatment of neuroendocrine lung tumors remains unclear, and more
appropriate molecular classification criteria and therapeutic
strategies against BP-NETs have yet to be clearly established.
These facts prompted us to investigate whether mTOR and its
upstream effector p-AKT were expressed across the whole spectrum of
BP-NETs, including tumorlets.
In the present study, the expression of p-AKT and
p-mTOR was analyzed in a large retrospective series of 210 patients
whose tumors were classified according to low and high p-AKT and
p-mTOR expression.
Other studies have also investigated the
p-AKT/p-mTOR pathway in BP-NETs, but their analysis of the
percentage of immunoreactive tumor cells was combined with the
staining intensity of the cells (26–29).
We decided to analyze our cases using only the percentage of
positive cells, as our experience has indicated that the scoring
system is highly subjective.
In our study, AKT and mTOR were widely expressed in
the entire series of BP-NETs, including tumorlets. Significantly
higher expression of p-AKT was observed in tumorlets and
carcinoids, both typical and atypical, than in high-grade
neuroendocrine carcinomas, LCNECs and SCLCs (p=0.0001).
Additionally, significantly higher
immunohistochemical expression of p-mTOR was revealed in tumorlets,
TCs and ACs compared to LCNECs and SCLCs (p=0.0002).
Furthermore, a correlation was identified between
p-mTOR expression and tumor size (T) in SCLCs and LCNECs. Patients
with high tumor sizes exhibited significantly lower levels of
p-mTOR expression, compared to patients with smaller tumors.
Dobashi et al reported immunohistochemical
expression of activated AKT and mTOR in 14/30 (46.7%) and in 3/30
(10%) SCLC specimens, respectively (28). They failed to reveal correlations
between immunohistochemical expression of the two markers and the
clinicopathological characteristics of patients. In our study,
p-AKT and p-mTOR expression levels were observed in a larger number
of SCLC cases (84.7 and 57.6%, respectively), probably due to the
larger number of patients analyzed.
Our results agreed with the conclusions of the study
conducted on 218 clinically malignant BP-NETs by Righi et
al, who revealed a statistically significant higher expression
level of p-mTOR in well-differentiated neuroendocrine tumors
compared to high-grade carcinomas (29).
Since the mTOR pathway controls protein synthesis,
cell growth and proliferation, lower expression of p-AKT in
high-grade tumors was an unexpected result. One possible
explanation could be that in tumors with low or no mTOR expression,
another signaling pathway, such as Erk, could be activated
(30). Another potential
explanation is that the activity of mTOR may be controlled at the
post-translational level (31).
Moreover, experimental studies have shown that the mTOR pathway
regulates cell growth at the expense of proliferation (32). However, the functions of mTOR are
more complex than translational control alone, and cross-talk
between pathways could alter its oncogenic potential.
However, the differences in the expression of p-AKT
and p-mTOR among the various subsets of neuroendocrine tumors
agrees with the molecular and genetic data indicating that TCs and
ACs are more closely associated to each other than they are to
LCNECs and SCLCs, which are themselves closely related. In addition
to differences in clinical characteristics between the two groups,
abnormalities in several genetic markers, such as p53, bcl2/bax,
cyclin D1, RB loss and LOH at 3p, are observed in a high percentage
of both SCLCs and LCNECs with minimal and intermediate percentages
of TCs and ACs, respectively, exhibiting these abnormalities
(9,33).
Currently, the only potentially curative treatment
option for patients with pulmonary carcinoids is surgical resection
(34). Unfortunately, effective
therapeutic options for patients with unresectable disease are
lacking, since radiotherapy, chemotherapy and biotherapy have
exhibited only limited success (8). Promising results have been obtained
in experimental models of carcinoid tumors using PI3K/AKT
inhibitors (18). These findings,
in addition to the results of the present study revealing high
p-AKT and p-mTOR in lung carcinoids, indicate that innovative
therapies that block the PI3K/AKT/mTOR signaling pathway could
represent new treatment options for patients with unresectable
pulmonary carcinoid disease.
Regarding inhibition of the PI3K/AKT/mTOR pathway in
SCLCs, results achieved in pre-clinical models (12,28,29)
were not confirmed in a clinical trial (25). However, this disappointing result
could be the result of different activation status of the AKT/mTOR
pathway in distinct SCLC patients (12). Indeed, in our study, high p-AKT and
p-mTOR expression levels were observed in only 36.5 and 30.6% of
SCLCs, respectively. In this sense, the putative use of AKT/ mTOR
inhibitors in clinical treatments should be preceded by analysis of
the status of the AKT/mTOR signaling pathway.
In conclusion, the expression of activated AKT and
mTOR was examined in a large series of BP-NETs. The
immunohistochemical expression levels observed indicate that this
pathway plays an important role in this group of lung tumors.
Moreover, the differences in the expression of these markers in the
various types of neuroendocrine tumors confirm that low to
intermediate tumors are more closely associated with each other
than with high-grade tumors, despite sharing common classification
and a common origin from neuroendocrine cells. Our results provide
new knowledge of the biological characterization of these tumors
and offer new potential therapeutic opportunities for the treatment
of BP-NETs.
References
|
1.
|
Travis WD, Brambilla E, Muller-Hermelink
HK and Harris CC: World Health Organization Classification of
Tumours. Pathology and Genetics of Tumours of the Lung, Pleura,
Thymus and Heart. IARC Press; Lyon: 2004
|
|
2.
|
Travis WD, Rush W, Flieder DB, Falk R,
Fleming MV, Gal AA and Koss MN: Survival analysis of 200 pulmonary
neuroendocrine tumors with clarification of criteria for atypical
carcinoid and its separation from typical carcinoid. Am J Surg
Pathol. 22:934–944. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Canessa PA, Santini D, Zanelli M and
Capecchi V: Pulmonary tumourlets and microcarcinoids in
bronchiectasis. Monaldi Arch Chest Dis. 52:138–139. 1997.PubMed/NCBI
|
|
4.
|
Watanabe H, Kobayashi H, Honma K, Ohnishi
Y and Iwafuchi M: Diffuse panbronchiolitis with multiple tumorlets.
A quantitative study of the Kultschitzky cells and the clusters.
Acta Pathol Jpn. 35:1221–1231. 1985.PubMed/NCBI
|
|
5.
|
Granberg D, Wilander E, Oberg K and
Skogseid B: Prognostic markers in patients with typical bronchial
carcinoid tumors. J Clin Endocrinol Metab. 85:3425–3430.
2000.PubMed/NCBI
|
|
6.
|
Cooper WA, Thourani VH, Gal AA, Lee RB,
Mansour KA and Miller JI: The surgical spectrum of pulmonary
neuroendocrine neoplasms. Chest. 119:14–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Asamura H, Kameya T, Matsuno Y, Noguchi M,
Tada H, Ishikawa Y, Yokose T, Jiang SX, Inoue T, Nakagawa K, Tajima
K and Nagai K: Neuroendocrine neoplasms of the lung: a prognostic
spectrum. J Clin Oncol. 24:70–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Gustafsson BI, Kidd M, Chan A,
Malfertheiner MV and Modlin IM: Bronchopulmonary neuroendocrine
tumors. Cancer. 113:5–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Onuki N, Wistuba II, Travis WD, Virmani
AK, Yashima K, Brambilla E, Hasleton P and Gazdar AF: Genetic
changes in the spectrum of neuroendocrine lung tumors. Cancer.
85:600–607. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Debelenko LV, Brambilla E, Agarwal SK, et
al: Identification of MEN1 gene mutations in sporadic carcinoid
tumors of the lung. Hum Mol Genet. 6:2285–2290. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Marinov M, Ziogas A, Pardo OE, Tan LT,
Dhillon T, Mauri FA, Lane HA, Lemoine NR, Zangemeister-Wittke U,
Seckl MJ and Arcaro A: AKT/mTOR pathway activation and BCL-2 family
proteins modulate the sensitivity of human small cell lung cancer
cells to RAD001. Clin Cancer Res. 15:1277–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Bjornsti MA and Houghton PJ: The TOR
pathway: a target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar
|
|
15.
|
Peterson RT, Beal PA, Comb MJ and
Schreiber SL: FKBP12-rapamycin-associated protein (FRAP)
autophosphorylates at serine 2481 under translationally repressive
conditions. J Biol Chem. 275:7416–7423. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Akcakanat A, Sahin A, Shaye AN, Velasco MA
and Meric-Bernstam F: Comparison of Akt/mTOR signaling in primary
breast tumors and matched distant metastases. Cancer.
112:2352–2358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Marinov M, Fischer B and Arcaro A:
Targeting mTOR signaling in lung cancer. Crit Rev Oncol Hematol.
63:172–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Pitt SC, Chen H and Kunnimalaiyaan M:
Phosphatidylinositol 3-kinase-Akt signaling in pulmonary carcinoid
cells. J Am Coll Surg. 209:82–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zatelli MC, Minoia M, Martini C, Tagliati
F, Ambrosio MR, Schiavon M, Buratto M, Calabrese F, Gentilin E,
Cavallesco G, Berdondini L, Rea F and degli Uberti EC: Everolimus
as a new potential antiproliferative agent in aggressive human
bronchial carcinoids. Endocr Relat Cancer. 17:719–729. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tsurutani J, West KA, Sayyah J, Gills JJ
and Dennis PA: Inhibition of the phosphatidylinositol
3-kinase/Akt/mammalian target of rapamycin pathway but not the
MEK/ERK pathway attenuates laminin-mediated small cell lung cancer
cellular survival and resistance to imatinib mesylate or
chemotherapy. Cancer Res. 65:8423–8432. 2005. View Article : Google Scholar
|
|
21.
|
Mamane Y, Petroulakis E, LeBacquer O and
Sonenberg N: mTOR, translation initiation and cancer. Oncogene.
25:6416–6422. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Sobin LH, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; NJ: 2009
|
|
23.
|
Vignot S, Faivre S, Aguirre D and Raymond
E: mTOR-targeted therapy of cancer with rapamycin derivatives. Ann
Oncol. 16:525–537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Krystal GW, Sulanke G and Litz J:
Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks
growth, promotes apoptosis, and enhances sensitivity of small cell
lung cancer cells to chemotherapy. Mol Cancer Ther. 1:913–922.
2002.PubMed/NCBI
|
|
25.
|
Pandya KJ, Dahlberg S, Hidalgo M, Cohen
RB, Lee MW, Schiller JH and Johnson DH; Eastern Cooperative
Oncology Group (E1500): A randomized, phase II trial of two dose
levels of temsirolimus (CCI-779) in patients with extensive-stage
small-cell lung cancer who have responding or stable disease after
induction chemotherapy: a trial of the Eastern Cooperative Oncology
Group (E1500). J Thorac Oncol. 2:1036–1041. 2007. View Article : Google Scholar
|
|
26.
|
Massion PP, Taflan PM, Shyr Y, Rahman SM,
Yildiz P, Shakthour B, Edgerton ME, Ninan M, Andersen JJ and
Gonzalez AL: Early involvement of the phosphatidylinositol
3-kinase/Akt pathway in lung cancer progression. Am J Respir Crit
Care Med. 170:1088–1094. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Shah T, Hochhauser D, Frow R, Quaglia A,
Dhillon AP and Caplin ME: Epidermal growth factor receptor
expression and activation in neuroendocrine tumours. J
Neuroendocrinol. 18:355–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Dobashi Y, Suzuki S, Matsubara H, Kimura
M, Endo S and Ooi A: Critical and diverse involvement of
Akt/mammalian target of rapamycin signaling in human lung
carcinomas. Cancer. 115:107–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Righi L, Volante M, Rapa I, Tavaglione V,
Inzani F, Pelosi G and Papotti M: Mammalian target of rapamycin
(mTOR) signaling activation patterns in neuroendocrine tumors of
the lung. Endocr Relat Cancer. 17:977–987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Chadha KS, Khoury T, Yu J, Black JD, Gibbs
JF, Kuvshinoff BW, Tan D, Brattain MG and Javle MM: Activated Akt
and Erk expression and survival after surgery in pancreatic
carcinoma. Ann Surg Oncol. 13:933–939. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Gingras AC, Raught B and Sonenberg N:
Regulation of translation initiation by FRAP/mTOR. Genes Dev.
15:807–826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Montagne J, Stewart MJ, Stocker H, Hafen
E, Kozma SC and Thomas G: Drosophila S6 kinase: a regulator of cell
size. Science. 285:2126–2129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Gugger M, Burckhardt E, Kappeler A,
Hirsiger H, Laissue JA and Mazzucchelli L: Quantitative expansion
of structural genomic alterations in the spectrum of neuroendocrine
lung carcinomas. J Pathol. 196:408–415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Pinchot SN, Holen K, Sippel RS and Chen H:
Carcinoid tumors. Oncologist. 13:1255–1269. 2008. View Article : Google Scholar : PubMed/NCBI
|