Introduction
Early gastric cancer (EGC) is defined as gastric
carcinoma confined to the mucosa and/or submucosa irrespective of
lymph node involvement and tumor size, according to the Japanese
Classification of Gastric Carcinoma (JCGC) (1). Compared to advanced gastric cancer
(AGC), EGC has a more favorable prognosis after curative resection;
the 5-year survival rate is higher than 90% in Japan (2), Korea (3), and slightly lower in Italy, France
and the US (4–6). In spite of the very favorable
prognosis of EGC, recurrence or second primary cancers present in
certain patients after curative surgery, with the recurrence or
second primary cancer rate varying from 1.4 to 13.7% (5,7–11).
It is crucial to understand the prognostic factors and features of
recurrence in patients with EGC and to identify who are at high
risk and when; a subsequent follow-up schedule or adjuvant therapy
may be considered accordingly.
The incidence of EGC is approximately 10% among
in-patients with gastric carcinoma in China. However, due to the
relatively low incidence of recurrence and favorable prognosis
after curative resection for EGC, relevant studies have been
limited. Furthermore, most previous reports have focused on
short-term survival results and few have investigated survival over
10 years (4,5,7,9–11).
In the present study, we assessed the long-term survival profiles
of patients with EGC at our single institute through 0.3–33 years
of follow-up. Survival rate, characteristics of recurrence and
causes of death were analyzed, and prognostic factors were also
discussed, enabling us to provide a more tailored follow-up
schedule and treatment for high-risk patients.
Patients and methods
Between February 1970 and June 2005, a consecutive
series of 3,144 gastric cancer patients underwent curative
gastrectomy at the Department of Surgical Oncology, First
Affiliated Hospital, China Medical University. Among them, 344
patients (10.9%) were diagnosed with EGC. The outcome of all of the
patients was followed up by outpatient visits, telephone and mail
contact and death certificates. At the end of the follow-up in June
2008, 21 patients were excluded from our study due to incompleted
data collection. The rate of follow-up was 96%, and a total of 323
patients were enrolled in the present study after providing
informed consent. The median and mean follow-up period for the
survivors was 9 and 10.6 years (range 0.3–33), respectively. The
loss of follow-up cases and deaths from any other causes other than
cancer were treated as censored data for the analysis of
disease-free survival. Only patients who died of gastric cancer or
second primary cancer were considered as tumor-related deaths.
The pattern of recurrence and second primary cancer
were confirmed by physical findings, computed tomography (CT),
abdominal ultrasonography, bone scan, endoscopy, needle biopsy or
surgery, respectively. Each second primary cancer was
geographically separate, distinct and consisted of a single lesion
to adequately exclude the possibility that the second tumor
represented metastasis. In addition, occurrence of a second tumor
was at least 6 months after resection of EGC (12). The study protocol was approved by
the Ethics Committee of the China Medical University.
Statistical analysis
Data were analyzed by using the SPSS statistical
software program (SPSS Inc., Chicago, IL, USA). The Cox
proportional hazards regression model was applied to identify
prognostic factors. Disease-free and overall survival rates were
calculated using Kaplan-Meier estimation and the log-rank test. The
correlation between hematogenous metastasis and clinicopathological
factors was evaluated by using logistic regression. p<0.05 was
considered statistically significant.
Results
Clinicopathological features
The age of the patients (mean ± SD) was 54.2±12
years (range 19–80); 49 patients were ≤40 years of age, 242
patients were 41–69 years and 32 patients were ≥70 years. More men
than women (236 men vs. 87 women) participated in the study.
Carcinoma was located in the lower third of the stomach (L/LM) in
240 patients, in the middle third (M/ML/MU) in 62 patients, in the
upper third (U/UM) in 13 patients and in the whole stomach (UML) in
8 patients. Lymphadenectomy was executed based on the JCGC. D1
and/or D1 plus no. 7, 8a, 9 lymph node dissection was performed in
98 patients, D2 lymphadenectomy in 154 patients and more than D2
lymphadenectomy in 71 patients.
Mucosa carcinoma was diagnosed in 138 patients
(42.7%) and submucosa carcinoma in 185 (57.3%), based on the depth
of tumor invasion. Protruded type (I a and II a) was found in 26
patients (8%), flat type (II b) in 20 (6.2%), depressed type (II c
and III) in 238 (73.7%) and mixed type in 39 patients (12.1%),
respectively. The tumor diameter ranged from 0.5 to 18 cm
(3.4±2.0); ≤1 cm in 24 patients (7.4%), 1–3 cm in 167 patients
(51.7%), 3–6 cm in 93 patients (28.8%) and ≥6 cm in 39 patients
(12.1%). Well and/or moderately differentiated tumors were found in
117 patients (36.2%) and poorly differentiated tumors in 206
patients (63.8%). Vessel involvement occurred in 25 patients
(7.7%). Lymph node metastasis was detected in 51 cases (15.8%) and
the median number of MLNs was 2 (range 1–16). The
clinicopathological terminology in this study follows the JCGC.
Survival rate, cause and time of
death
The 5-, 10-, 15- and 20-year disease-free survival
rates in the patients were 97.0, 91.3, 87.1 and 82.5%,
respectively, while the overall survival rates for the different
periods above were 93.9, 80.7, 65.5 and 45.9%, respectively.
A total of 22 patients (6.8%) died of recurrence, 9
patients (2.8%) died of second primary cancers and 65 patients
(20.1%) died of comorbid diseases, such as ischemic heart disease,
cerebrovascular disease, respiratory disease, hepatic cirrhosis and
accidents, throughout the 0.3–33 years of follow-up. Among the
relapsed cases, hematogenous metastasis was the major pattern of
recurrence (77.3%, 17/22), often presenting in the lung, liver,
brain and bone marrow. Peritoneal dissemination and lymph node
metastases were also noted in 3 and 2 patients, respectively.
Notably, 15 out of 17 hematogenous metastases occurred within the
first decade and 8 metastases occurred within the first 5 years
after surgery. Recurrence occurring in the first decade accounted
for 86.4% (19/22), the earliest case presented itself as brain
metastasis 1 year after surgery. The time and patterns of
recurrence are summarized in Table
I.
 | Table ITime and patterns of recurrence in 22
patients with EGC. |
Table I
Time and patterns of recurrence in 22
patients with EGC.
| Time | Liver | Lung | Brain | Bone | Lymph node | Peritoneal
dissemination | Liver/lung | Liver/lung/bone | Total |
|---|
| ≤ 5 years | 2 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 8 |
| >5 to ≤ 10
years | 1 | 1 | 0 | 0 | 1 | 3 | 5 | 0 | 11 |
| >10 years | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 3 |
| Total | 4 | 4 | 1 | 1 | 2 | 3 | 6 | 1 | 22 |
The site distribution and time to occurrence of
second primary cancers are shown in Table II. Most second primary cancers
(6/9) developed 10 years after the original treatment and often
presented in the remnant stomach, liver, lung and colon. The latest
case happened at 30 years after surgery as a remnant gastric
cancer. There was no significant clinicopathological factor
associated with second primary cancer through univariate and
multivariate analysis (data not shown).
 | Table IISite distribution and occurrence of
second primary cancers in 9 patients with EGC. |
Table II
Site distribution and occurrence of
second primary cancers in 9 patients with EGC.
| Second primary
cancers | Time to occurrence
(years)
|
|---|
| ≤5 | >5 and ≤10 | >10 |
|---|
| Liver (n=2) | | | 2 |
| Lung (n=2) | | 1 | 1 |
| Colon (n=1) | | | 1 |
| Ureter (n=1) | 1 | | |
| Remnant stomach
(n=3) | | 1 | 2 |
Prognostic factors for disease-free
survival in early gastric cancer
Univariate analysis of the ten potential prognostic
factors was carried out, and the results are summarized in Table III. Significantly worse prognoses
were found in patients with good/moderate differentiation, vessel
involvement, lymph node metastasis and older age compared to their
counterparts, by univariate analysis (p<0.05). There was no
significant difference among other factors on prognosis
(p>0.05).
 | Table IIIUnivariate analysis of the prognosis
of patients with EGC. |
Table III
Univariate analysis of the prognosis
of patients with EGC.
| Factors | Disease-free survival
rate (%)
|
|---|
| Total | Deceased | 5-year | 10-year | 15-year | 20-year | P-value |
|---|
| Gender | | | | | | | |
| Male | 236 | 21 | 97.3 | 92.1 | 86.5 | 84.5 | 0.3270 |
| Female | 87 | 10 | 96.3 | 89.1 | 89.1 | 69.6 | |
| Age (years) | | | | | | | |
| ≤40 | 49 | 4 | 95.5 | 92.8 | 87.4 | - | 0.0460a |
| 41–69 | 242 | 22 | 98.3 | 92.0 | 88.8 | 83.2 | |
| ≥70 | 32 | 5 | 88.9 | 83.9 | 67.1 | - | |
| Site | | | | | | | |
| L/LM | 240 | 23 | 96.9 | 90.2 | 85.0 | 83.3 | 0.7650 |
| M/ML/MU | 62 | 7 | 96.7 | 93.7 | 90.4 | 73.9 | |
| U/UM | 13 | 1 | 100.0 | 91.7 | - | - | |
| UML | 7 | 0 | - | - | - | - | |
| Tumor size
(cm) | | | | | | | |
| ≤1.0 | 24 | 2 | 100.0 | 94.4 | 80.9 | - | 0.5700 |
| 1.1–3.0 | 167 | 14 | 96.1 | 92.0 | 86.5 | 83.9 | |
| 3.1–5.9 | 93 | 13 | 97.7 | 88.3 | 86.2 | 75.5 | |
| ≥6 | 39 | 2 | 97.4 | 93.8 | - | - | |
| Depth of
invasion | | | | | | | |
| Mucosal | 138 | 11 | 97.8 | 94.9 | 89.9 | 83.9 | 0.1990 |
| Submucosal | 185 | 20 | 96.5 | 88.2 | 84.9 | 82.4 | |
|
Differentiation | | | | | | | |
|
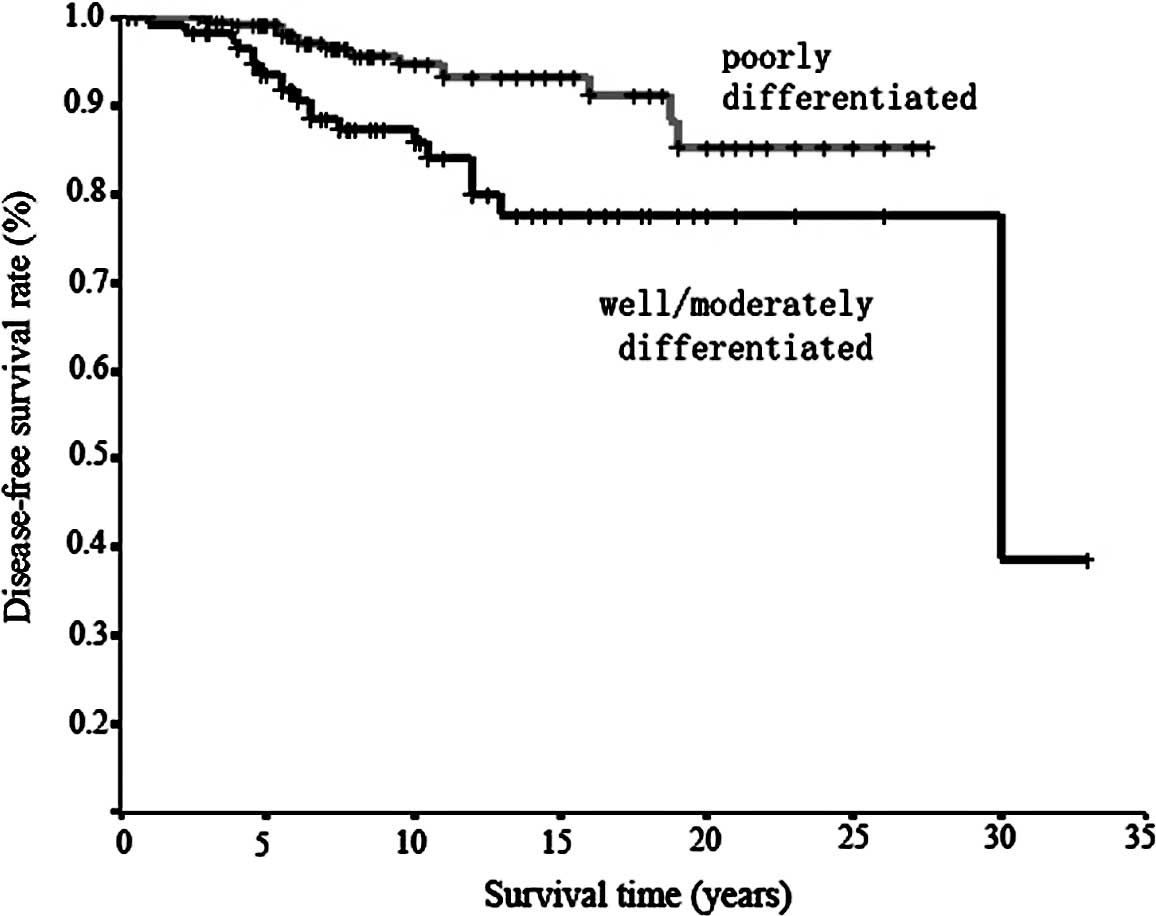
Well/moderate | 117 | 19 | 93.7 | 85.8 | 77.5 | - | 0.0090a |
| Poor | 206 | 12 | 98.9 | 94.6 | 93.3 | 85.3 | |
| Macroscopic
type | | | | | | | |
| Protruded | 26 | 4 | 100.0 | 86.5 | - | - | 0.7460 |
| Flat | 20 | 3 | 100.0 | 87.5 | 87.5 | 77.7 | |
| Depressed | 238 | 22 | 96.4 | 92.1 | 85.6 | 80.2 | |
| Mixed | 39 | 2 | 97.4 | 93.8 | - | - | |
| Vessel
involvement | | | | | | | |
| Negative | 298 | 24 | 97.2 | 92.9 | 89.3 | 86.1 | 0.0003a |
| Positive | 25 | 7 | 95.2 | 69.8 | 59.8 | 44.9 | |
| Lymph node
metastasis | | | | | | | |
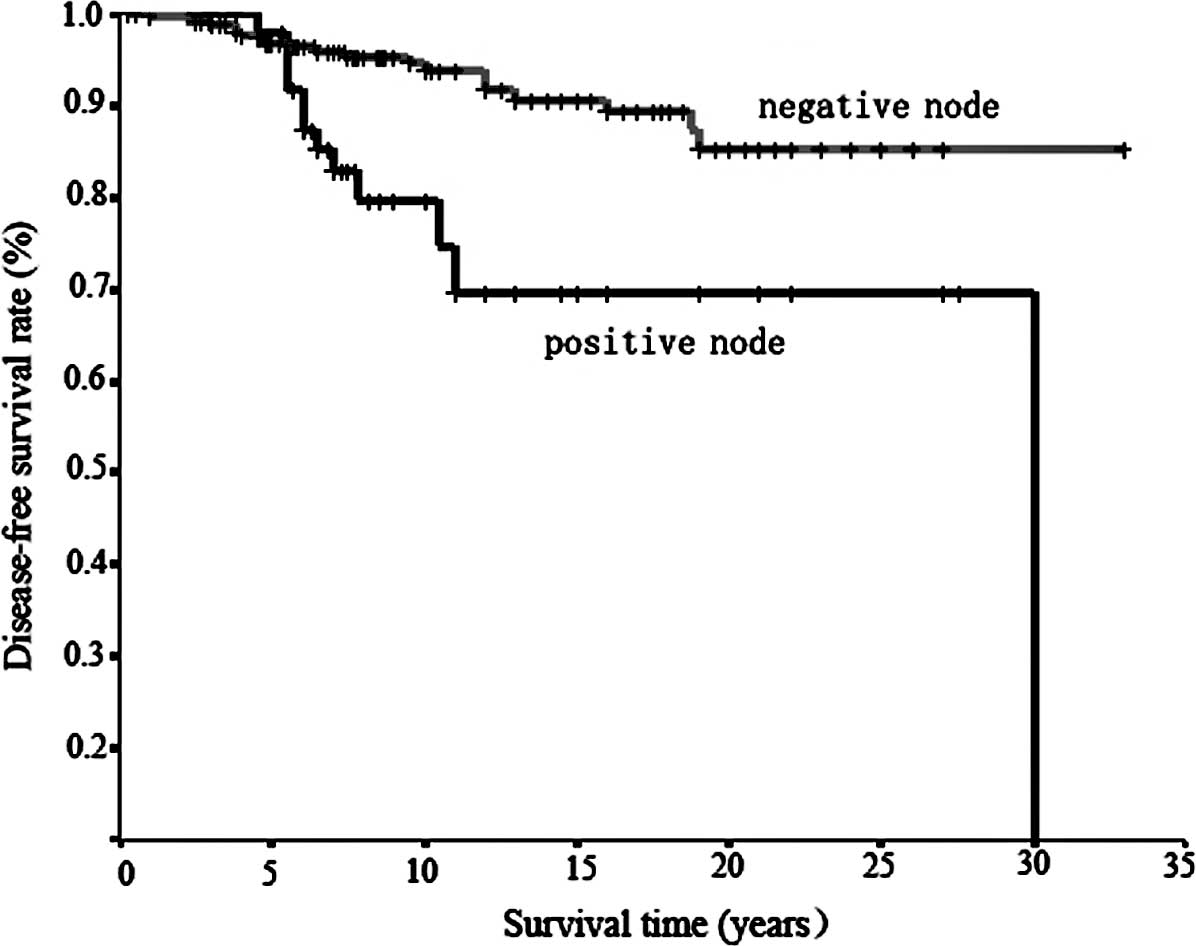
| Negative | 272 | 19 | 96.8 | 93.8 | 90.7 | 85.2 | 0.0006a |
| Positive | 51 | 12 | 97.9 | 79.7 | 69.7 | - | |
| Scope of lymph node
dissection | | | | | | | |
| D1/D1+ | 98 | 11 | 97.5 | 87.7 | 82.6 | 74.3 | 0.6590 |
| D2 | 154 | 12 | 95.9 | 93.5 | 89.5 | 86.1 | |
| D2+/D3/D3+ | 71 | 8 | 98.6 | 91.1 | 87.9 | 83.9 | |
Only differentiation, vessel involvement and nodal
status were identified as independent prognostic factors using Cox
regression analysis. Worse survival was observed for patients with
well/moderately differentiated tumors than with poorly
differentiated tumors (HR=3.52 vs. 1), for patients with positive
vessel involvement than with negative involvement (HR=3.40 vs. 1),
and with lymph node metastasis than without metastasis (HR=4.20 vs.
1) (Table IV). Age was excluded by
multivariate analysis. The survival curves stratified according to
different clinicopathological factors are shown in Figs. 1–3.
 | Table IVMultivariate analysis of prognostic
factors for disease-free survival in ECG.a |
Table IV
Multivariate analysis of prognostic
factors for disease-free survival in ECG.a
| Factors | β-coefficient | SE | Wald | P-value | HR | 95% CI for HR
|
|---|
| Lower | Upper |
|---|
|
Differentiation | 1.26 | 0.40 | 10.01 | 0.0020 | 3.52 | 1.61 | 7.66 |
| Vessel
involvement | 1.22 | 0.44 | 7.91 | 0.0050 | 3.40 | 1.45 | 7.98 |
| Lymph node
metastasis | 1.44 | 0.40 | 12.89 | <0.0001 | 4.20 | 1.92 | 9.20 |
Prognostic factors for overall survival
in early gastric cancer
Among the ten clinicopathological factors, only
gender, age and differentiation were independent influencing
factors for overall survival in patients with EGC using
multivariate analysis. The HR for death was significantly higher in
males than in females, in elders than in younger patients, and in
patients with well/moderately differentiated tumors than in those
with poorly differentiated ones (p<0.05). Worse overall survival
was also observed with an increase in tumor size, but this was not
statistically significant (p=0.071) (Table V).
 | Table VMultivariate analysis of prognostic
factors for overall survival in ECG.a |
Table V
Multivariate analysis of prognostic
factors for overall survival in ECG.a
| Factors | β-coefficient | SE | Wald | P-value | HR | 95% CI for HR
|
|---|
| Lower | Upper |
|---|
| Gender | −0.50 | 0.25 | 4.04 | 0.044 | 0.61 | 0.37 | 0.99 |
| Age | 0.05 | 0.01 | 18.18 | <0.001 | 1.05 | 1.03 | 1.07 |
| Size | 0.08 | 0.04 | 3.25 | 0.071 | 1.08 | 0.99 | 1.18 |
|
Differentiation | 0.48 | 0.22 | 4.94 | 0.026 | 1.62 | 1.06 | 2.47 |
Influencing factors for hematogenous
recurrence of early gastric cancer
As hematogenous metastasis was the predominant
recurrence pattern, its influencing factors were further
investigated. Differentiation and nodal status were identified by
using multivariate analysis. When the relative risks for recurrence
after gastrectomy were set as 1 for patients with poorly
differentiated tumors and without lymph node metastasis, the HR of
hematogenous metastasis increased to 3.65 (1.27–10.51, p=0.016) in
patients with well/moderately differentiated tumors, and increased
to 5.66 (1.92–16.67, p=0.002) in patients with lymph node
metastasis (Table VI).
 | Table VIMultivariate analysis of factors
influencing hematogenous metastasis in ECG.a |
Table VI
Multivariate analysis of factors
influencing hematogenous metastasis in ECG.a
| Factors | β-coefficient | SE | Wald | P-value | HR | 95% CI for HR
|
|---|
| Lower | Upper |
|---|
|
Differentiation | 1.29 | 0.54 | 5.75 | 0.016 | 3.65 | 1.27 | 10.51 |
| Nodal status | 1.73 | 0.55 | 9.87 | 0.002 | 5.66 | 1.92 | 16.67 |
Discussion
In the present study, EGC accounted for only 10.9%
of all resectable gastric carcinomas, which is similar to Western
countries, but is much lower than Japan and Korea, with an
incidence ranging from 40 to 60% (7,10,13–17).
However, the 10-year disease-free survival rate was 91.3%,
suggesting a good prognosis for patients treated at our institute,
which closely resembles studies from Japan and Korea.
As indicated by long-term follow-up, recurrence and
second primary cancers occurred in 6.8 and 2.8% of the patients
with EGC, respectively, slightly higher than that in Japan and
Korea, and lower compared to Western countries (7–11,18,19).
Moreover, most recurrence occurred within 10 years after the
original surgical treatment (86.4%, 19/22). Hematogenous spreading
was the major pattern of recurrence (77.3%, 17/22), including 8
cases of early recurrence (≤5 years) and 9 cases of late recurrence
(>5 years). Our findings corroborate those of Sano et al
(9) and Kunisaki et al
(10), where hematogenous
spreading was also predominant at 65% (13/20) and 71.4% (15/21),
respectively. To note, remnant gastric cancer as well as other
second primary cancers, including hepatoma and lung cancer,
commonly occurred beyond the first decade after surgery, resembling
other reports. Yamamoto et al reported that the average
interval from surgery to development of a second primary cancer was
7.1 years (18). This may reflect
multicenter tumorigenesis. In this study, CT, ultrasonography and
endoscopy were the most useful tools for detecting not only
recurrence, but also second primary cancers. More than 80% of
recurrences and second primary cancers were detected by CT.
Therefore, these tools may be beneficial for early detection of
recurrence and second primary cancers; thus a closer follow-up
program should be executed.
Since hematogenous metastasis was the main pattern
of recurrence, the influencing factors were further discussed in
this study. Nodal status was confirmed as an independent risk
factor for hematogenous spreading by multivariate analysis. Seven
out of 17 patients with hematogenous metastasis had lymph node
metastasis and 10 out of 51 patients with lymph node metastasis had
vessel invasion, showing a positive correlation between them. This
discovery supports the hypothesis that hematogenous recurrence of
EGC may be caused by vessel involvement by malignant cells in the
mucosal and submucosal layers (20). Histological differentiation status
also strongly correlated with hematogenous metastasis (HR=3.65),
with well/moderately differentiated tumors more frequently involved
in hematogenous metastasis. This is also supported by studies by
Sano et al (9) and Adachi
et al (21), where
hematogenous metastasis was more frequent in the gastric cancer
patients with well-differentiated tumors compared to those with
poorly differentiated tumors (68 and 34%, 41 and 6%, respectively).
Although the underlying mechanism of hematogenous metastasis in EGC
remains elusive, vessel invasion by cancer cells may be the first
step, and histological differentiation may contribute to this
course. Further studies based on genomics may elucidate this issue
in the future.
Concerning prognostic factors, lymph node metastasis
has been well recognized as an important prognostic factor in both
EGC and AGC (22,23), which was also confirmed in this
study. In addition to nodal status, another two significant
prognostic factors identified were histological type and vessel
involvement. This may be explained by the fact that patients with
well/moderately differentiated tumors and positive nodes are prone
to hematogenous metastasis. While many studies have found tumor
size and depth of tumor invasion to be significant prognostic
factors (5,10,15,16),
we did not observe this in our cases. However, tumor size and depth
of tumor invasion were slightly correlated with lymph node
metastasis (data not shown). Therefore, they may influence
prognosis through lymph node metastasis indirectly.
Among the patients who died during the follow-up
period, death from comorbid diseases was the most common (20.1%),
followed by recurrence and second primary cancers. With respect to
overall survival, age, as well as gender and tumor differentiation,
were identified as powerful prognostic indicators in EGC by Cox
regression analysis. The rate of death from comorbid diseases
increased significantly with age (≥70 years). In addition, a poorer
prognosis was observed in male patients than in female ones. These
results were also supported by studies of Kunisaki et al
(10) and Bando et al
(24). This suggests that patients
with curatively resected EGC tend to die of comorbid diseases more
frequently at an increased age. Therefore, in order to improve
survival and therapeutic outcomes, treatment of comorbid diseases
may be quite important, in addition to a periodic follow-up
schedule for detecting recurrence and second primary cancers in
patients with EGC.
In conclusion, hematogenous recurrence is the major
type of relapse in the first decade after surgery, with
differentiation and nodal status as independent influencing
factors. A second primary cancer commonly occurred in the second
decade after surgery. Prognosis is generally worse in EGC patients
with vessel involvement, lymph node metastasis or well/moderately
differentiated lesions. Death from comorbid diseases was common in
patients of increased age. Treatment of comorbid diseases and a
periodic follow-up schedule may contribute to the improved
prognosis of patients with EGC.
Abbreviations:
|
EGC,
|
early gastric cancer;
|
|
AGC,
|
advanced gastric cancer;
|
|
MLN,
|
metastatic lymph node;
|
|
HR,
|
hazard ratio;
|
|
CT,
|
computed tomography
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 30873043 and no. 81071956).
References
|
1
|
Japanese Gastric Cancer Association:
Japanese Classification of Gastric Carcinoma. 2nd English edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shiozawa N, Kodama M, Chida T, Arakawa A,
Tur GE and Koyama K: Recurrent death among early gastric cancer
patients: 20 years experience. Hepatogastroenterology. 41:244–247.
1994.PubMed/NCBI
|
|
3
|
Kim JP, Hur YS and Yang HK: Lymph node
metastasis as a significant prognostic factor in early gastric
cancer; analysis of 1,136 early gastric cancers. Ann Surg Oncol.
2:308–313. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Degiuli M and Calvo F: Survival of early
gastric cancer in a specialized European center. Which
lymphadenectomy is necessary? World J Surg. 30:2193–2203. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borie F, Rigau V, Fingerhut A and Millat
B; French Association for Surgical Research: Prognostic factors for
early gastric cancer in France: Cox regression analysis of 332
cases. World J Surg. 28:686–691. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith JW, Shiu MH, Kelsey L and Brennan
MF: Morbidity of radical lymphadenectomy in the curative resection
of gastric cancer. Arch Surg. 126:1469–1473. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee HJ, Kim YH, Kim WH, Lee KU, Choe KJ,
Kim JP and Yang HK: Clinicopathological analysis for recurrence of
early gastric cancer. Jpn J Clin Oncol. 33:209–214. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ikeda Y, Saku M, Kishihara F and Maehara
Y: Effective follow-up for recurrence or a second primary cancer in
patients with early gastric cancer. Br J Surg. 92:235–239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sano T, Sasako M, Kinoshita T and Maruyama
K: Recurrence of early gastric cancer. Follow-up of 1475 patients
and review of the Japanese literature. Cancer. 72:3174–3178. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kunisaki C, Akiyama H, Nomura M, et al:
Significance of long-term follow-up of early gastric cancer. Ann
Surg Oncol. 13:363–369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Basili G, Nesi G, Barchielli A, Manetti A
and Biliotti G: Pathologic features and long-term results in early
gastric cancer: report of 116 cases 8–13 years after surgery. World
J Surg. 27:149–152. 2003.PubMed/NCBI
|
|
12
|
Moertel CG, Dockerty MB and Baggenstoss
AH: Multiple primary malignant neoplasms. III. Tumors of
multicentric origin. Cancer. 14:238–248. 1961. View Article : Google Scholar
|
|
13
|
Nishi M, Ishihara S, Nakajima T, Ohta K,
Ohyama S and Ohta H: Chronological changes of characteristics of
early gastric cancer and therapy: experience in the Cancer
Institute Hospital of Tokyo, 1950–1994. J Cancer Res Clin Oncol.
121:535–541. 1995.PubMed/NCBI
|
|
14
|
Maehara Y, Kakeji Y, Oda S, Takahashi I,
Akazawa K and Sugimachi K: Time trends of surgical treatment and
the prognosis for Japanese patients with gastric cancer. Br J
Cancer. 83:986–991. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park IS, Lee YC, Kim WH, Noh SH, Lee KS
and Kim H: Clinicopathologic characteristics of early gastric
cancer in Korea. Yonsei Med J. 41:607–614. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hochwald SN, Brennan MF, Klimstra DS, Kim
S and Karpeh MS: Is lymphadenectomy necessary for early gastric
cancer? Ann Surg Oncol. 6:664–670. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Everett SM and Axon AT: Early gastric
cancer in Europe. Gut. 41:142–150. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto M, Yamanaka T, Baba H, Kakeji Y
and Maehara Y: The postoperative recurrence and the occurrence of
second primary carcinomas in patients with early gastric carcinoma.
J Surg Oncol. 97:231–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Youn HG, An JY, Choi MG, Noh JH, Sohn TS
and Kim S: Recurrence after curative resection of early gastric
cancer. Ann Surg Oncol. 17:448–454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saka M, Katai H, Fukagawa T, Nijjar R and
Sano T: Recurrence in early gastric cancer with lymph node
metastasis. Gastric Cancer. 11:214–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adachi Y, Yasuda K, Inomata M, Sato K,
Shiraishi N and Kitano S: Pathology and prognosis of gastric
carcinoma: well versus poorly differentiated type. Cancer.
89:1418–1424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maehara Y, Emi Y, Baba H, Adachi Y,
Akazawa K, Ichiyoshi Y and Sugimachi K: Recurrences and related
characteristics of gastric cancer. Br J Cancer. 74:975–979. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bando E, Kojima N, Kawamura T, Takahashi
S, Fukushima N and Yonemura Y: Prognostic value of age and sex in
early gastric cancer. Br J Surg. 91:1197–1201. 2004. View Article : Google Scholar : PubMed/NCBI
|