Introduction
The World Health Organization (WHO) defines
large-cell neuroendocrine carcinoma (LCNEC) as a tumor with a large
nucleus and a tendency to have neuroendocrine differentiation, and
it defines ovarian LCNECs as miscellaneous tumors (1). In Japan, the term LCNEC was described
for the first time in The General Rules for Clinical and
Pathological Management of Ovarian Tumors by the Japan Society of
Obstetrics and Gynecology and the Japanese Society of Pathology in
December 2009. Until these classifications were established, LCNEC
had been classified as anaplastic or poorly differentiated
carcinoma and was synonymous with undifferentiated carcinoma of the
non-small cell neuroendocrine type (1,2).
According to the WHO classification of lung cancer (3), the pathological structures by H&E
staining reveal a characteristic organization, such as round nest
and sheet-like formation with frequently central coagulative
necrosis, and rosette-like formation of tumor cells is often
observed everywhere. The nucleus is large with granular or coarse
chromatin, a prominent nucleolus and moderate or abundant
cytoplasm. Additionally, neuroendocrine differentiation by
immunohistochemical analysis, such as chromogranin A, synaptophysin
and CD56, is required to confirm the diagnosis of LCNEC. This
cancer is also generally accompanied by surface epithelial-stromal
tumors, and the LCNEC components metastasize relatively early,
resulting in poor prognosis (1).
To date, only 29 cases of ovarian LCNEC have been reported
(4–17). Thus, we describe our 4 recently
encountered cases and evaluate the clinicopathological features of
ovarian LCNEC from 33 primary cases, including previously reported
cases.
Materials and methods
Initially, we described the clinical courses of our
4 cases. Then, for all 33 available LCNEC cases, we summarized the
clinicopathological findings, such as patient age, FIGO stage,
tumor marker values, operation and intra-abdominal findings,
chemotherapy regimens and outcomes (4–17).
The Kaplan-Meier overall survival rate was estimated by analyzing
the survival time data reported in the literature with STAT view
software for Windows. Routine H&E staining and
immunohistochemical staining for CD56, synaptophysin and
chromogranin A as neuroendocrine markers were performed in sections
of both LCNEC and epithelial carcinoma components. The antibody
clones, dilutions and sources that we used are as follows: CD56
(1B6, 1/1; Nichirei), chromogranin A (DAK-A3, 1/100; Dako) and
synaptophysin (SY38, 1/100; Dako).
Case reports
Case 1
A 66-year-old, gravida 2, parity 2, post-menopausal
woman was found to have multiple lung nodules in a chest X-ray
obtained during a routine medical examination. She was referred to
us because of her pelvic mass. The patient also had a metastatic
tumor in her vagina, which was diagnosed as undifferentiated
carcinoma based on histological examination. A computed tomography
(CT) examination revealed that the pelvic tumor was composed of
cystic and solid components, suggesting malignancy. Her CA125 level
was elevated to 6,595 U/ml; therefore, we clinically defined this
case as FIGO (International Federation of Gynecology and
Obstetrics) clinical stage IV ovarian cancer. Consequently, we
initiated paclitaxel/carboplatin (TC) chemotherapy in the
neoadjuvant setting. Clinical partial response (PR), including
complete remission of multiple lung disease, was acquired by RECIST
criteria after 4 cycles of chemotherapy; therefore,
interval-debulking surgery was performed, including bilateral
salpingo-oophorectomy, hysterectomy, omentectomy and peritoneal
biopsy. There were no malignant findings, except for the existing
viable cancer cells in the right ovary. Although adjuvant and
maintenance chemotherapy were continued, brain metastasis was
observed 17 months after the start of initial treatment.
Neurosurgeons resected the tumor and the brain tumor pathology was
similar to not only the primary vaginal tumor, but also to ordinary
lung LCNEC. Immunohistochemical analyses for neuroendocrine markers
were performed, and positive staining for chromogranin A was
acquired. As a result, the brain tumor was diagnosed as metastatic
LCNEC that had originated from the ovary, despite lacking an
epithelial component. The patient received whole-brain radiation
therapy, and during the 64 months post initial treatment she did
not experience tumor recurrence.
Case 2
An 80-year-old, gravida 2, parity 2, post-menopausal
woman was found to have an abdominal mass during a routine medical
examination. The fist-sized, cystic tumor was palpable near the
left side of the uterus. Ultrasonography, CT and magnetic resonance
imaging (MRI) examinations revealed a 7-cm left ovarian tumor with
solid and cystic components, and hydrosalpinx. Her CA125 level was
relatively high at 204.3 U/ml. Her Pap cervical cancer screening
was normal. As part of her complete surgical treatment, she
underwent the following: bilateral salpingo-oophorectomy,
hysterectomy, pelvic lymphadenectomy, omentectomy and appendectomy,
which macroscopically resulted in no residual tumor. Para-aortic
lymph nodes were not swollen upon palpation. Histopathologically,
this tumor was diagnosed as LCNEC with an endometrioid
adenocarcinoma component. The tumor had already ruptured and
invaded into the left fallopian tube and the parametrium with
positive peritoneal cytology, and it was classified as FIGO
surgical stage IIc ovarian cancer (pT2cN0M0). Six courses of
postoperative TC [175 mg/m2 paclitaxel and carboplatin
(CBDCA) at AUC 6.0] chemotherapy were carried out and during the 40
months post initial treatment there were no recurrent signs.
Case 3
A 65-year-old, nulligravida, post-menopausal woman
complained of nausea and abdominal distension with continuous pain.
She was referred to our hospital because of an abdominal mass. CT
and MRI examinations revealed an 11-cm cystic abdominal ovarian
tumor with enhanced nodule formation. Her CA125 and CA19-9 levels
were 77.0 and 776.5 U/ml, respectively. Her Pap smear of the
uterine cervix and endometrium was negative. Owing to her
continuous abdominal pain, we performed an emergency operation,
including bilateral salpingo-oophorectomy, hysterectomy and
omentectomy; macroscopically, there was no residual tumor. Pelvic
and para-aortic lymph nodes were not swollen upon palpation. The
tumor had spontaneously ruptured, which was suggested as the cause
of her abdominal pain. Peritoneal washing cytology was negative. On
pathological examination, most of the tumor had a characteristic
sheet or nest formation with central and peripheral coagulative
necrosis, and the diagnosis of LCNEC was confirmed by CD56-positive
immunohistochemistry. The remaining tumor was diagnosed as
endometrioid adenocarcinoma with squamous differentiation. Although
we planned postoperative adjuvant chemotherapy for this FIGO
surgical stage Ic ovarian cancer (pT1cNxM0), the patient was morbid
with nausea, vomiting and pain as a result of her abdominal
distension within 2 weeks of the operation. CT examination revealed
an occlusive ileus due to the 10-cm recurrent pelvic mass, and
liver metastasis and regional lymphadenopathy had already appeared.
The patient died 2 months after the surgery and exhibited no
response to the TC chemotherapy.
Case 4
A 42-year-old, gravida 3, parity 3 woman with a
regular menstrual cycle was discovered to have a lower abdominal
mass at a local clinic; she was then referred to our hospital. CT
and MRI examinations revealed an ovarian tumor composed of cystic
and solid parts. Her CA125 and STN levels were elevated to 775.2
and 139.3 U/ml, respectively. Pap smears of the uterine cervix and
endometrium were both negative. The tumor disseminated to the
abdominal cavity, particularly the omentum and the Douglas
peritoneum; as a result, optimal debulking surgery was performed,
including bilateral salpingo-oophorectomy, hysterectomy with
peritoneum resection of the Douglas pouch, omentectomy and
retroperitoneal lymphadenectomy. Histopathological diagnosis was
LCNEC, which contained a component of endometrioid adenocarcinoma
with weakly positive staining for CD56 and chromogranin A. All
disseminated tumors in the right ovary, parametrium, uterus,
Douglas peritoneum and omentum were histologically diagnosed as
LCNEC. The patient received 6 cycles of postoperative TC (175
mg/m2 paclitaxel and CBDCA at AUC 6.0) chemotherapy for
FIGO surgical stage IIIb ovarian cancer (pT3bN0M0), and during the
32 months after initial therapy she was alive with no signs of
recurrence.
Pathological findings
Macroscopic findings on the cut surface of the
tumors were not specific compared to the usual type of ovarian
cancer. The solid component was slightly elastic with a light gray
color and had hemorrhage and necrosis. All cases had characteristic
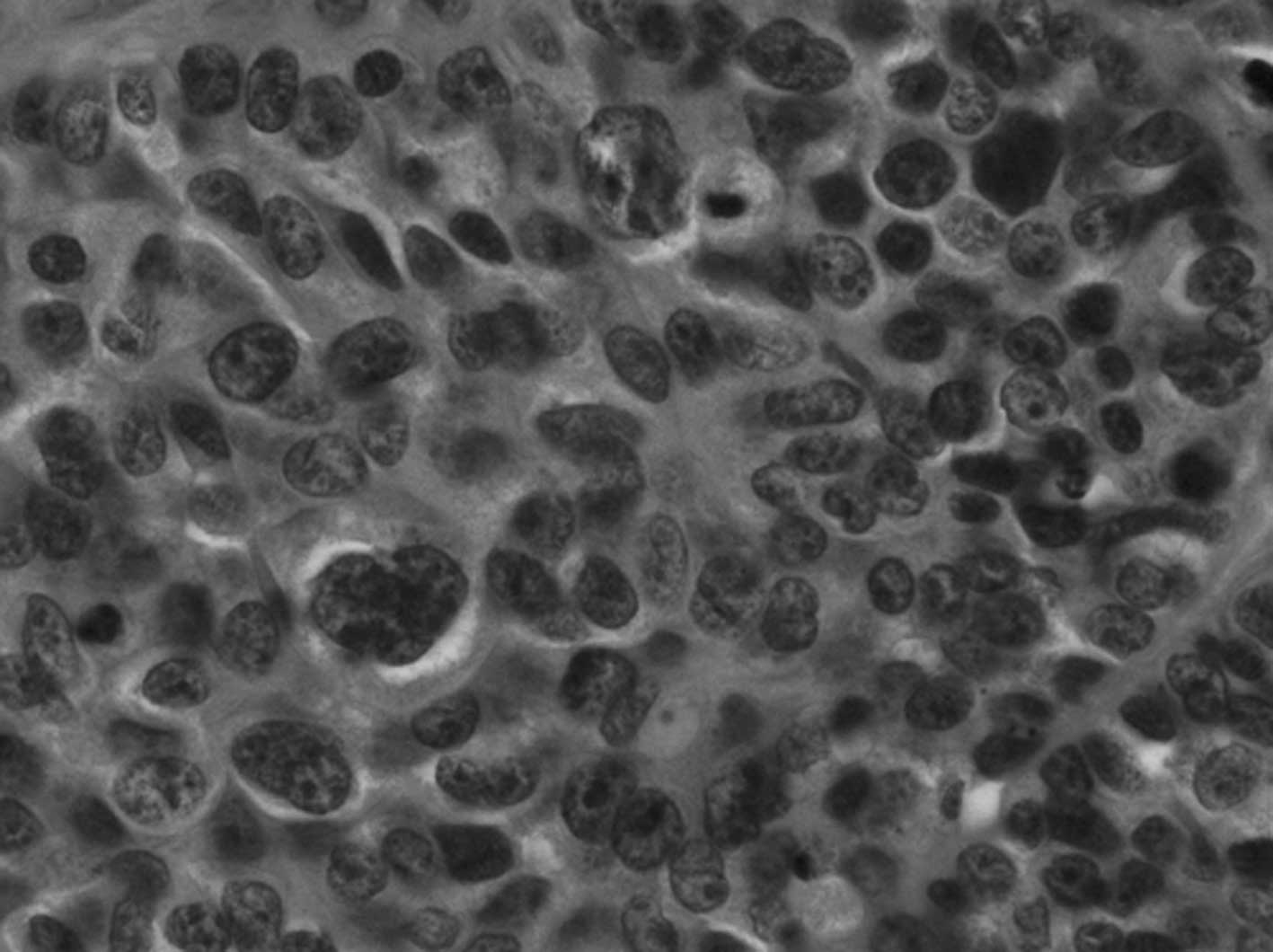
findings on microscopic examination of the H&E-stained slides
(Fig. 1A–D). Sheet or nest
formations were observed, which had central coagulative necrosis
with scanty stroma or massive hemorrhage surrounding the nest.
Tumor cells were arranged in a palisading pattern at the periphery
of the nests, and frequently showed rosette-like formation
(Fig. 2A and B). The tumor cells
had large round-to-oval nuclei, sometimes with prominent nucleoli,
granular or coarse chromatin and relatively abundant basophilic
cytoplasm. In particular, the nuclear polymorphism in Case 3 was
very strong with high mitotic activity. In addition, adenocarcinoma
components were observed adjacent to the LCNEC component. For
example, Case 2 showed well differentiated endometrioid
adenocarcinoma, Case 3 had poorly differentiated endometrioid
adenocarcinoma with squamous differentiation and mucinous
adenocarcinoma, and Case 4 had poorly differentiated endometrioid
adenocarcinoma. At the time of diagnosis, these H&E findings
led us to initially doubt that the tumors were LCNECs; however, we
finally defined the tumors more decisively based on positive
immunohistochemical results for neuroendocrine markers, such as
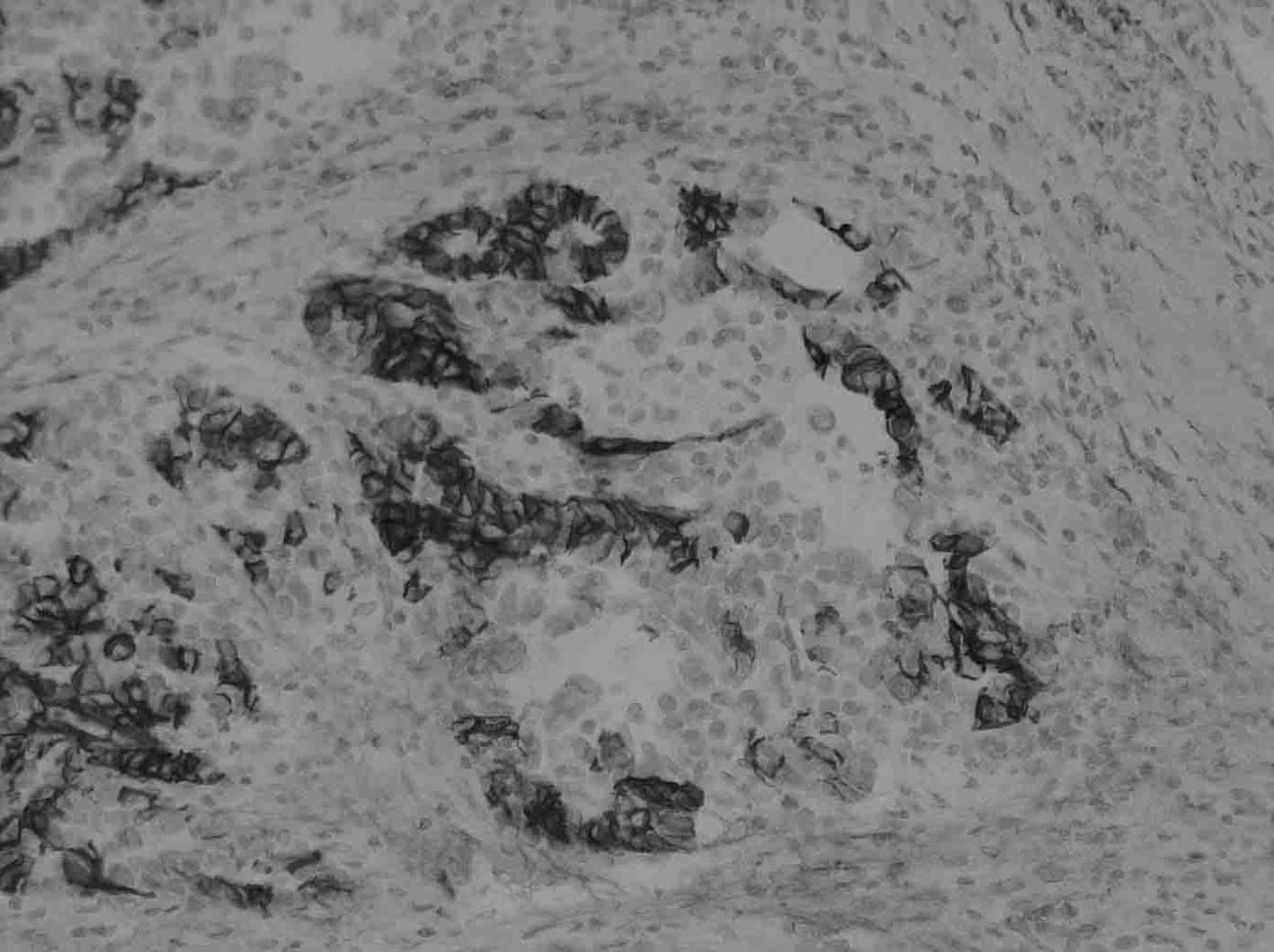
CD56, chromogranin A and synaptophysin. In the LCNEC component, 3
of 4 cases (75%) were partially or diffusely positive for CD56, 3
of 4 cases (75%) were partially positive for chromogranin A, and
all of the cases were negative for synaptophysin (Fig. 3A and B). On the other hand, in the
adjacent carcinoma component, a similar staining pattern was
observed compared to the LCNEC component.
Clinicopathological features of LCNEC –
literature review
The clinicopathological summary of the 33 LCNEC
cases is presented in Table I
(4–17). The median age of the patients was
55 years (range 22–81). The FIGO surgical stages were: 15 stage I,
3 stage II, 8 stage III, 6 stage IV and 1 unknown stage. There was
no laterality, and the median tumor diameter was 14 cm (range
5–30). The CA125 level was elevated in 11 cases, including our 4
cases, similar to what is usually found in ovarian cancer (4,6,9,10,14,16,17).
The epithelial components in 29 cases of ovarian LCNEC, excluding
the 4 pure type LCNEC cases, were as follows: 17 cases (56.7%) were
mucinous tumors (benign, borderline malignancy and malignancy), 8
cases (26.7%) were endometrioid adenocarcinomas, 3 cases (10%) were
mature cystic teratomas, 2 cases (6.7%) were adenocarcinomas, not
otherwise specified, 2 cases (6.7%) were serous adenocarcinomas and
1 case (3.3%) was a benign ovarian cyst (4–14).
In the 21 cases described for which the patients had undergone
surgical treatment, 16 (76.2%) cases had complete surgery, 4
(19.0%) had optimal surgery and 1 (4.8%) had suboptimal surgery.
Platinum-based chemotherapy was performed in most of the cases. The
most popular recurrence sites were both pelvis ± abdomen and liver,
found in 5 out of the 11 cases (45.4%) described in the literature.
The next most popular sites were as follows: lymph nodes (4 out of
11 cases, 36.4%), brain (3 out of 11 cases, 27.2%) and bone (2 out
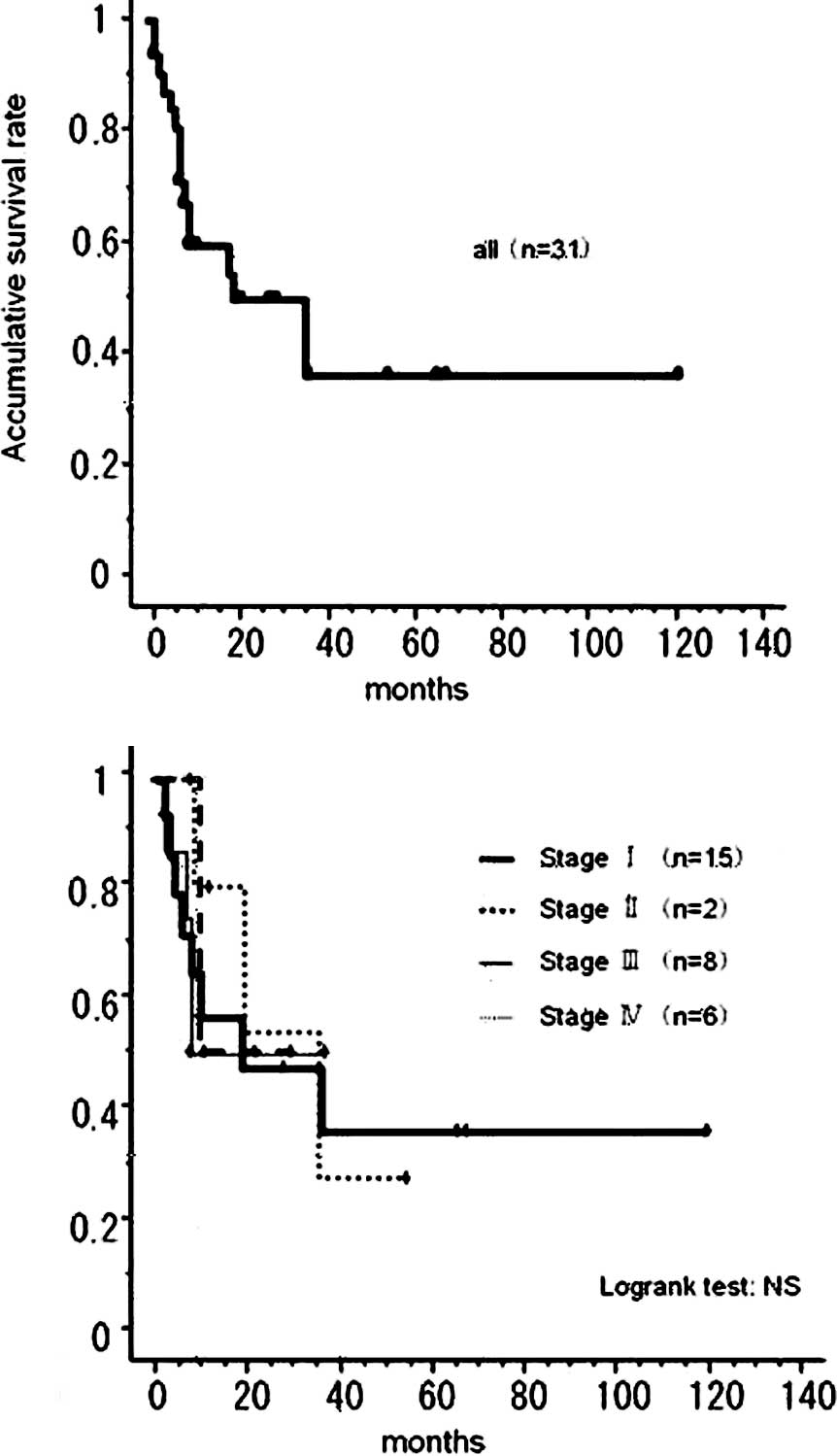
of 11 cases, 18.2%). A Kaplan-Meier survival curve of the 33 cases
is shown in Fig. 4. Twelve
patients died within 12 months, such that the total 5-year survival
was 34.9%, a value that was 35.3% even in stage I patients.
 | Table IClinicopathological features of 33
LCNEC cases. |
Table I
Clinicopathological features of 33
LCNEC cases.
| Ref. | Age (years) | FIGO stage | Findings | Tumor markers | Epithelial
componenta | Surgeryb | Completion of
surgery | Chemotherapyc | Recurrence | Outcomed |
|---|
| (4) | 34 | Ic | Left, 16×11×8 cm | CA125, 591.8
U/ml | Mucinous cystadenoma
(BLM) and mucinous adenocarcinoma | TAH/BSO/OM | Complete | CP | Liver, brain, PAN,
pelvis | DOD (8 months) |
| (5) | 22 | Ia | Right, 2,100 g,
21×15×12 cm | Unclear | Mucinous cystadenoma
(BLM) and mucinous adenocarcinoma | RSO/AP | Complete | CP (J) | Liver | DOD (3 months) |
| (6) | 65 | Ia | Left, 1,086 g,
16.5×13.6×9 cm | CA125, 215 U/ml | Mucinous
cystadenoma |
TAH/BSO/LNBx2OM/AP | Complete | No | Liver, abdomen | DOD (10 months) |
| (7) | 77 | Ia | 15 cm | Unclear | Endometrioid
adenocarcinoma (G1/3) | TAH/BSO/LN/peritoneal
Bx | Complete | Refused | Abdomen, bone,
lung | DOD (19 months) |
| (7) | 36 | Ia | Right, 10 cm | Unclear | Mucinous adenoma
(G2/3) | RSO/AP, prior
TAH | Complete | Yes | | Recent |
| (7) | 45 | Ib | Right, 18 cm | Unclear | Mucinous cystadenoma
(BLM) and partial intraepithelial carcinoma | TAH/BSO/OM | Complete | Yes | Unclear | DOD (36 months) |
| Left
micrometastasis |
| (7) | 68 | IIa | Right, 9 cm | Unclear | Mucinous
adenocarcinoma (G2/3) | TAH/LSO/OM/peritoneal
Bx | Complete | Unclear | Unclear | LFT ? |
| Left tubal
metastasis |
| (7) | 58 | IIIb | Left, 30 cm,
appendix, peritoneal disseminations | Unclear | Mucinous cystadenoma
(BLM) and partial intraepithelial carcinoma |
TAH/BSO/OM/AP/LN/peritoneal Bx | Optimal | Yes | Unclear | DOD (8 months) |
| (8) | 73 | IIIc | Left, 309 g, 11×10×7
cm, peritoneal disseminations, lymphadenopathy | Unclear | Microinvasive
mucinous adenocarcinoma | BSO/OM/LNBx, prior
TAH | Optimal | TP→ADM | Retroperitoneal
space, bone, liver | DOD (8 months) |
| (8) | 44 | Ia | Right, 24 cm | Unclear | Mucinous
intraepithelial carcinoma | TAH/BSO/OM | Complete | TC | Retroperitoneal
space | DOD (4 months) |
| (9) | 33 | Ic | Left, 11 cm | CA19-9, 4303
U/ml
CA125, 4681 U/ml
Ca, 17.3 mg/dl | Endometrioid
adenocarcinoma (G1) | LSO/ROVBx/pOM | Complete | CPT-11/Ned | PAN | DOD (6 months) |
| (10) | 56 | IIc | Right, 2,050 g, 18
cm | CA125, 190
U/ml
CA72-4, 21 U/ml | Mucinous
adenocarcinoma and teratoma |
TAH/BSO/peritonectomy/PLA | Complete | Refused | Unclear | DOD (10 months) |
| (10) | 35 | Ic | | Unclear | Mucinous adenoma | TAH/BSO/OM | Complete | CDDP (iP), HDC | None | NED (36
months) |
| (11) | 27 | Ia | Left, 1,310 g,
17×15.8×7.5 cm | Unclear | N/A |
LSO/ROVBx/pOM/AP/PALA/peritoneal Bx | Complete | TC | None | NED (10
months) |
| (12) | 31 | Unclear | 15×12×11 cm | Unclear | Mucinous
adenoma | TAH/BSO | Unclear | Unclear | Unclear | Unclear |
| (13) | 71 | IIIb | Right, 6.5×5.0×4.5
cm, peritoneal disseminations | Unclear | Serous
carcinoma | TAH/BSO | Optimal | TC | None | NED (8 months) |
| (14) | 64 | Ia | Right, 780 g, 14
cm | CA125, 380
U/ml
CEA, 36 ng/ml | N/A | TAH/BSO/OM | Complete | BEP | None | NED (9 months) |
| (15) | 39 | IV | Left, 26 cm | Unclear | Mucinous
adenocarcinoma | TAH/BSO | Unclear | Pt-based CT | Unclear | AWD (8 months) |
| Right ovarian
metastasis |
| (15) | 55 | I | Right, 26 cm | Unclear | Mucinous LMP with
intraepithelial carcinoma | TAH/BSO | Unclear | Pt-based CT | None | NED (68
months) |
| (15) | 55 | I | Right, 26 cm | Unclear | Mucinous LMP with
intraepithelial carcinoma | TAH/BSO | Unclear | Pt-based CT | None | NED (68
months) |
| (15) | 42 | IV | Unclear | Unclear | Benign cyst and
teratoma in contralateral ovary | TAH/BSO | Unclear | Pt-based CT | Unclear | DOD (20
months) |
| (15) | 53 | III | Left, 14.5 cm | Unclear | Endometrioid
adenocarcinoma | TAH/BSO | Unclear | Pt-based CT | None | NED (37
months) |
| (15) | 47 | III | Right, 14 cm | Unclear | Adenocarcinoma, NOS
and teratoma | TAH/BSO | Unclear | Pt-based CT | None | NED (11
months) |
| (15) | 25 | IV | Right, 5 cm | Unclear | Mature cystic
teratoma | BSO/OM/AP | Unclear | Pt-based CT | Unclear | DOD (36
months) |
| (15) | 55 | III | Right, 13.5 cm | Unclear | Mucinous LMP | TAH/BSO | Unclear | Pt-based CT | Unclear | DOD (2 months) |
| (15) | 54 | I | Right, 14 cm | Unclear | Mucinous carcinoma,
endometrioid adenocarcinoma | TAH/BSO | Unclear | Pt-based CT | None | NED (66
months) |
| (15) | 63 | IV | Right, 14 cm | Unclear | Endometrioid
adenocarcinoma | TAH/RSO | Unclear | Pt-based CT | Unclear | DOD (9 months) |
| (15) | 59 | I | Left, 14 cm | Unclear | High-grade
adenocarcinoma, NOS | BSO | Unclear | Pt-based CT | None | NED (28
months) |
| (16) | 73 | IV | Left, 9×7×7 cm | CA125, 94
U/ml
CA19-9, 133 U/ml
NSE, 23 ng/ml | N/A |
TAH/BSO/OM/Nephrectomy
Resection of brain metastasis | Complete | TC→γ-knife | Brain | NED (12
months) |
| (17) | 68 | IIIc | 18 cm | CA125, 1,235
U/ml | Serous
carcinoma | IDS: BSO/OM | Suboptimal | TC→Doxil | Abdomen | DOD (7 months) |
| Right 7 cm, left 5
cm after NAC |
| Case 1 | 64 | IV | Right, 11 cm | CA125, 6,595
U/ml
CA19-9, 7.9 U/ml
CEA, 1.0 ng/ml | N/A | IDS:
BSO/TAH/OM/Bx | N/A | TC (as NAC) | Brain | NED (64
months) |
| Case 2 | 80 | IIc | Left, 7 cm | CA125, 204.3
U/ml
CA19-9, 22.1 U/ml
CEA, 2.4 ng/ml | Endometrioid
adenocarcinoma |
TAH/BSO/PLA/OM/AP | Complete | TC | None | NED (40
months) |
| Case 3 | 65 | Ic | Left, 15 cm | CA125, 77.0
U/ml
CA19-9, 776.5 U/ml
CEA, 1.7 ng/ml | Endometrioid
adenocarcinoma with squamous differentiation mucinous
adenocarcinoma | TAH/BSO/OM | Complete | TC | Pelvis, liver,
lymph node | DOD (2 months) |
| Case 4 | 42 | IIIb | Left, 13 cm | CA125, 775.2
U/ml
CA19-9, 7.0 U/ml
CEA, 0.4 ng/ml | Endometrioid
adencarcinoma |
TAH/BSO/PLA/PALA/OM | Optimal | TC | None | NED (32
months) |
| Rt ovarian
metastasis, peritoneal disseminations |
Discussion
Only 33 primary ovarian LCNEC cases have been
reported in the literature, including our 4 cases. LCNEC is a
relatively new classification, and gynecologic oncologists are
still not familiar with its name. The most difficult differential
diagnoses are thought to be poorly differentiated carcinoma and
undifferentiated carcinoma. In diagnosing LCNEC, both the presence
of an epithelial component and morphological neuroendocrine
differentiation, such as rosette formation, are useful. In the
confusing case of distinguishing LCNEC from poorly differentiated
adenocarcinoma, it is first necessary to doubt in favor of LCNEC by
characteristic H&E findings, and then to confirm its diagnosis
through positive immunohistochemical results for neuroendocrine
markers (3). On the other hand, in
a case that lacks an epithelial component, undifferentiated
carcinoma is the most important differential diagnosis. Although
LCNEC is synonymous with undifferentiated carcinoma of the
non-small cell neuroendocrine type according to the WHO
classification (1), we posit that
finding morphologic neuroendocrine differentiation, such as
rosette-like formation, requires classification as LCNEC. Of
course, it is important that secondary ovarian LCNEC from the lung
is excluded clinically. In Case 1, we made the clinical diagnosis
of ovarian, rather than lung LCNEC because of both multiple nodules
and the larger size of the ovarian tumor nodules.
Ovarian LCNEC is also characterized by the presence
of surface epithelial-stromal tumors with benign, borderline or
malignant behavior, and the presence of only LCNEC components at
the metastatic site (1,9). In fact, both metastatic brain tumors
in Case 1 and the widely disseminated tumor to the abdominal cavity
in Case 4 were diagnosed not as epithelial, but LCNEC components.
Mucinous tumors and endometrioid adenocarcinoma were obviously
predominant. However, in 2 cases with serous adenocarcinoma, both
Draganova-Tacheva et al (17) and Choi et al (13) concluded that each LCNEC component
and serous adenocarcinoma component had different origins, meaning
they arose independently, on the basis of the immunohistochemical
pattern or microsatellite instability (MSI) analysis. Notably,
neuroendocrine markers, such as chromogranin, synaptophysin and
NSE, were detected in the epithelial component as well as in the
LCNEC component in the same patient by immunohistochemistry
(4,5,7–9,17).
These results are shown in Table
II. This suggests that the LCNEC and epithelial components have
similar biological characteristics, even though they differ in
regards to morphology.
 | Table IIImmunohistochemical analysis of 33
LCNEC cases. |
Table II
Immunohistochemical analysis of 33
LCNEC cases.
| Ref. | LCNEC component
| Epithelial
component
|
|---|
| Epithelial
markera | Neuroendocrine
markersb | Epithelial
markera | Neuroendocrine
markersb |
|---|
|
|
|
|
|---|
| CK | CD56 | CG | SP | NSE | CK | CD56 | CG | SP | NSE |
|---|
| (4) | + | N/A | + | N/A | ± | + | N/A | + | N/A | N/A |
| (5) | + | − | + | + | N/A | + | N/A | + | N/A | N/A |
| (6) | + | N/A | + | + | ± | N/A | N/A | N/A | N/A | N/A |
| (7) | + | N/A | + | N/A | + | + | N/A | ± | N/A | + |
| (7) | + | N/A | + | N/A | + | + | N/A | + | N/A | ± |
| (7) | + | N/A | + | N/A | + | + | N/A | + | N/A | + |
| (7) | + | N/A | + | N/A | + | + | N/A | + | N/A | + |
| (7) | + | N/A | + | N/A | + | + | N/A | + | N/A | + |
| (8) | + | N/A | + | + | N/A | + | N/A | + | − | N/A |
| (8) | + | N/A | + | + | N/A | + | N/A | + | − | N/A |
| (9) | N/A | N/A | + | + | + | N/A | N/A | ± | − | − |
| (10) | N/A | N/A | + | + | + | N/A | N/A | N/A | N/A | N/A |
| (10) | N/A | N/A | + | ± | + | N/A | N/A | N/A | N/A | N/A |
| (11) | + | N/A | + | N/A | N/A | | Absence of
epithelial component |
| (12) | + | N/A | + | + | N/A | N/A | N/A | N/A | N/A | N/A |
| (13) | ± | + | + | + | N/A | + | − | − | − | N/A |
| (14) | + | + | + | + | + | | Absence of
epithelial component |
| (15) | + | + | + | + | N/A | N/A | N/A | N/A | N/A | N/A |
| (15) | N/A | N/A | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| (15) | + | + | + | + | N/A | N/A | N/A | N/A | N/A | N/A |
| (15) | + | N/A | + | + | N/A | N/A | N/A | N/A | N/A | N/A |
| (15) | + | + | − | + | N/A | N/A | N/A | N/A | N/A | N/A |
| (15) | + | − | ± | ± | N/A | N/A | N/A | N/A | N/A | N/A |
| (15) | ± | N/A | − | N/A | + | N/A | N/A | N/A | N/A | N/A |
| (15) | + | − | + | + | N/A | N/A | N/A | N/A | N/A | N/A |
| (15) | + | − | − | ± | N/A | N/A | N/A | N/A | N/A | N/A |
| (15) | + | + | ± | + | N/A | N/A | N/A | N/A | N/A | N/A |
| (16) | + | + | + | + | N/A | | Absence of
epithelial component |
| (17) | ± | N/A | + | + | N/A | + | N/A | − | − | N/A |
| Case 1 | N/A | − | ± | − | N/A | | Absence of
epithelial component |
| Case 2 | N/A | + | − | − | N/A | N/A | + | − | − | N/A |
| Case 3 | N/A | + | ± | − | N/A | N/A | + | ± | + | N/A |
| Case 4 | N/A | ± | ± | − | N/A | N/A | ± | − | − | N/A |
The prognosis of ovarian LCNEC is recognized to be
extremely poor (1); our survey
also revealed that the total 5-year survival was 34.9%, and still
only 35.3% for stage I cases, even though in over 95% of these
cases complete or optimal surgery was performed. There was such a
high incidence in recurrence not only to the abdominal cavity, but
also to specific sites that differed from the usual ovarian cancer
distribution. This suggests that LCNEC may have strong
lymph-vascular space invasion, which contributes to its poor
prognosis. Case 3 showed extremely rapid progression with pelvic
mass formation, liver metastasis and pelvic lymphadenopathy within
only 2 weeks after primary surgery; moreover, TC chemotherapy was
not effective in this patient. Similar cases have been previously
reported (4,5,8) and
provide evidence that ovarian LCNEC is a malignant neoplasm with
aggressive behavior.
However, we found that, except for the
aforementioned aggressive case, our other cases were sensitive to
TC chemotherapy and have to date demonstrated relatively good
outcomes. Veras et al conducted a study at the MD Anderson
Cancer Center and reported that 3 stage I cases of LCNEC acquired
long-term survival for 22–68 months, and even stage III or IV cases
revealed similar prognosis with standard surgery that was followed
by adjuvant platinum-based chemotherapy (15). As a result, we suggest that most
LCNEC cases are as chemotherapy-sensitive as common ovarian cancer.
When these tumors are poorly responsive to TC chemotherapy,
second-line chemotherapy regimens, such as cisplatin/vinorelbine,
cisplatin/etoposide, cisplatin/vinblastine, cisplatin/gemcitabine
and cisplatin/docetaxel, should be taken into consideration
according to the National Comprehensive Cancer Network (NCCN)
guidelines for primary lung LCNEC (18). Moreover, to our surprise, in 2
LCNEC cases that included brain metastasis, both tumor resection
and adjuvant radiotherapy resulted in patient survival, to date
(16). Thus, in cases of local
recurrence, the combination of chemotherapy with aggressive surgery
and adjuvant radiation therapy should be taken into consideration
as a possible treatment strategy.
In summary, only 33 ovarian LCNEC cases have been
reported to date. The term LCNEC has been unfamiliar to gynecologic
oncologists not only in Japan, but also worldwide, and its criteria
for diagnosis may be considered vague. As a result, certain LCNEC
cases may have been inaccurately classified as undifferentiated or
poorly differentiated adenocarcinoma. Generally, the prognosis of
LCNEC has been recognized as extremely poor owing to its
biologically aggressive behavior. However, we suggest that some
ovarian LCNECs reveal more favorable prognosis than previously
reported, particularly because of their ordinary chemo-sensitivity.
A retrospective survey to elucidate the prognostic factors and
prospective clinical studies to evaluate the efficacy of treatment
modalities of ovarian LCNEC are necessary, particularly for
aggressive LCNEC cases.
References
|
1.
|
Tavassoli FA and Devilee P: World Health
Organization Classification of Tumours. Pathology and Genetics of
Tumours of the Breast and Female Genital Organs. IARC Press; Lyon:
2003
|
|
2.
|
Scully RE, Young RH and Clement PB: Atlas
of Tumor Pathology. Tumors of the Ovary, Maldeveloped Gonads,
Fallopian Tube, and Broad Ligament. Armed Forces Institute of
Pathology; Washington, DC: 1996
|
|
3.
|
Travis WD, Brambilla E, Muller-Hermelink
HK, et al: World Health Organization Classification of Tumours.
Pathology and Genetics of Tumours of the Lung, Pleura, Thumus and
Heart. IARC Press; Lyon: 2004
|
|
4.
|
Collins RJ, Cheung A, Ngan HY, et al:
Primary mixed neuroendocrine and mucinous carcinoma of the ovary.
Arch Gynecol Obstet. 248:139–143. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Khurana KK, Tornos C and Silva EG: Ovarian
neuroendocrine carcinoma associated with a mucinous neoplasm. Arch
Pathol Lab Med. 118:1032–1034. 1994.PubMed/NCBI
|
|
6.
|
Jones K, Diaz JA and Donner LR:
Neuroendocrine carcinoma arising in an ovarian mucinous
cystadenoma. Int J Gynecol Pathol. 15:167–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Eichhorn JH, Lawrence WD, Young RH, et al:
Ovarian neuroendocrine carcinomas of non-small-cell type associated
with surface epithelial adenocarcinomas. A study of five cases and
review of the literature. Int J Gynecol Pathol. 15:303–314. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chen KT: Composite large-cell
neuroendocrine carcinoma and surface epithelial-stromal neoplasm of
the ovary. Int J Surg Pathol. 8:169–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ohira S, Itoh K, Shiozawa T, et al:
Ovarian non-small cell neuroendocrine carcinoma with paraneoplastic
parathyroid hormone-related hypercalcemia. Int J Gynecol Pathol.
23:393–397. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hirasawa T: Ovarian neuroendocrine
carcinoma associated with mucinous carcinoma and teratoma. Nippon
Rinsho. 62:973–978. 2004.PubMed/NCBI
|
|
11.
|
Behnam K, Kabus D and Behnam M: Primary
ovarian undifferentiated non-small cell carcinoma, neuroendocrine
type. Gynecol Oncol. 92:372–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ahmed Z, Aftab K and Kayani N: Ovarian
primary neuroendocrine carcinoma of non-small cell type: report of
an extremely rare neoplasm. J Pak Med Assoc. 55:82–84.
2005.PubMed/NCBI
|
|
13.
|
Choi YD, Lee JS, Choi C, et al: Ovarian
neuroendocrine carcinoma, non-small cell type, associated with
serous carcinoma. Gynecol Oncol. 104:747–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lindboe CF: Large cell neuroendocrine
carcinoma of the ovary. APMIS. 115:169–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Veras E, Deavers MT, Silva EG, et al:
Ovarian nonsmall cell neuroendocrine carcinoma: a clinicopathologic
and immunohistochemical study of 11 cases. Am J Surg Pathol.
31:774–782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Dundr P, Fischerová D, Povýsil C, et al:
Primary pure large-cell neuroendocrine carcinoma of the ovary.
Pathol Res Pract. 204:133–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Draganova-Tacheva RA, Khurana JS, Huang Y,
et al: Large cell neuroendocrine carcinoma of the ovary associated
with serous carcinoma with mucin production: a case report and
literature review. Int J Clin Exp Pathol. 2:304–309.
2009.PubMed/NCBI
|
|
18.
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN guidelines TM for Non-Small Cell Lung Cancer).
(http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdfuri).
|