Introduction
Pleural effusion is one of the most common clinical
manifestations of pleural diseases (1–3).
According to clinical risk factors and prognosis, pleural effusion
can be divided into two categories, benign and malignant (4). The most common cause of benign
pleural effusion is tuberculosis (TB), and that of malignant
pleural effusion (MPE) is lung and breast cancer (5). Due to different risk factors and
prognosis, it is necessary to differentiate between them. However,
this remains a major clinical problem. Although the presence of
tumor cells in pleural effusion is a diagnostic marker of MPE, the
probability of finding them is low. For cytology-negative pleural
effusion, some of the currently used indices, such as lactate
dehydrogenase (LDH), adenosine dehydrogenase (ADA) and
carcinoembryonic antigen (CEA), have a certain extent of
differential value, however, their specificity and sensitivity are
limited. Due to the fear of possible trauma caused by thoracoscopy,
some patients are not keen to agree on such a procedure. Therefore,
searching for new indices is very important.
The improved understanding of pleural effusion
immunopathogenesis could lead to the development of new
immunodiagnostic tools to facilitate its differential diagnosis.
The main cellular components in both tuberculous pleural effusions
(TPEs) and MPEs are lymphocytes. Previous research data have
reported that lymphocytes play an important regulatory role in the
pathogenesis of pleural effusion (1,2).
Inflammation leads to the accumulation of lymphocytes in the
pleural cavity, which release a variety of mediators and cytokines
influencing pleural capillary permeability, resulting in pleural
effusion (6,7). Large scale studies have reported that
CD4+ T lymphocytes play an important role in the
pathogenesis of pleural effusion (7). CD4+ T cells can be
differentiated into Th1, Th2, Th17 and Treg cells. Th17 cells,
which form a distinct subset of T helper cells, produce unique
cytokines, including interleukin (IL)-17A, IL-17F and IL-22. These
cytokines stimulate defensin production and the recruitment of
neutrophils and monocytes at the site of inflammation. They are
also involved in the early phase of host defence (8–12).
Wang et al revealed that Th17 cells can be found in the
pleural effusion of patients with TB, suggesting their potential
role in immunity against Mycobacterium tuberculosis
(13).
Certain research data have shown that Th17 cells are
one of the major sources of IL-22. IL-22 is a member of the IL-10
family and is mainly expressed in activated T and natural killer
cells. Its biological targets are epithelial or parenchymal cells
in the gut, lungs, skin and kidneys (14–16).
In pancreatitis, psoriasis, inflammatory bowel disease, asthma and
other inflammatory diseases, IL-22 may play an important regulatory
role (17–20). Previous studies have reported that
in TPE and MPE, the levels of IL-22 are high (21,22).
However, whether there is a significant difference in their
expression levels, and whether we could distinguish them based on
this difference, remains unknown.
Based on the above problems, the idea was to collect
samples of TPE and MPE, determine the expression of IL-22 by ELISA,
and explore its value in the differential diagnosis between TPE and
MPE.
Materials and methods
Pleural effusion samples
In this study, samples of pleural effusions were
collected from 56 patients who were hospitalized in the Department
of Respiratory Medicine, Tongji Hospital Affiliated to Tongji
Medical College, Huazhong University of Science and Technology,
between April 2009 and May 2011. Written consent was obtained from
all the patients concerned in order to perform this study. Pleural
effusions were divided into TPE (28 cases; 24 males and 4 females;
41.46±3.34 years of age) and MPE groups (28 cases; 10 males and 14
females; 58.71±2.1 years of age).
Diagnostic criteria for pleural
effusions
The pleural effusions were firstly diagnosed as
exudates using Light's criteria. The diagnostic criteria for MPE
were: Cytological evidence of malignant cells present in pleural
effusion or from biopsies taken. TPE was diagnosed according to the
following principle: Identification of M. tuberculosis,
pleural biopsy revealing granulomatous tissue, positive PPD test
and positive response to anti-TB treatment.
Samples collection
Pleural effusions were collected before any
treatment was initiated within 24 h after hospitalization. Some of
the pleural fluids were sent to the hospital laboratory to detect
levels of total protein (Pro), LDH, ADA and CEA. Some other pleural
effusions (100 ml) were centrifuged at 4°C 1200 r/min for 15 min,
and the supernatants were immediately frozen with 500 μl Ep tubes
at −80°C.
Measurement of IL-22
The concentration of IL-22 in pleural effusions was
measured by the enzyme-linked immunosorbent assay kit (ELISA)
according to the manufacturer's protocol (Bender, Austrilian). All
samples were assayed in duplicate.
Statistical analysis
Data were expressed as the means ± SEM. Difference
in data was analyzed by the Student's t-test or the χ2
test, using receiver operating curve (ROC) analysis to evaluate the
threshold value of IL-22 and CEA in differentiating TPE from MPE.
For each ROC, a cut-off point was determined as the value of the
parameter that maximized the sum of specificity and sensitivity. A
value of P<0.05 was considered significant. Statistical analysis
was carried out using SPSS 17.0 software.
Results
General characteristics of the pleural
effusions
Significant differences were observed in age, as
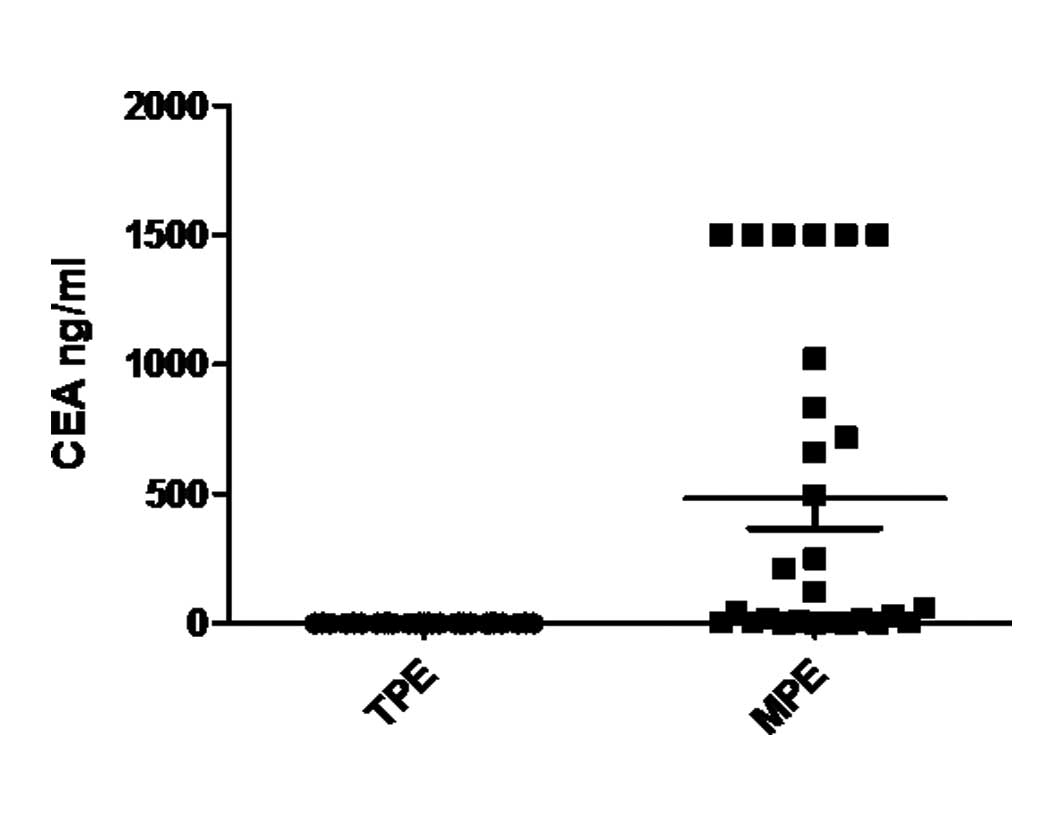
well as CEA and ADA levels in TPE and MPE (P<0.01). We did not
find a significant difference in the concentration of Pro and LDH
between TPE and MPE (P>0.05, Table
I).
 | Table IDescriptive statistics of each pleural
effusion group. |
Table I
Descriptive statistics of each pleural
effusion group.
| Groups | TPE (n=28) | MPE (n=28) |
|---|
| Age | 41.46±3.34a | 58.71±2.10 |
| Gender
(male/female) | 24/4 | 10/18 |
| CEA (ng/ml) | 1.29±0.11a | 482.86±115.26 |
| Pro (g/l) | 48.47±1.52 | 44.14±1.71 |
| LDH (U/l) | 317.86±36.54 | 557.25±117.35 |
| ADA (IU/l) | 54.75±5.03a | 23.45±6.40 |
IL-22 concentration in pleural
effusions
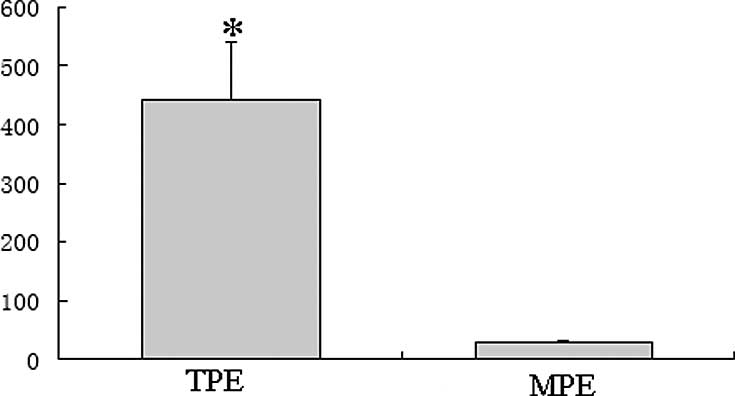
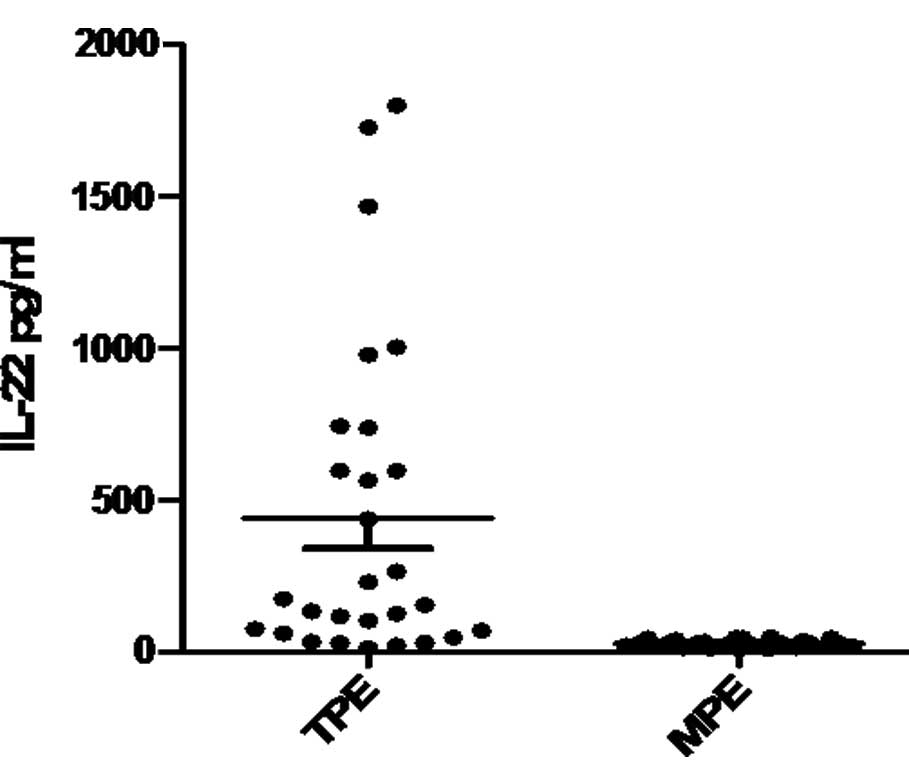
As shown in Fig. 1,
the concentration of IL-22 in the TPE group (441.91±99.34 pg/ml)
was significantly higher compared to the MPE group (29.81±2.15
pg/ml; P<0.01).
Diagnostic value of IL-22 in TPE and
MPE
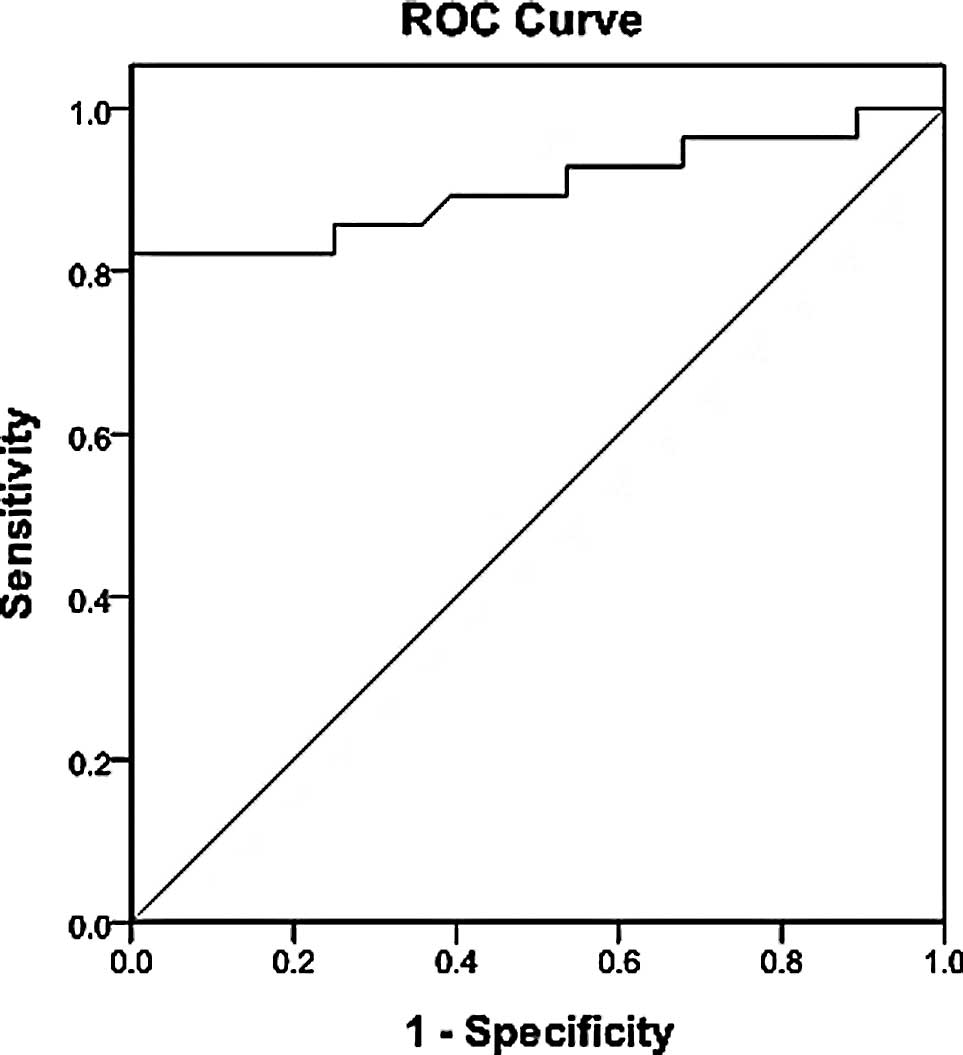
The diagnostic threshold afforded by the ROC
analysis for IL-22 was 49 pg/ml (Fig.
2). The area under the IL-22 ROC was 0.902. Using a threshold
value of 49 pg/ml, IL-22 had a sensitivity of 82.14% (23/28), a
specificity of 96.43% (27/28), an accuracy of 89.29% (50/56), a
positive predictive value of 95.8% (23/24) and a negative
predictive value of 84.4% (27/32) (Fig. 3).
Diagnostic value of combined detection of
IL-22 and CEA in TPE and MPE
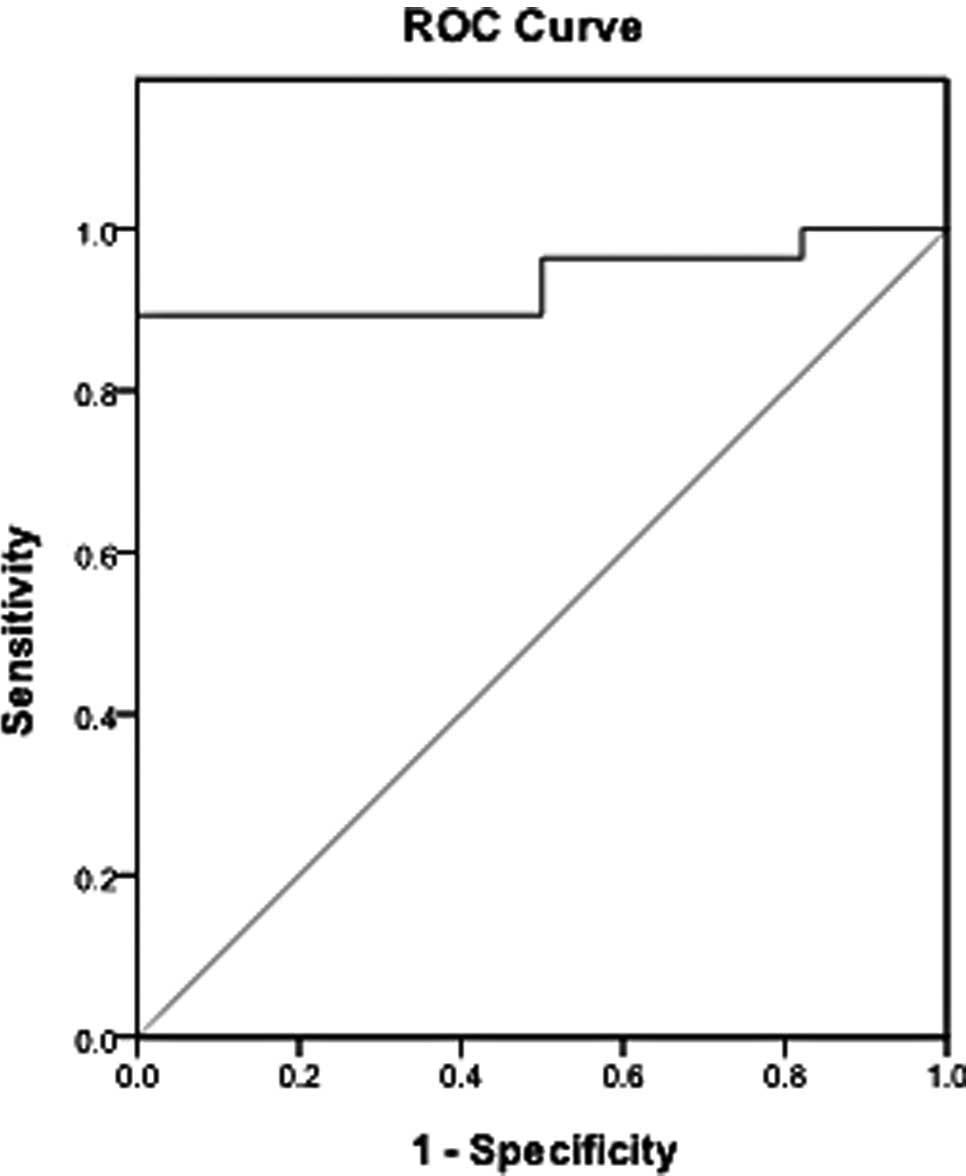
Firstly, CEA levels were detected and the diagnostic
value in TPE and MPE was analyzed. The diagnostic threshold
afforded by the ROC analysis for CEA was 2.42 ng/ml. The area under
the CEA ROC was 0.935. It was higher compared to the areas of IL-22
(Fig. 4). With a threshold value
of 2.42 ng/ml, CEA had a sensitivity of 89.3% (25/28), a
specificity of 96.43% (27/28), an accuracy of 92.86% (52/56), a
positive predictive value of 96.15% (25/26) and a negative
predictive value of 90% (27/30) (Fig.
5). The sensitivity of IL-22 was lower compared to CEA. Between
the studied parameters, IL-22 and CEA, no significant differences
were found with respect to the specificity.
The combined diagnostic value of IL-22 and CEA in
TPE and MPE was further detected. The results showed that the
combined detection of these two indices had a sensitivity of 100%
(28/28) and a specificity of 96.43% (27/28). The sensitivity was
higher compared to the two separate tests for TPE and MPE. However,
no significant differences were found with respect to
specificity.
Discussion
In this study, the concentration of IL-22 was
significantly increased in TPE and MPE, similar to results from
other studies (22).
Simultaneously, we found that IL-22 levels were significantly
higher in TPE compared to MPE, suggesting more pleural sources of
IL-22 in TB patients and a high differential diagnostic value of
IL-22 among TPE and MPE. Using the combined detection of IL-22 and
CEA, the diagnostic value was more accurate compared to the single
index.
Clinically, the presence of tumor cells in pleural
effusion is the gold standard for MPE. Nevertheless, for
cytology-negative effusion, the main problem was to differentiate
benign pleural effusion from MPE and to decide on further
treatment. In our results, we found that certain indices, such as
LDH and Pro in pleural effusion had no significant differential
values. However, CEA and IL-22 expression in TPE and MPE was
significantly different, which might be helpful in distinguishing
between them.
Reports regarding the association of IL-22
expression with pleural effusions are few and varied. Qiao et
al reported that the expression of IL-22 was significantly
increased in TPE (21). Zhang
et al also demonstrated this view. However, they found that
IL-22 levels in MPE were higher compared to TPE (22). This differs from our results. Such
a contradiction can be justified and explained by the fact that
more pleural effusion samples were collected and used in our
study.
IL-22 was found in T lymphocytoma by Dumoutier et
al in 1999 (23). IL-22
belongs to IL-10-related cytokines, whose receptor complex consists
of two chains, IL-22R1 and IL-10R2. Aujla et al demonstrated
that IL-22 is a crucial immune mediator produced by T cells and a
critical mediator in mucosal host defense (24). Previous studies have reported that
IL-22 can induce the synthesis of acute phase proteins in acute
hepatitis and pancreatitis (25,17).
Other data have demonstrated that IL-22 can also play an important
regulatory role in the process of asthma and allergic diseases
(26). In addition, IL-22 is also
involved in the pathogenesis of autoimmune diseases. Ikeuchi et
al found that IL-22 promoted the proliferation of synovial
fibroblasts and increased the secretion of various inflammatory
mediators and cytokines, which participate in the pathogenesis of
rheumatoid arthritis (27).
Schmechel et al found that IL-22 serum levels in Crohn's
patients were significantly higher compared to the normal controls
and closely related with disease activity (19). Wolk et al found that the
expression of IL-22 was unusually high in psoriatics patients
(18).
In this study, IL-22 expression was significantly
higher in TPE when compared to MPE, indicating the involvement of
IL-22 in the pathogenesis of pleural effusion. However, the
specific mechanism of IL-22 in the pathogenesis of pleural effusion
was unclear. Th17 cells are a major source of IL-22. In addition to
the traditional Th1 and Th2 subsets, Th17 cells belong to a
recently identified T helper subset. Th17 cells play an important
regulatory role in the pathogenesis of infections and autoimmune
diseases by releasing IL-17A, IL-17F, IL-22 and other cytokines
(16). Therefore, we presumed that
acute or chronic inflammation causes the accumulation of
lymphocytes which release a variety of inflammatory mediators and
cytokines such as IL-22, increasing the pleural capillary
permeability, thus resulting in pleural effusion.
Clinically, although lymphocytic cellular components
are the base of TPE and MPE, the lymphocyte subsets are different.
This causes a difference in the concentration of inflammatory
mediators and cytokines and may explain the higher expression of
IL-22 in TPE found in our study. However the specific role of IL-22
in the pathogenesis of TPE and MPE requires further research.
CEA has been studied extensively and has been found
to have a differential value in distinguishing benign pleural
effusion from MPE. Boucher et al reported that CEA
concentration in malignant tissues was on average 60-fold higher
compared to the non-malignant tissues (28). CEA was one of the most commonly
used clinically identified indices in benign pleural effusion and
MPE. In our study, the diagnostic value of CEA in TPE and MPE was
2.42 ng/ml. The threshold value of the diagnosis of TPE and MPE had
a sensitivity of 89.3% (25/28) and a specificity of (27/28). These
results are consistent with those from the study by Shi et
al (29). In particular, we
discovered that the combined IL-22 and CEA detection had a
sensitivity of 100% (28/28) and a specificity of 96.43% (27/28).
The diagnostic value was higher compared to the single index. These
results may provide a new approach with a higher diagnostic value
in TPE and MPE.
In summary, our results suggest that IL-22 is higher
in TPE compared to MPE. The critical value of IL-22 protein for
diagnosing TPE and MPE was 49 pg/ml. The combined detection of CEA
and IL-22 had a higher sensitivity compared to the single index.
This may effectively improve the diagnostic performance.
Acknowledgements
This study was supported in part by a
research grant from the Hubei Programs for Science and Technology
Development (2002AA301D11).
References
|
1
|
Doelken P: Clinical implications of
unexplainable lung due to pleural disease. Am J Med Sci. 335:21–25.
2008. View Article : Google Scholar
|
|
2
|
Khaleeq G and Musani AI: Emerging
paradigms in the management of malignant pleural effusions. Respir
Med. 102:939–948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Musani AI: Treatment options for malignant
pleural effusion. Curr Opin Pulm Med. 15:380–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spector M and Pollak JS: Management of
malignant pleural effusions. Semin Respir Crit Care Med.
29:405–413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heffner JE: Diagnosis and management of
malignant pleural effusions. Respirology. 13:5–20. 2008.
|
|
6
|
Yang HB and Shi HZ: T lymphocytes in
pleural effusion. Chin Med J (Engl). 121:579–580. 2008.PubMed/NCBI
|
|
7
|
Qin XJ, Shi HZ, Liang QL, Huang LY and
Yang HB: CD4+CD25+ regulatory T lymphocytes
in tuberculous pleural effusion. Chin Med J (Engl). 121:581–586.
2008.
|
|
8
|
Infante-Duarte C, Horton HF, Byrne MC and
Kamradt T: Microbial lipopeptides induce the production of IL17 in
Th cells. J Immunol. 165:6107–6115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4+ T cells regulates tissue inflammation by
producing interleukin 17. Nat Immunol. 6:1133–1141. 2005.
|
|
10
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT:
Interleukin17-producing CD4+ effector T cells develop
via a lineage distinct from the T helper type 1 and 2 lineages. Nat
Immunol. 6:1123–1133. 2005.PubMed/NCBI
|
|
11
|
Harrington LE, Mangan PR and Weaver CT:
Expanding the effector CD4 T-cell repertoire: the Th17 lineage.
Curr Opin Immunol. 18:349–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weaver CT, Harrington LE, Mangan PR,
Gavrieli M and Murphy KM: Th17: an effector CD4 T cell lineage with
regulatory T cell ties. Immunity. 24:677–688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang T, Lv M, Qian Q, Nie Y, Yu L and Hou
Y: Increased frequencies of T helper type 17 cells in tuberculous
pleural effusion. Tuberculosis. 91:231–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolk K, Witte E, Witte K, Warszawska K and
Sabat R: Biology of interleukin-22. Semin Immunopathol. 32:17–31.
2010. View Article : Google Scholar
|
|
15
|
Dumoutier L, Lejeune D, Colau D and
Renauld JC: Cloning and characterization of IL-22 binding protein,
a natural antagonist of IL-10-related T cell-derived inducible
factor/IL-22. J Immunol. 166:7090–7095. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolk K and Sabat R: Interleukin-22: a
novel T and NK cell-derived cytokine that regulates the biology of
tissue cells. Cytokine Growth Factor Rev. 17:367–380. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aggarwal S, Xie MH, Maruoka M, Foster J
and Gurney AL: Acinar cells of the pancreas are a target of
interleukin-22. J Interferon Cytokine Res. 21:1047–1053. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolk K, Witte E, Wallace E, Döcke WD, Kunz
S, Asadullah K, Volk HD, Sterry W and Sabat R: IL-22 regulates the
expression of genes responsible for antimicrobial defense, cellular
differentiation, and mobility in keratinocytes: a potential role in
psoriasis. Eur J Immunol. 36:1309–1323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmechel S, Konrad A, Diegelmann J, Glas
J, Wetzke M, Paschos E, Lohse P, Göke B and Brand S: Linking
genetic susceptibility to Crohn's disease with Th17 cell function:
IL-22 serum levels are increased in Crohn's disease and correlate
with disease activity and IL23R genotype status. Inflamm Bowel Dis.
14:204–212. 2008.
|
|
20
|
Schnyder B, Lima C and Schnyder-Candrian
S: Interleukin-22 is a negative regulator of the allergic response.
Cytokine. 50:220–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiao D, Yang BY, Li L, Ma JJ, Zhang XL,
Lao SH and Wu CY: ESAT-6- and CFP-10-specific Th1, Th22 and Th17
cells in tuberculous pleurisy may contribute to the local immune
response against Mycobacterium tuberculosis infection. Scand J
Immunol. 73:330–337. 2011. View Article : Google Scholar
|
|
22
|
Zhang W, Chen Y, Wei H, Zheng C, Sun R,
Zhang J and Tian Z: Antiapoptotic activity of autocrine
interleukin-22 and therapeutic effects of interleukin-22-small
interfering RNA on human lung cancer xenografts. Clin Cancer Res.
14:6432–6439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dumoutier L, Lejeune D, Colau D and
Renauld JC: Cloning and characterization of IL-10 related T cell
derived inducible factor (IL-TIF), a novel cytokine structurally
related to IL-10 and inducible by IL-9. J Immunol. 164:1814–1819.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aujla SJ and Kolls JK: IL-22: a critical
mediator in mucosal host defense. J Mol Med1. 87:451–454. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dumoutier L, van Roost E, Colau D and
Renauld JC: Human interleukin-10-related T cell-derived inducible
factor: molecular cloning and functional characterization as a
hepatocyte-stimulating factor. Proc Natl Acad Sci USA.
97:10144–10149. 2000. View Article : Google Scholar
|
|
26
|
Kotenko SV, Izotova LS, Mirochnitchenko
OV, Esterova E, Dickensheets H, Donnelly RP and Pestka S:
Identification of the functional interleukin-22 (IL-22) receptor
complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both
the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor,
IL-TIF) receptor complexes. J Biol Chem. 276:2725–2732. 2001.
View Article : Google Scholar
|
|
27
|
Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko
Y, Hiromura K, Ueki K and Nojima Y: Expression of interleukin-22 in
rheumatoid arthritis: potential role as a proinflammatory cytokine.
Arthritis Rheum. 52:1037–1046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boucher D, Cournoyer D, Stanners CP and
Fuks A: Studies on the control of gene expression of the
carcinoembryonic antigen family in human tissue. Cancer Res.
49:847–852. 1989.PubMed/NCBI
|
|
29
|
Shi HZ, Liang QL, Jiang J, Qin XJ and Yang
HB: Diagnostic value of carcinoembryonic antigen in malignant
pleural effusion: a meta-analysis. Respirology. 13:518–527. 2008.
View Article : Google Scholar : PubMed/NCBI
|