Introduction
Previous studies have demonstrated that the major
components of the cholinergic system, such as acetylcholinesterase
(AChE), acetylcholine (ACh) and choline acetyltransferase (ChAT),
are closely associated with important biological events, including
neuronal maturation and plasticity, axon guidance, regulation of
gene expression, and cell survival (1). Brain-derived neurotrophic factor
(BDNF) and nerve growth factor (NGF) are neurotrophic agents that
support the growth, differentiation and survival of neurons
(2). The reciprocal regulation of
NGF and BDNF in hippocampal neurons by cholinergic activity and the
release of ACh from presynaptic nerve terminals by neurotrophins
represent a novel positive mechanism for controlling synaptic
efficiency in the cholinergic system (3).
AChE plays a key role in terminating the synaptic
action of ACh at cholinergic synapses (1). Inhibition of AChE can result in the
potentiation of central cholinergic activity by increasing the
amount of ACh available for neurotransmission. Huperzine A (HupA),
a Lycopodium alkaloid isolated from the Chinese medicinal
herb Huperzia serrata, is a potent, specific and reversible
AChE inhibitor. It is an important regulator of cholinergic
signaling in the hippocampus (4).
Previous studies have reported that HupA inhibition of AChE
activity in the brain lasts for approximately 6 h and that there is
a wide fluctuation between peaks and troughs after oral
administration of a HupA tablet (HT) (5,6). A
recent study from our laboratory indicated that after an
intramuscular injection of HupA sustained-release microspheres
(HSMs) in mice, the plasma concentration of HupA reached a maximum
on the 4th day and then slowly decreased until the drug was
undetectable in plasma on the 12th day. The AChE activity continued
to be significantly inhibited on the 14th day after treatment with
HSM (7,8).
The changes induced by treatment of AChE inhibitors
in the central cholinergic system have been widely studied in aged
brain tissue. However, little research has evaluated
neurobiological changes, particularly changes in the levels of
neurotrophic factors associated with AChE activity, in the
cholinergic system of the hippocampus in normal juvenile mice. As
the brain of juvenile animals is vulnerable to neuroactive
chemicals during multiple developmental roles of neurotransmitters,
the immature nervous system may be more susceptible to AChE
inhibitors (9). A few studies have
reported that AChE inhibitors, such as donepezil or
organophosphates, can activate the central cholinergic transmission
and enhance the expression of neurotrophic factors (10). However, previous studies
investigated the effects of AChE inhibitors that provide fluctuant
inhibition, but not steady inhibition, of AChE activity.
The hippocampus receives an abundance of projections
from cholinergic neurons of the medial septum. The central
cholinergic pathway in the hippocampus plays a critical role in
regulating numerous vital functions, including memory, learning and
movement (11). The present study
investigated whether steady versus fluctuant methods of AChE
inhibition has different effects on the cholinergic system and
neurotrophic factor levels in the hippocampus of juvenile mice. In
addition, the effect of AChE inhibitors on learning in juvenile
mice was evaluated.
Materials and methods
Animals
Juvenile (2-week-old) male Swiss mice were purchased
from the Experimental Animal Center of the Shandong Engineering
Research Center for Natural Drugs (Yantai, Shandong, China).
Animals were housed in a climate-controlled room, maintained on a
12-h light cycle, and given food and water ad libitum. The
Institutional Animal Care and Use Committee approved the study
protocols. The animals were maintained according to the Guidelines
for the Care and Use of Laboratory Animals of Yantai University.
All efforts were made to minimize the number of animals used and to
minimize their suffering.
Drug dosing schedule
Sixty-three mice were randomly divided into the
following three groups (n=21 in each group): vehicle group (VEH),
HT group (0.2 mg/kg/day), and HSM group (3 mg/ kg/ 15 days). The
mice in the VEH group were given sodium carboxymethyl cellulose.
HTs (Henan Tailong Pharmaceutical Co., Ltd., Zhengzhou, Henan,
China) were dissolved in 2.5% (w/v) sodium carboxymethyl cellulose
and were given intragastrically in a volume of 0.2 ml/10 g body
weight daily. HSMs (Luye Pharmaceutical Co., Yantai, Shandong,
China) were dissolved in 2.5% (w/v) sodium carboxymethyl cellulose
and injected intramuscularly in a volume of 0.1 ml/10 g body weight
(HSM group). To induce steady inhibition of AChE, the HSM group was
dosed on the 1st, 16th and 31st day. To induce fluctuant inhibition
of AChE, the HT group was dosed daily for 45 days. The dosages of
HupA were determined by previous studies of Tang et al
(5). In addition, the dosages of
HSM were designed according to the pharmacokinetics and
pharmacodynamics of HSM reported by Chu et al (7).
Morris water maze task
After a 45-day course of drug treatment, the spatial
learning ability of the mice was measured using the Morris water
maze for 6 days (Institute of Materia Medica, Academy of Medical
Science, China). Before initiating the Morris water maze test, the
mice were allowed to swim freely in a pool of water (diameter, 90
cm; depth, 19 cm; temperature, 26±1°C) for 60 sec without an escape
platform. Afterward, a platform (diameter, 5 cm) was placed 1 cm
below the surface of the water. Learning consisted of 4 trials/day
for 5 consecutive days. In each trial, the starting location was
randomized to 1 of 4 starting positions (north, east, south or
west), and the latency to escape onto the platform was recorded.
Mice that were unable to find the platform within 60 sec were
placed on the platform for 20 sec, and their escape latency was
recorded as 60 sec. An automated tracking system analyzed the swim
path of each subject, and the mean escape latency was calculated
(the time between being placed in the water and finding the hidden
platform).
Biochemical analyses
The mice were sacrificed by decapitation 6 days (the
duration of the Morris water maze test) after the 45-day course of
treatment. The hippocampus was separated on ice and homogenized
with ice-cold saline to yield a 10% (w/v) homogenate. The
homogenate was centrifuged at 3,500 x g for 10 min at 4°C, after
which the supernatant was stored at −80°C until subsequent
analyses. The total protein concentration was estimated by a
previously described method (12).
AChE activity was determined according to the
methods of Ellman et al (13). ChAT activity was determined using
the spectrometric method of Chao and Wolfgram (14) with assay kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). The absorption of the
final solution was measured via an automated ELISA reader (Synergy
HT, USA). The levels of BDNF and NGF in the hippocampus were
measured using ELISA assay kits (R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer’s protocol. The
minimum detection limits of the kits are 3 ng/ml for BDNF and 15
pg/ ml for NGF. Each sample was analyzed in duplicate.
Statistical analyses
Data were analyzed using statistical product and
service solutions (SPSS 13.0) computer software (SPSS, Inc.,
Chicago, IL, USA). The main treatment effect on the escape latency
was analyzed using analysis of variance (ANOVA) with repeated
measures. Fisher’s least significant difference Post-hoc test was
used to test the differences between groups. One-way ANOVA was used
to analyze the biochemical data. A P-value <0.05 was considered
to represent a statistically significant difference. All values are
presented as the mean ± SEM.
Results
Effects of HTs and HSMs on AChE activity
in the hippocampus
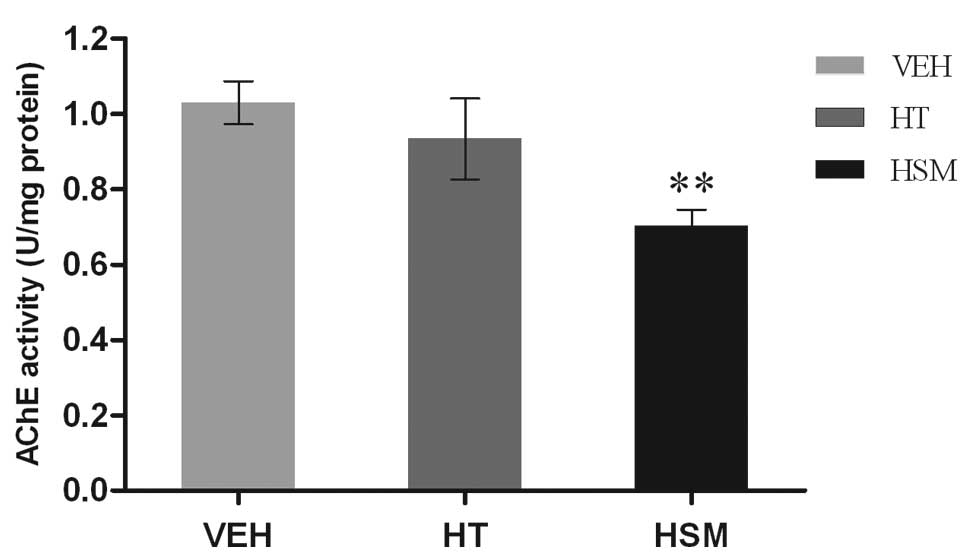
Compared with vehicle treatment, AChE activity in
HT-treated mice did not change significantly (P>0.05), while
HSM-treated mice had a significant decrease in AChE activity
(P<0.01) (Fig. 1).
Effects of HTs and HSMs on BDNF and NGF
levels in the hippocampus
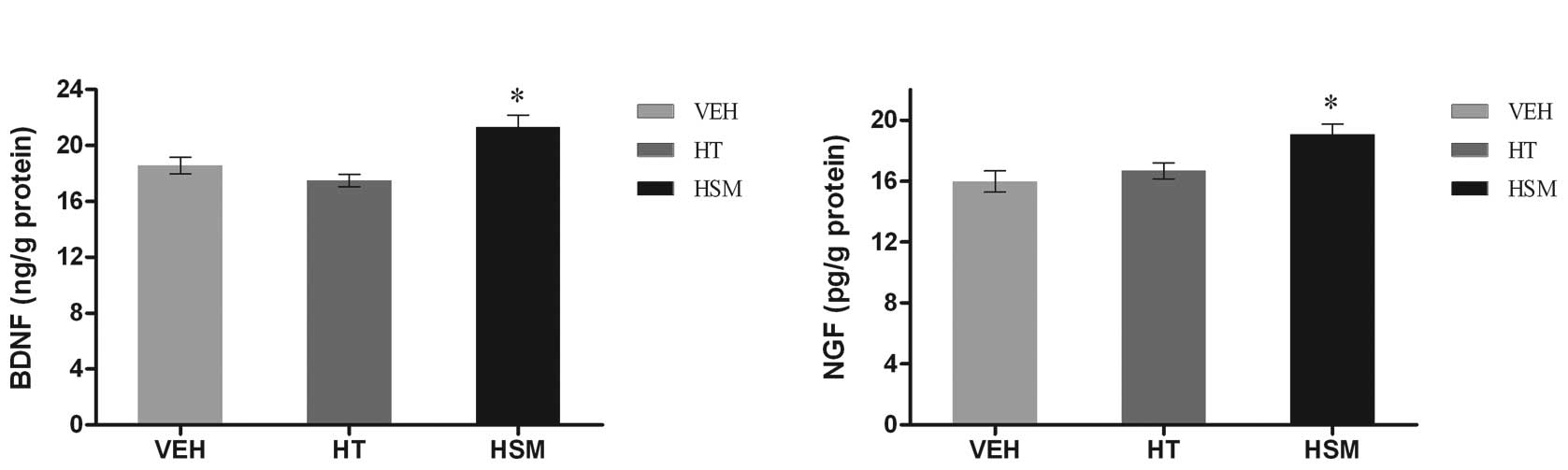
Compared with vehicle-treated mice, there were no
differences found in BDNF and NGF levels in the HT-treated mice.
However, treatment with HSMs resulted in a significant increase in
BDNF and NGF levels (P<0.05) (Fig.
2).
Effects of HTs and HSMs on ChAT activity
in the hippocampus
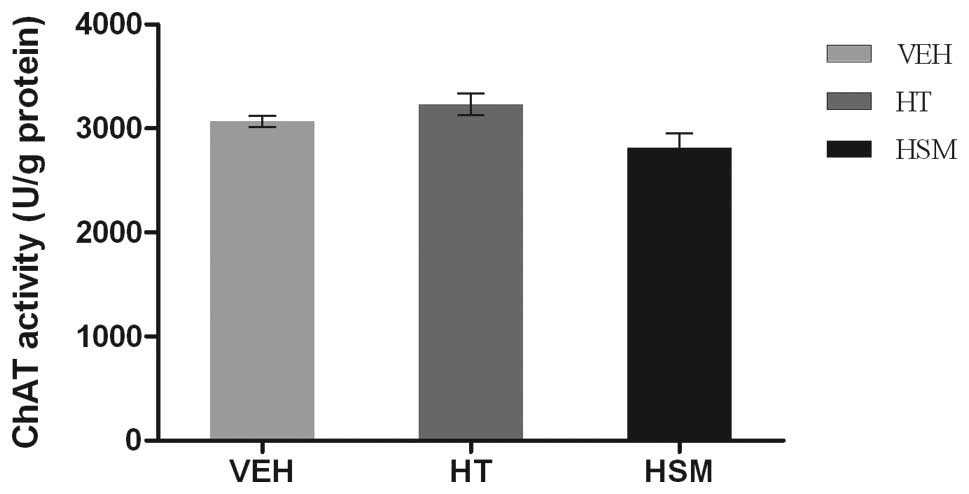
The effects of HTs and HSMs on ChAT activity in the
hippocampus of juvenile mice are shown in Fig. 3. The data revealed that neither HT
nor HSM had a significant effect on ChAT activity (P>0.05).
Effects of HTs and HSMs on escape latency
in juvenile mice
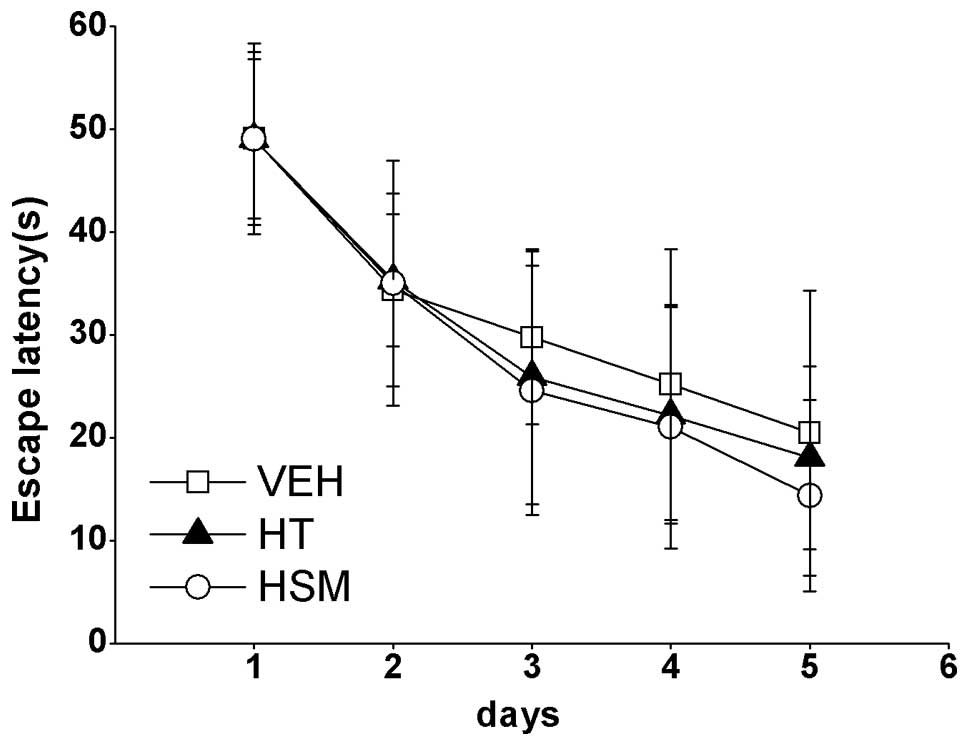
ANOVA revealed a significant effect of the testing
day on escape latency within the groups (F=548.86; P<0.01),
suggesting that all mice effectively improved their spatial
learning across the 5-day training period. However, no significant
main treatment effect on escape latency was found (F (2,40) =0.315;
P>0.05), demonstrating that neither fluctuant nor steady
inhibition of AChE had any effect on spatial learning in juvenile
mice (P>0.05) (Fig. 4).
Discussion
AChE is found in presynaptic (cholinergic) and
postsynaptic (cholinoceptive) components of the central cholinergic
pathways, where it terminates the synaptic action of ACh (1). The AChE inhibition provided by AChE
inhibitors should strengthen the ACh effect by allowing more ACh
molecules to bind to the nicotinic and muscarinic ACh receptors,
leading to the activation of downstream signaling pathways.
As an AChE inhibitor, HupA was rapidly absorbed and
eliminated in vivo after an oral administration of HT; thus,
the plasma concentration of HupA was fluctuant. However, previous
experiments in vivo demonstrated that HSM in mice can
maintain a steady-state HupA concentration for 14 days after a
single intramuscular administration of HSM (7). Thus, HT and HSM treatments can
provide steady and fluctuant cholinergic stimulation,
respectively.
The present study revealed that AChE activity was
still significantly inhibited 6 days after HSM treatment.
Furthermore, no marked changes were found in the HT group compared
with the VEH group. The results indicate that the steady versus
fluctuant AChE inhibition exerted different effects on AChE
activity in the hippocampus.
BDNF and NGF provide trophic support to cholinergic
neurons that project mainly to the hippocampus and the cortex. It
has been demonstrated that NGF levels critically regulate
cholinergic neuron size, cholinergic hippocampal innervation, and
neurochemical differentiation during development (15,16).
In addition, BDNF can increase the size of cholinergic neurons and
the maturation of cholinergic projections to the hippocampal
formation (17). Most importantly,
it has been reported that in the hippocampus, there is positive
feedback between cholinergic activity and neurotrophins, such as
NGF and BDNF (17,18). In the central cholinergic system,
studies of conventional AChE inhibitors have demonstrated that
fluctuant inhibition of AChE can upregulate the expression of BDNF
and NGF in the hippocampus (19,20).
In the present study, 6 days after withdrawal of steady and
fluctuant AChE inhibition, the levels of BDNF and NGF in the
hippocampus were investigated. Both BDNF and NGF levels increased
after the steady inhibition of AChE in HSM-treated mice. In
contrast, BDNF and NGF levels did not show any significant changes
after the fluctuant inhibition of AChE in HT-treated mice. These
results differ from previous studies, which concluded that AChE
inhibitors increase neurotrophic factors levels (18,20–22).
Regarding the contradictory results, it must be noted that the
neurotrophic factor levels in the previous studies were measured
immediately (4 to 24 h) after administration of AChE inhibitors.
Our findings demonstrated that steady AChE inhibition caused a
long-term increase in the levels neurotrophic factors in the
hippocampus. The present study also indicated a consistent
correlation between low AChE activity and the levels of BDNF and
NGF.
ChAT, an enzyme that catalyzes the biosynthesis of
ACh, has been used as a specific marker for cholinergic neurons
(23). The chronic administration
of AChE inhibitors may modify the ACh recycling pathway. However,
neither the HSM group nor the HT group showed any alterations in
ChAT activity in the hippocampus. The absence of changes in ChAT
activity may be attributed to other components of the cholinergic
system, which need to be investigated further.
AChE inhibitors can improve cognitive function in
dementia animals with cholinergic dysfunction. However, it is
unknown whether AChE inhibitors affect learning and memory in
normal juvenile animals. Using the Morris water maze test, the
present study demonstrated that compared with the VEH group, mice
in the HT and HSM groups did not show any improvement in learning.
These results suggest that the actions of AChE inhibitors on
learning differ between normal and dementia model mice.
In conclusion, the present study indicated that
steady AChE inhibition leads to a decrease in AChE activity and an
increase in BDNF and NGF levels in the hippocampus. However,
fluctuant AChE inhibition did not increase the BDNF or NGF levels.
The present findings also suggest that AChE inhibitors do not
improve learning in normal juvenile mice.
Acknowledgements
This study was supported by the 11th
Five-Year Key Program for Science and Technology Development of
China (no. 2009ZX09102-125). The authors also thank Professor Lon
Clark for the English language revision.
References
|
1.
|
Y Abreu-VillacaCC FilgueirasAC
ManhaesDevelopmental aspects of the cholinergic systemBehav Brain
Res221367378201010.1016/j.bbr.2009.12.049
|
|
2.
|
M BothwellFunctional interactions of
neurotrophins and neurotrophin receptorsAnnu Rev
Neurosci18223253199510.1146/annurev.ne.18.030195.0012557605062
|
|
3.
|
M KnipperM da Penha BerzaghiA BlochlH
BreerH ThoenenD LindholmPositive feedback between acetylcholine and
the neurotrophin nerve growth factor and brain-derived neurotrophic
factor in the rat hippocampusEur J
Neurosci6668671199410.1111/j.1460-9568.1994.tb00312.x
|
|
4.
|
R WangH YanXC TangProgress in studies of
huperzine A, a natural cholinesterase inhibitor from Chinese herbal
medicineActa Pharmacol
Sin27126200610.1111/j.1745-7254.2006.00255.x16364207
|
|
5.
|
XC TangP De SarnoK SugayaE GiacobiniEffect
of huperzine A, a new cholinesterase inhibitor, on the central
cholinergic system of the ratJ Neurosci
Res24276285198910.1002/jnr.4902402202585551
|
|
6.
|
JC YeS ZengGL ZhengGS ChenPharmacokinetics
of huperzine A after transdermal and oral administration in beagle
dogsInt J
Pharm356187192200810.1016/j.ijpharm.2008.01.00718289810
|
|
7.
|
D ChuJ TianW LiuZ LiY
LiPoly(lactic-co-glycolic acid) microspheres for the controlled
release of huperzine A: in vitro and in vivo studies and the
application in the treatment of the impaired memory of miceChem
Pharm Bull (Tokyo)55625628200710.1248/cpb.55.62517409558
|
|
8.
|
C WangT ZhangH MaJ LiuF FuK LiuProlonged
effects of poly(lactic-co-glycolic acid) microsphere-containing
huperzine A on mouse memory dysfunction induced by scopolamineBasic
Clin Pharmacol
Toxicol100190195200710.1111/j.1742-7843.2007.00041.x17309523
|
|
9.
|
TA SlotkinCholinergic systems in brain
development and disruption by neurotoxicants: nicotine,
environmental tobacco smoke, organophosphatesToxicol Appl
Pharmacol198132151200410.1016/j.taap.2003.06.001
|
|
10.
|
S KotaniT YamauchiT TeramotoH
OguraDonepezil, an acetylcholinesterase inhibitor, enhances adult
hippocampal neurogenesisChem Biol
Interact175227230200810.1016/j.cbi.2008.04.00418501884
|
|
11.
|
JT CoyleDL PriceMR DeLongAlzheimer’s
disease: a disorder of cortical cholinergic
innervationScience219118411901983
|
|
12.
|
OH LowryNJ RosebroughAL FarrRJ
RandallProtein measurement with the Folin phenol reagentJ Biol
Chem193265275195114907713
|
|
13.
|
GL EllmanKD CourtneyV Andres JrRM
Feather-StoneA new and rapid colorimetric determination of
acetylcholinesterase activityBiochem
Pharmacol78895196110.1016/0006-2952(61)90145-913726518
|
|
14.
|
LP ChaoF WolfgramSpectrophotometric assay
for choline acetyltransferaseAnal
Biochem46114118197210.1016/0003-2697(72)90401-05017650
|
|
15.
|
EC YuenCL HoweY LiDM HoltzmanWC
MobleyNerve growth factor and the neurotrophic factor
hypothesisBrain
Dev18362368199610.1016/0387-7604(96)00051-48891230
|
|
16.
|
C CrowleySD SpencerMC NishimuraMice
lacking nerve growth factor display perinatal loss of sensory and
sympathetic neurons yet develop basal forebrain cholinergic
neuronsCell7610011011199410.1016/0092-8674(94)90378-68137419
|
|
17.
|
NL WardT HaggBDNF is needed for postnatal
maturation of basal forebrain and neostriatum cholinergic neurons
in vivoExp Neurol162297310200010.1006/exnr.1999.734610739636
|
|
18.
|
M Da Penha BerzaghiJ CooperE
CastrenCholinergic regulation of brain-derived neurotrophic factor
(BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3)
mRNA levels in the developing rat hippocampusJ
Neurosci13381838261993
|
|
19.
|
N LindeforsP ErnforsT FalkenbergH
PerssonSeptal cholinergic afferents regulate expression of
brain-derived neurotrophic factor and beta-nerve growth factor mRNA
in rat hippocampusExp Brain Res887890199210.1007/BF02259130
|
|
20.
|
AM BetancourtNM FilipovRL CarrAlteration
of neurotrophins in the hippocampus and cerebral cortex of young
rats exposed to chlorpyrifos and methyl parathionToxicol
Sci100445455200710.1093/toxsci/kfm24817893397
|
|
21.
|
SJ FrenchT HumbyCH HornerMV SofroniewM
RattrayHippocampal neurotrophin and trk receptor mRNA levels are
altered by local administration of nicotine, carbachol and
pilocarpineBrain Res Mol Brain
Res67124136199910.1016/S0169-328X(99)00048-010101239
|
|
22.
|
LL TangR WangXC TangEffects of huperzine A
on secretion of nerve growth factor in cultured rat cortical
astrocytes and neurite outgrowth in rat PC12 cellsActa Pharmacol
Sin26673678200510.1111/j.1745-7254.2005.00130.x15916732
|
|
23.
|
Y OdaCholine acetyltransferase: the
structure, distribution and pathologic changes in the central
nervous systemPathol
Int49921937199910.1046/j.1440-1827.1999.00977.x10594838
|