Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common cancer and the third most common cause of cancer-related
death in the world (1,2). The number of new HCC cases is
estimated to be 564,000 per year, including 398,000 men and 166,000
women (3). It accounts for 80% of
all human liver cancers, and is responsible for 0.5–1 million
worldwide deaths annually (4). The
rising incidence and poor prognosis of HCC cases have prompted
extensive research on its pathogenesis for a better understanding
of HCC development.
Toxic industrial chemicals, air and water
pollutants, food additives and fungal toxins are major sources of
hepatocarcinogenesis (5). Although
these agents have been suspected, the molecular pathogenesis of HCC
remains unclear. As an established environmental hepatocarcinogen,
diethylnitrosamine (N-nitrosodiethylamine; DEN), which produces
primary metabolic activation resulting in initiation of liver
carcinogenesis (6) and formation
of liver tumors after repeated administration, is normally used to
induce liver cancer in several animal models (7,8).
DEN-induced hepatocarcinoma in animals serves as a standard model
to study the beneficial effects of many drugs and treatments on HCC
(9,10). Prior studies have shown the
histopathological similarities of human HCC and chemical-induced
experimental liver tumors in Wistar rats and Syrian golden hamsters
(11). The purpose of this study
was to define the characterization of DEN-induced liver
carcinogenesis in Syrian golden hamsters.
Materials and methods
Chemical reagents
DEN was purchased from Sigma (St. Louis, MO, USA)
and freshly diluted in saline to a final concentration before
use.
Animals
A total of 36 male Syrian hamsters (Slike
Experimental Animal Company, Shanghai, China) at 3 weeks of age and
weighing 75–100 g, were housed in accordance with the institutional
guidelines for animal experimentation and maintained under standard
laboratory conditions with a room temperature of 23±2°C, relative
humidity of 60±5% and a 12 h/12 h light/dark cycle. All animals
were fed with a standard diet and water ad libitum.
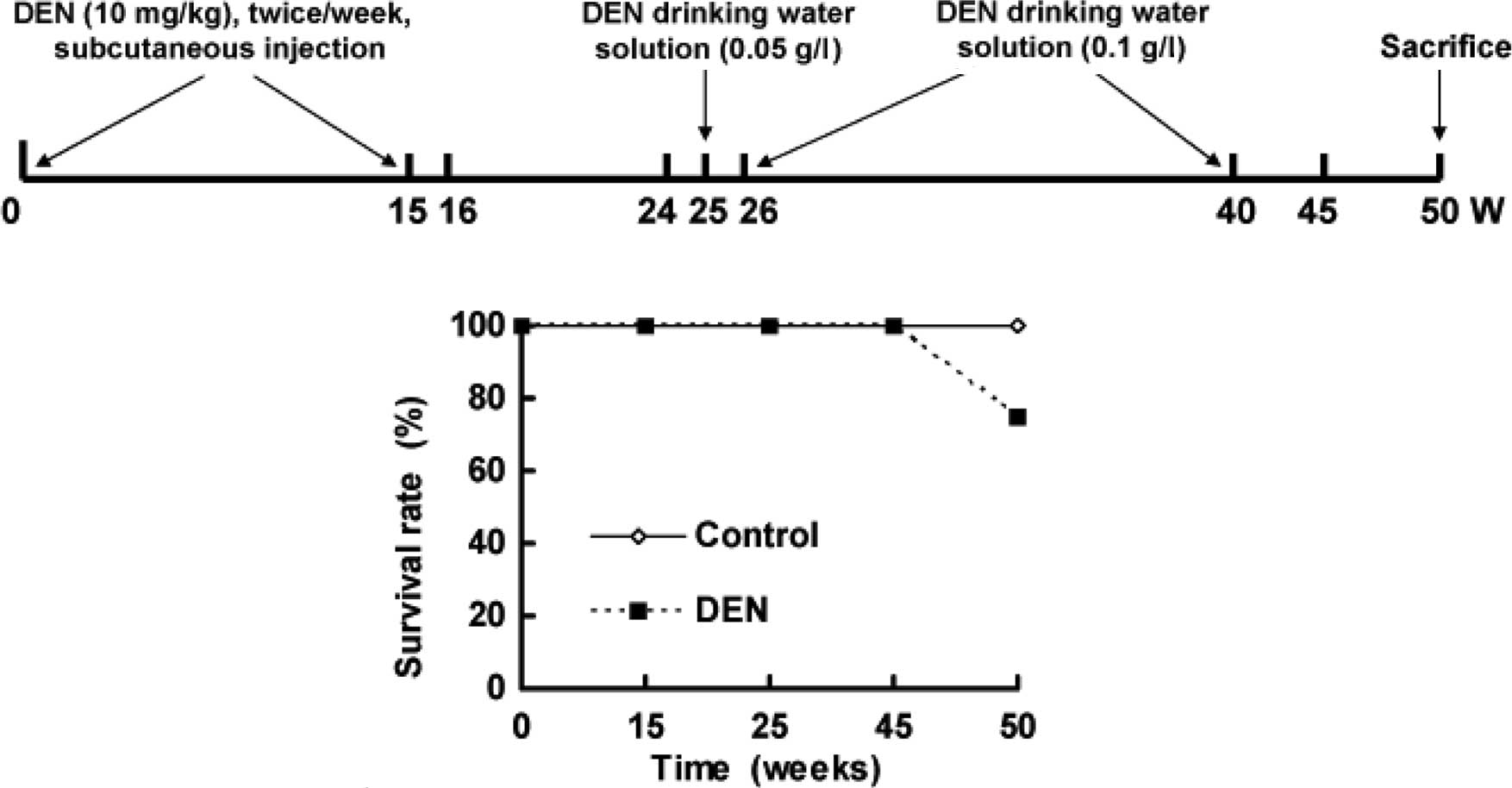
The hamsters were divided into two groups. Hamsters
(n=12) injected with normal saline and who drank distilled water
were used as control. Model hamsters (n=24) received DEN. DEN was
injected subcutaneously twice per week for 15 weeks, followed by
withdrawal and normal feeding at 16–24 weeks. From 25 to 40 weeks,
DEN was added to the drinking water with an initial concentration
of 0.05 g/l at 26 weeks and a final concentration of 0.1 g/l until
40 weeks. Normal drinking resumed from 41 to 50 weeks (Fig. 1A). Two animals from each group were
sacrificed at 15, 24 and 45 weeks for histopathological
examination. All hamsters were sacrificed by the end of 50 weeks
(Fig. 1A).
Histopathological examination
Animals were weighed weekly during the treatment
period and monthly thereafter. At the end of the 50th week, all
surviving animals were sacrificed with 1% sodium pentobarbital.
Liver, lung, spleen, kidney and heart were examined grossly and
microscopically for DEN-induced tumorigenicity. The organs were
fixed in 10% phosphate-buffered formalin and processed for
histological examination with H&E staining. H&E-stained
sections from multiple paraffin blocks (16 per animal) for each
period were examined by light microscopy. All nodular lesions were
diagnosed histopathologically and counted using serial
sections.
Immunohistochemical staining
Tissues were fixed in 10% phosphate-buffered
formalin and processed for α-fetoprotein (AFP) (Envison kit;
Maixin-Bio Company, Fuzhou, China). AFP immunostaining was
performed according to the steps described in the operational
manuals. Dilution of the primary antibody was 1:50. Human
AFP-positive HCC tissue served as the positive control, while the
negative control was diluted with normal mouse serum in PBS
solution as a replacement for the primary antibody control.
Cell culture and tumor
transplantation
The isolated tissue was immediately minced (1
mm3) by scalpel under sterile conditions and incubated
with 1 mg/ml collagenase IV (C5138; Sigma) for 30 min at 37°C under
gentle agitation (Sanyo, Japan) as previously described (12). Next, the cells were passed through
a 200-mesh screen and washed twice before spinning at 1,500 rpm for
5 min at 4°C. Cells were cultured in a culture flask (Corning) in
DMEM/F12 medium (Hyclone, Beijing, China) with the addition of 10%
fetal bovine serum (Gibco), 100 IU/ml penicillin (Amresco) and 50
μg/ml streptomycin (Amresco). The cell culture was monitored with
phase contrast microscopy.
Tumor tissue with high proliferating activity was
selected under sterile conditions, rinsed with iced normal saline
three times and cut into small pieces of 1×1 mm for xenografts.
Under direct vision through surgery, a small piece of tumor tissue
prepared within 2 h was inoculated in the left lobe of the liver of
male Syrian hamster, and sutured and disinfected conventionally.
Caesarean section was performed for observation at 7 and 10 days
after transplantation (Nikon ECLIPSE TS100).
Statistical analysis
All of the data were processed by statistical
software SPSS 16.0. Results were expressed as the means ± SE.
Statistical analysis was tested using one-way analysis of variance.
Differences were considered to denote statistical significance at
two-tailed p<0.05.
Results
Macronodular hepatocellular carcinoma in
Syrian hamsters
Fig. 1B shows the
survival rate of hamsters. A total of 6 animals treated with DEN
died between 45 and 50 weeks, whereas unexpected death was not
observed in the control hamsters.
No tumors were observed in the control animals. The
gross pathological findings of DEN-treated livers included ascites,
hepatomegaly, small hepatic cysts and multiple hepatic nodules.
Gross examination at 45 and 50 weeks of the DEN treatment
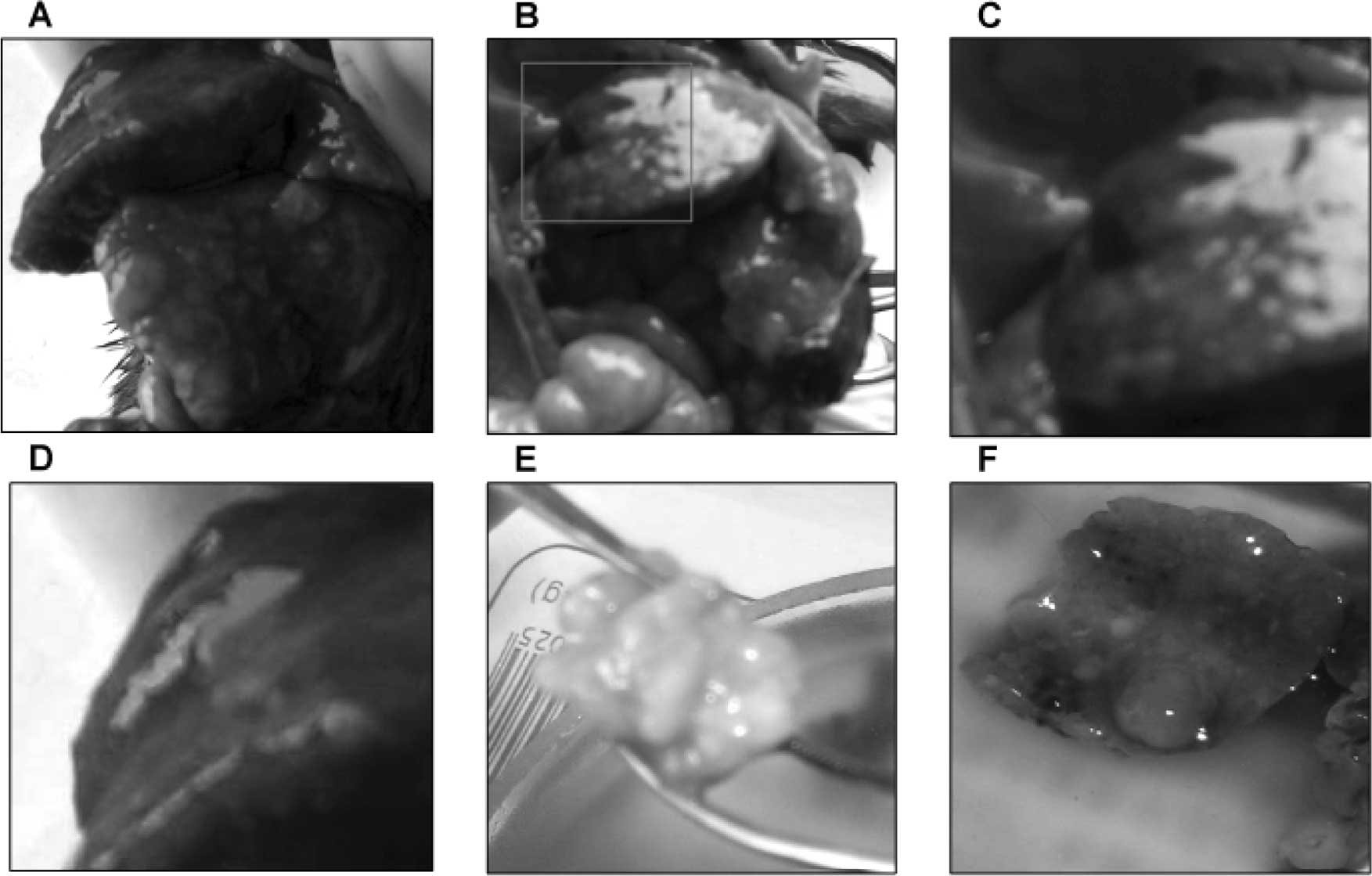
demonstrated macronodular HCC in 33.3% (4/12) of the DEN-treated
Syrian hamsters (Fig. 2). The
livers with HCC were enlarged with multiple small nodules of
0.2–0.5 cm in diameter (Fig.
2A–D), or with a single large nodule of 3 cm in diameter
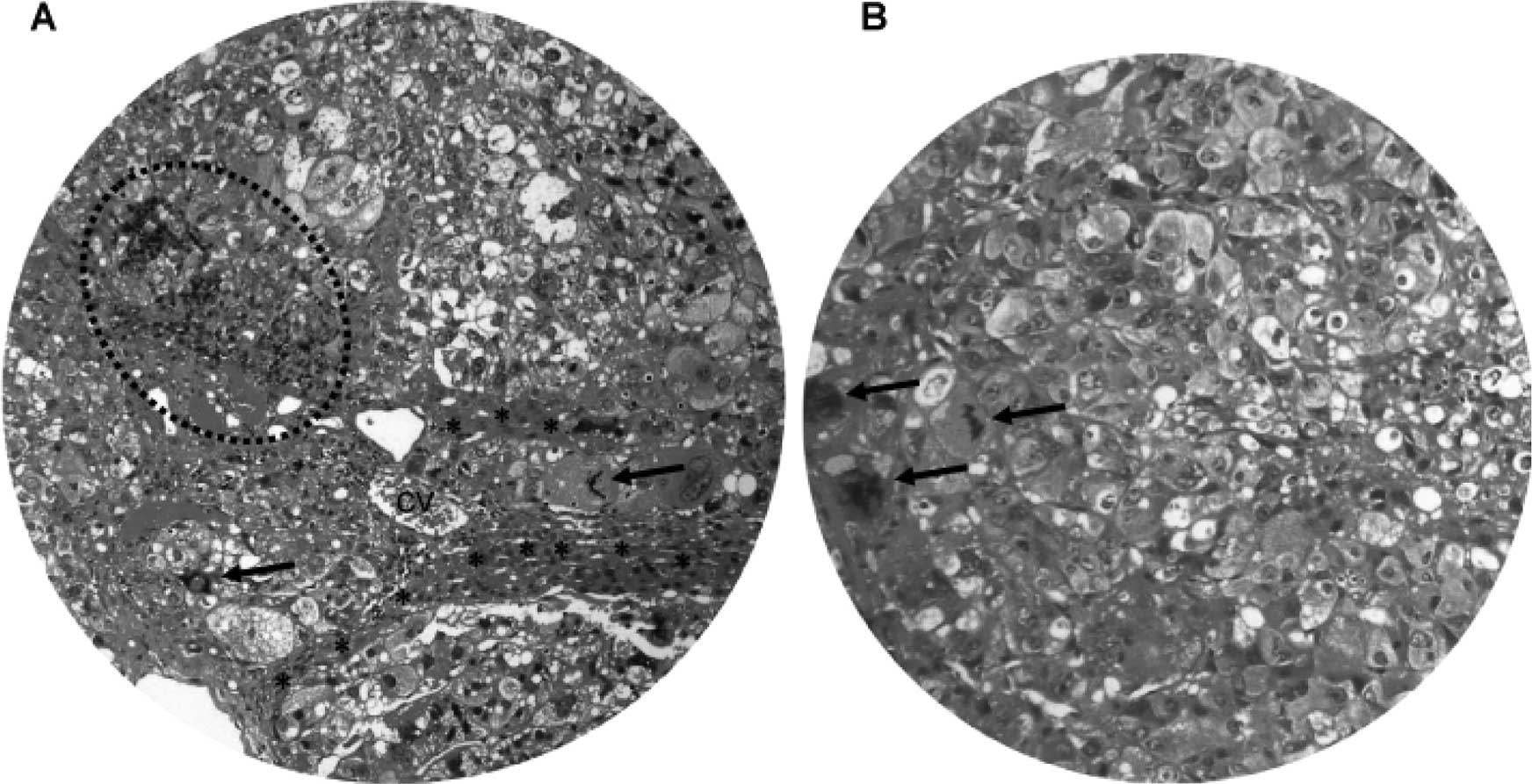
(Fig. 2E). Light microscopy and
H&E staining showed nodular HCC (Fig. 3A). There were frequent bizarre
neoplastic cells and abnormal mitotic figures (Fig. 3B). In addition, the HCC nodules
were accompanied by chronic inflammatory infiltrates (Fig. 3A) and separated by cords of
atrophic hepatocytes (Fig.
3A).
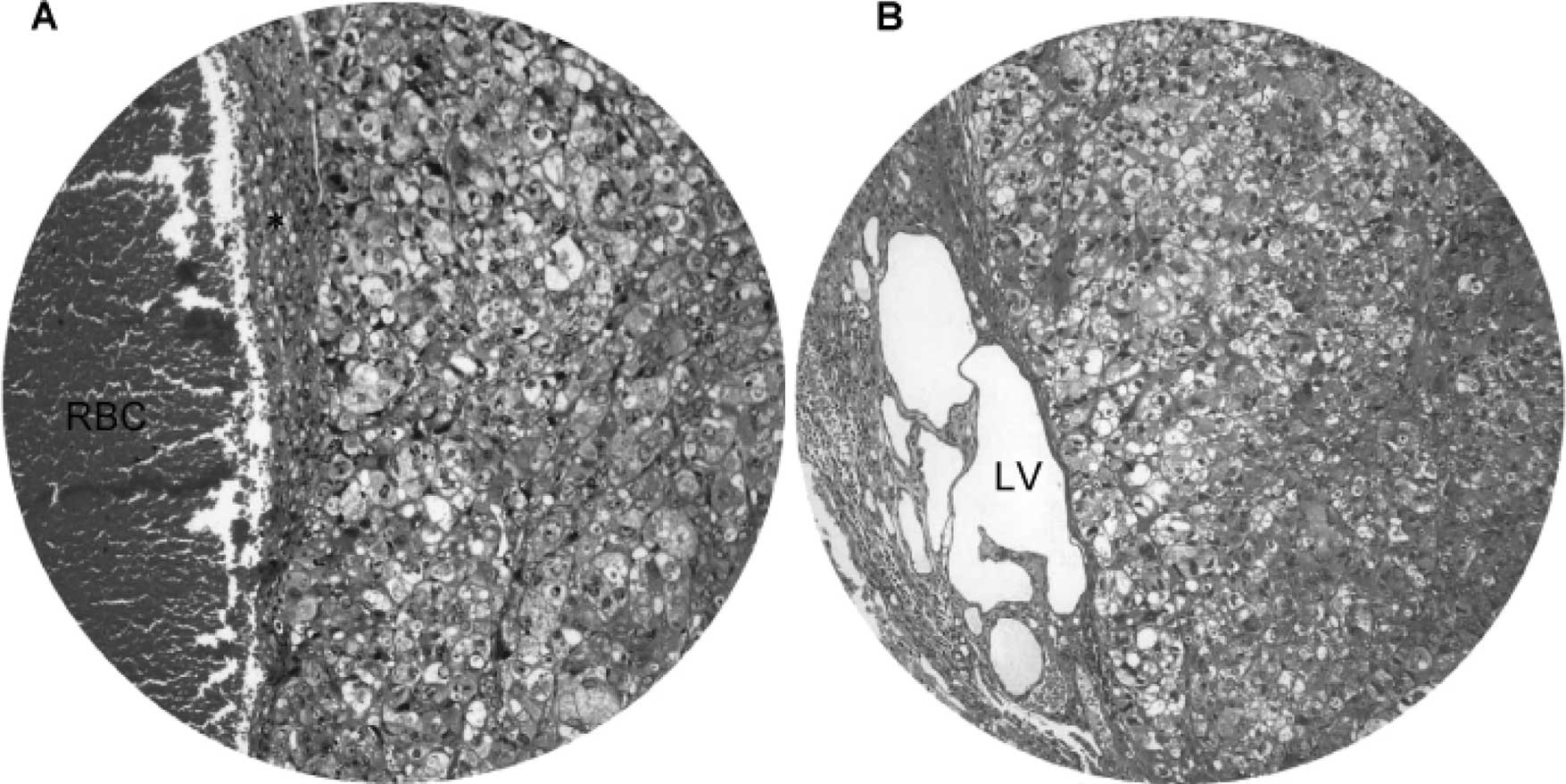
We also observed the invasiveness of macronodular
HCC (Fig. 4). Invasion of blood
vessels (Fig. 4A) and lymph
vessels (Fig. 4B) by HCC was
evident in hamsters treated with DEN for 45 weeks and onwards. The
blood vessels showed congestion, whereas the lymph vessels were
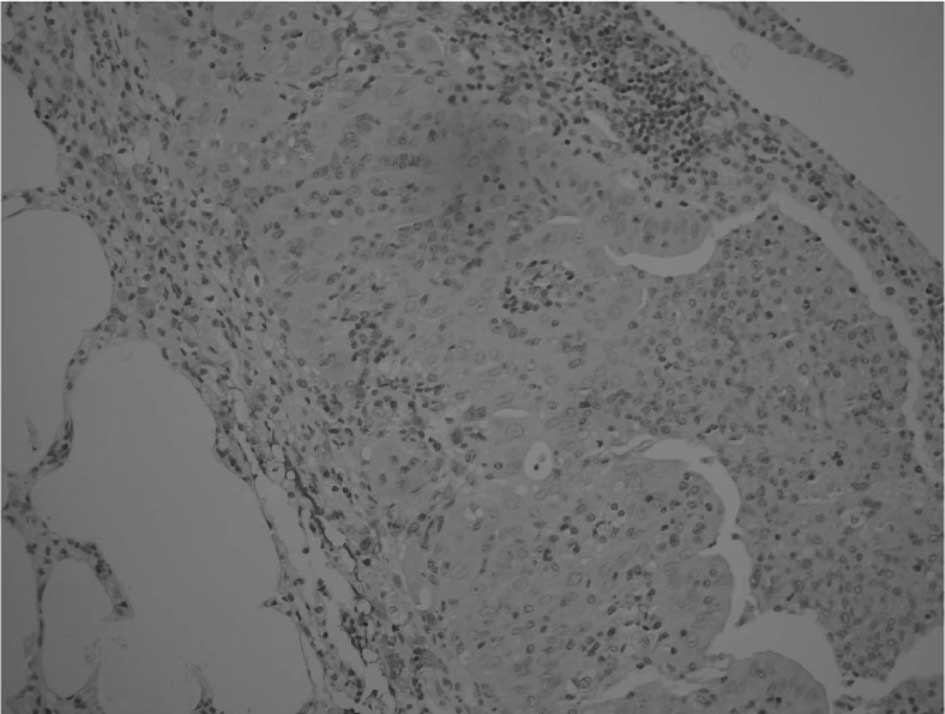
markedly dilated with multiple cystic changes. Immunohistochemical
staining revealed that approximately one-third of the HCC cells
were positive for AFP (Fig. 5),
and the immunoreactivity was localized in the cytoplasm and
membrane.
Primary cell culture and tumor
transplantation
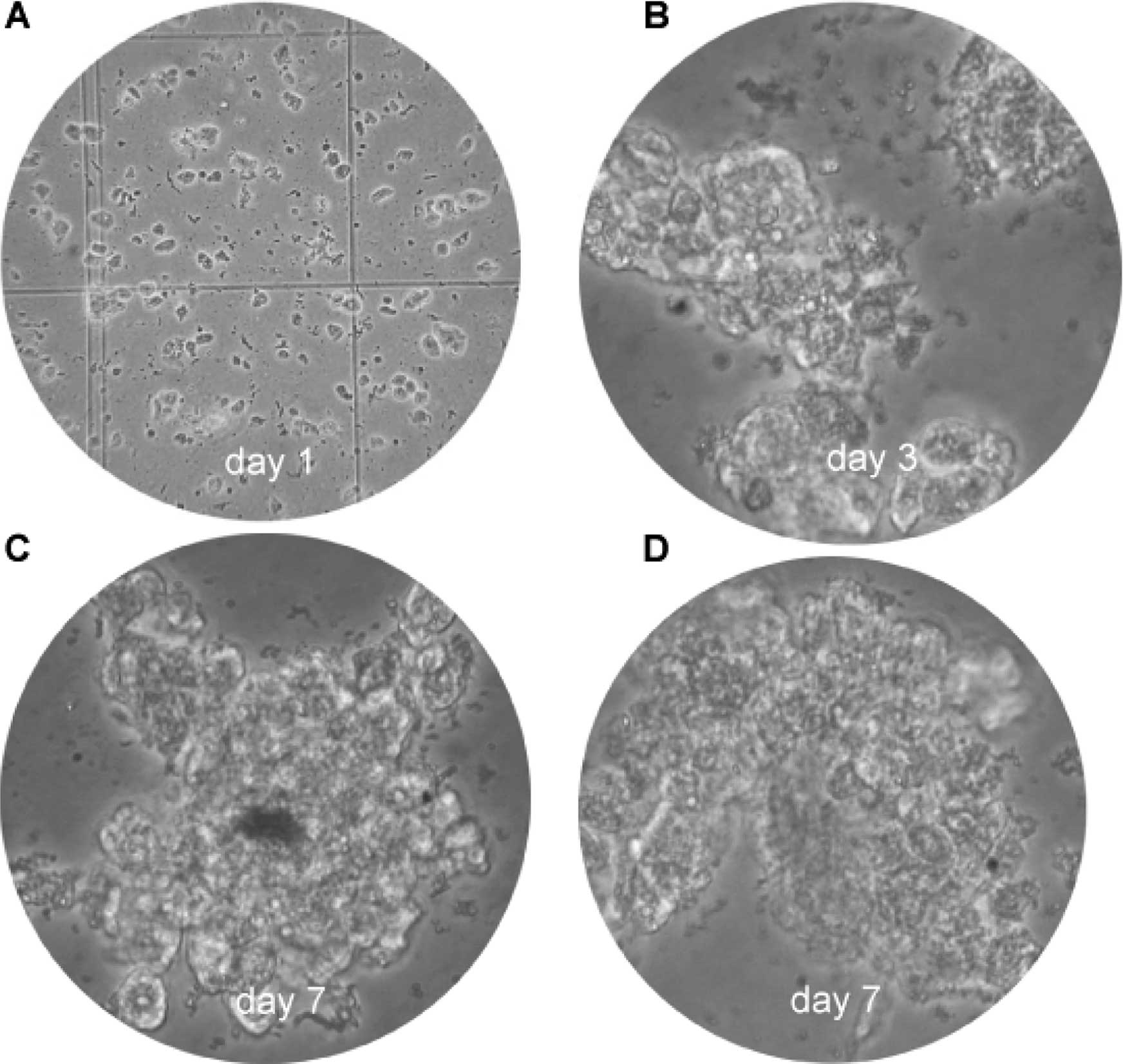
The use of primary cultures from tumor cells is a
promising approach for the development of new methods for the
diagnostics and therapy of cancer. Fig. 6 illustrates the cultured HCC cells
on day 1 (Fig. 6A), day 3
(Fig. 6B) and day 7 (Fig. 6C and D). Clone expansion and
confluence was time-dependent in this primary cell culture.
Pre-neoplastic changes in the liver
Changes in the DEN-treated livers in addition to HCC
were chronic inflammatory cell infiltrates, fatty metamorphosis,
ballooning degeneration, focal eosinophilic necrosis, hyperplastic
nodules and multilocular cysts (Figs.
7–10). Cirrhosis and
dilatation of the common bile duct were not observed in any
animals.
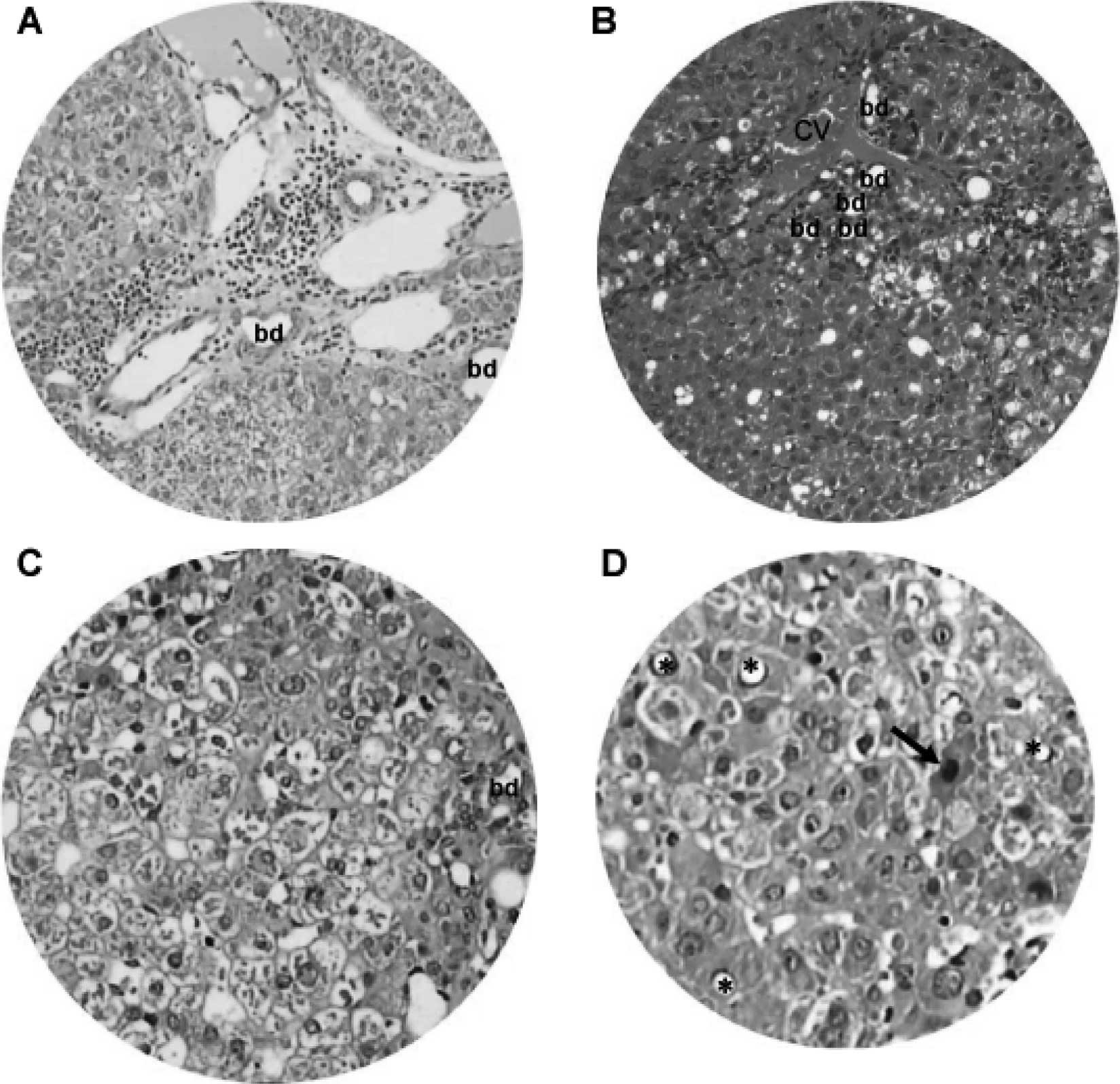
From 15 weeks onward, DEN-treated animals showed
chronic inflammatory infiltrates, dilated lymph vessels and
proliferating bile ducts in the portal area (Fig. 7A). In addition, fatty change and
clustering of proliferative bile ducts were found adjacent to the
central vein (Fig. 7B).
Hepatocytes in the DEN-treated hamsters also underwent degenerative
changes, such as nuclear vacuolation, ballooning degeneration
(Fig. 7C) and eosinophilic
necrosis (Fig. 7D).
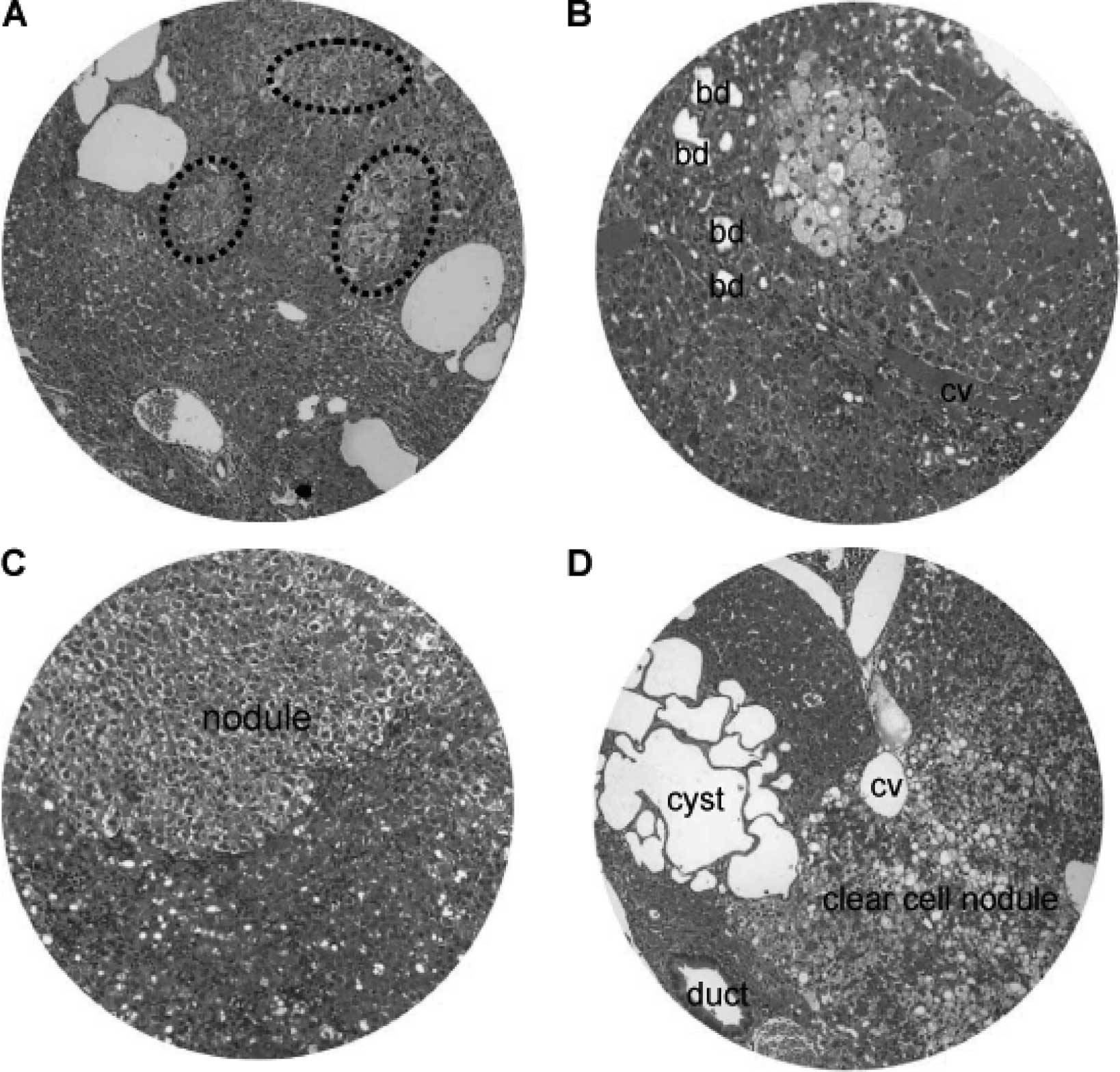
Nodular hyperplasia and hyperplastic nodules were
encountered in the hamsters at 16–24 weeks of the DEN treatment
(Fig. 8). The size of the nodular
lesions varied from a pinpoint to 1.5 cm in diameter. The nodular
lesions included patches of clear cell hyperplasia coexisting with
dilated blood vessels (Fig. 8A),
focal clear cell hyperplasia accompanying proliferating bile ducts
and chronic inflammatory cells adjacent to the central vein
(Fig. 8B), and well-defined
nodular hyperplasia arising from the background of chronic
inflammation and hepatocyte degeneration (Fig. 8C). In addition, clear cell nodules
and focal clustering of microcysts often existed (Fig. 8D).
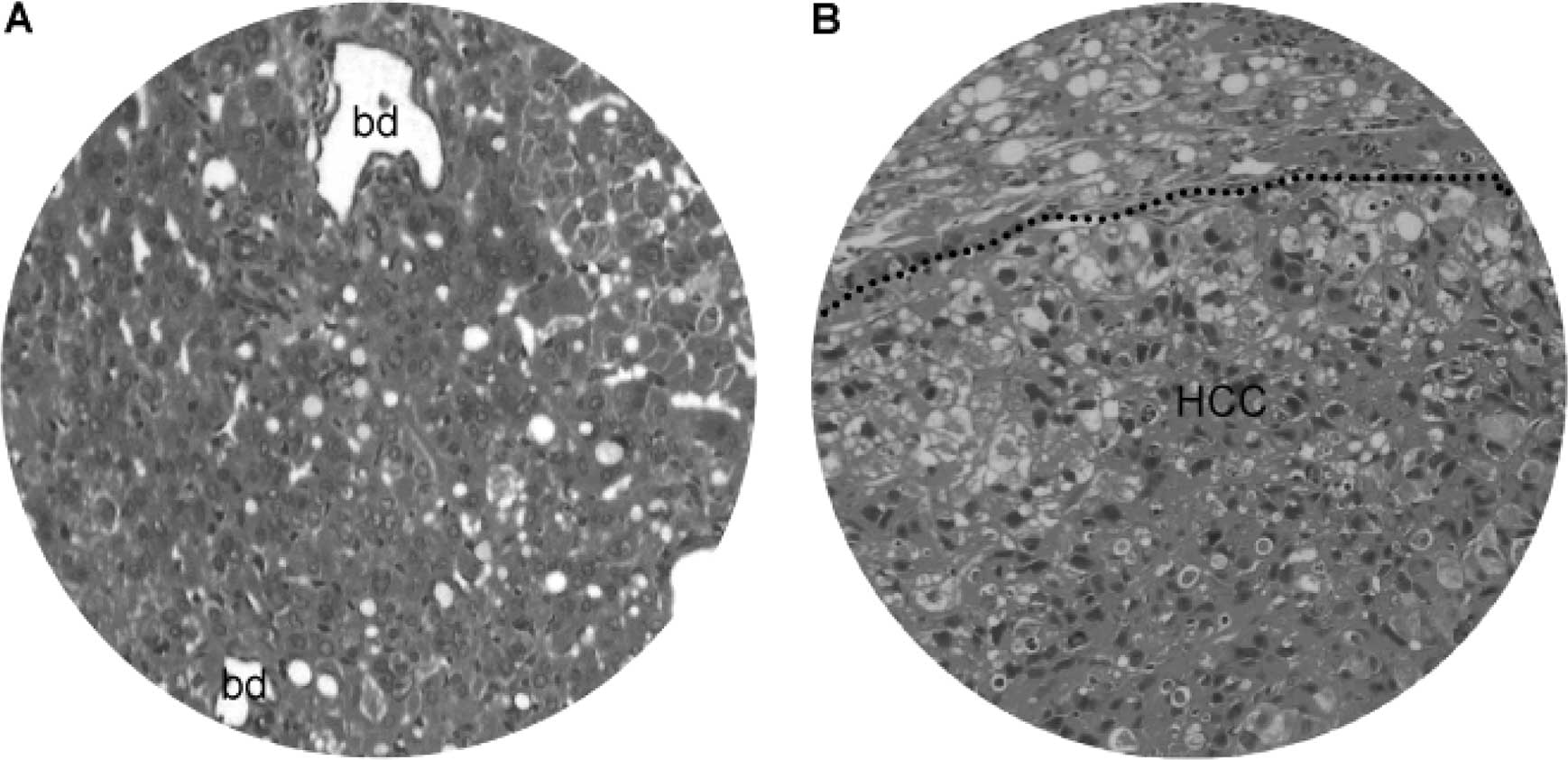
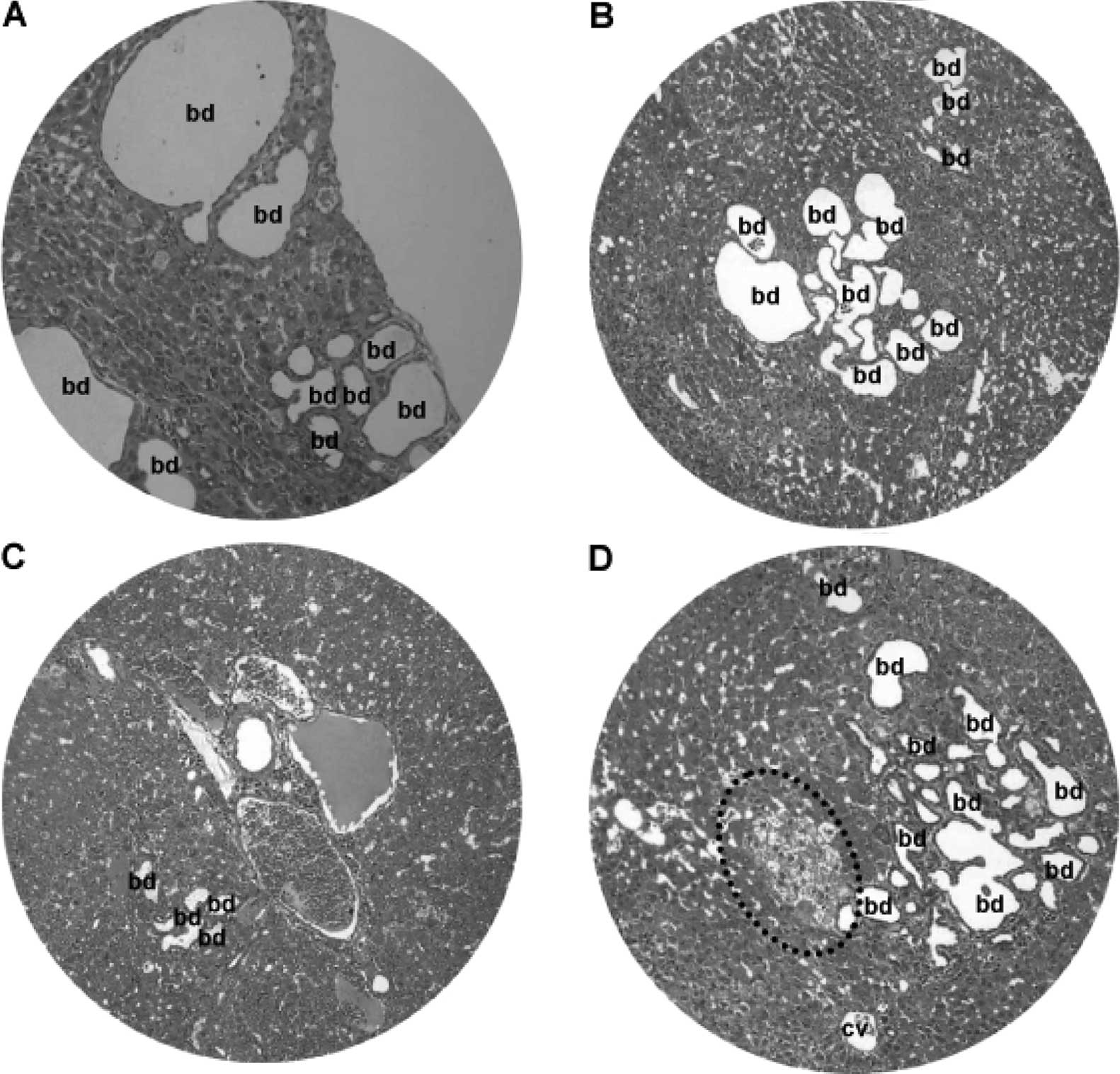
At 25–50 weeks of the DEN treatment, there were
focal bile duct hyperplasia (Fig.
9) and fatty changes (Fig.
10) adjacent to HCC lesions. Focal bile duct dysplasia, with
marked dilation and cystic formation (Fig. 9A), fatty changes (Fig. 9B), congestion (Fig. 9C) and clear cell hyperplasia
(Fig. 9D), often co-existed. Fatty
changes were observed in both early (Fig. 10A) and advanced (Fig. 10B) stage of HCC, suggesting an
association between fatty metamorphosis and the hepatic
carcinogenesis liver.
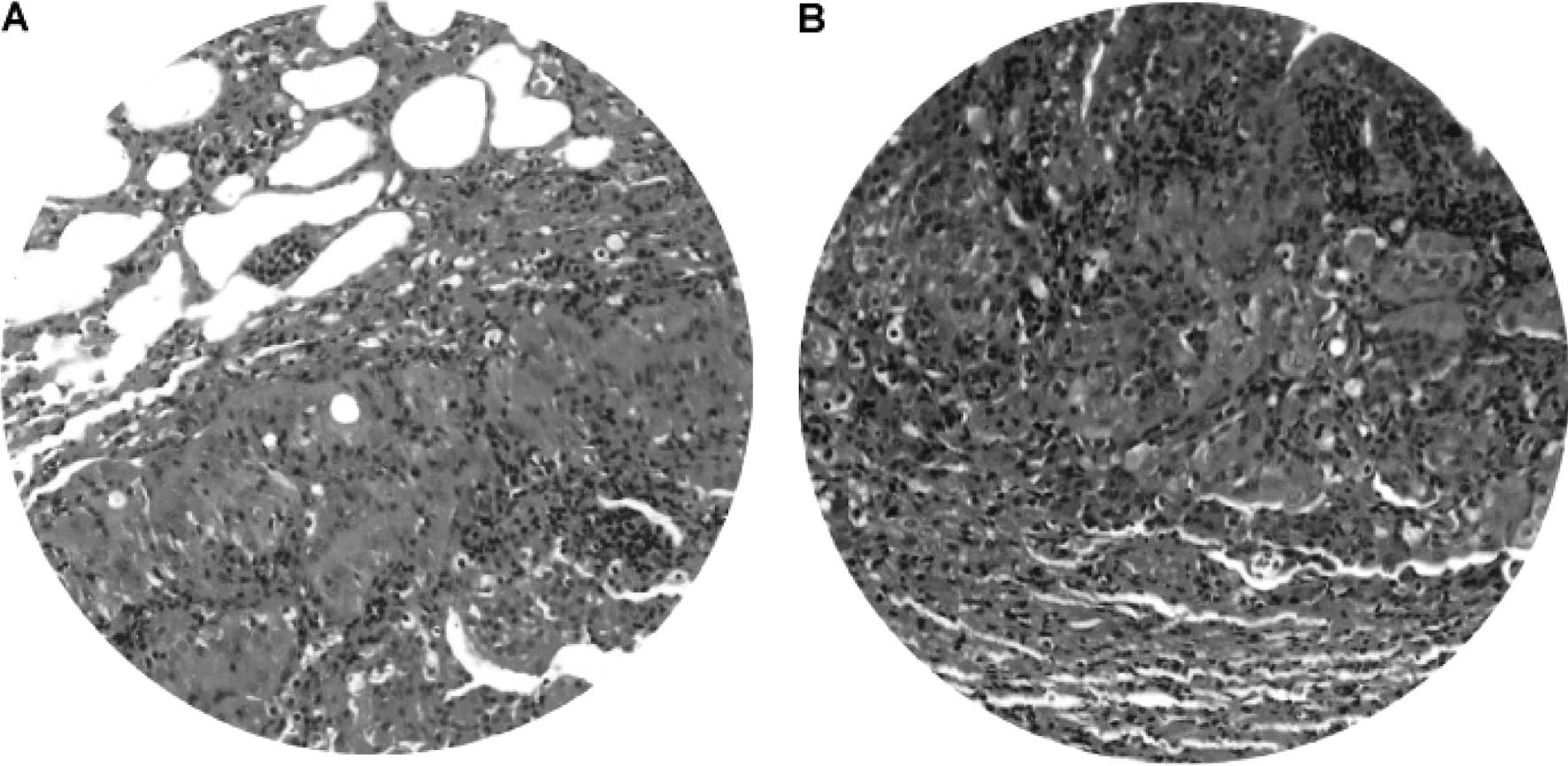
Lung tumors
Gross and microscopic examination revealed lung
tumors in 2/4 hamsters at 25–50 weeks of the DEN treatment. The
lung tumor mass was most frequently single rather than multiple,
and varied from 0.3 to 1.2 cm in diameter. Light microscopy and
H&E staining demonstrated well-differentiated neoplastic cells
(Fig. 11A) admixed with chronic
inflammatory cell infiltrates (Fig.
11B). The morphology of the neoplastic cells in the lung was
different compared to that of HCC. Consistently,
immunohistochemistry illustrated that only a few macrophages, but
not the neoplastic cells, were positive for AFP (Fig. 12), indicating that the lung tumors
may not be HCC metastasis. No tumors were detected in the kidney,
pancreas, spleen or other anatomical sites.
Discussion
This study describes the histopathological series of
events in hamsters administered DEN. The most important findings
were macronodular HCC with a potential for primary cell culture and
tumor transplantation, pre-neoplastic lesions that progress to HCC
and primary lung tumors.
HCC is a highly malignant tumor with poor prognosis
(13,14). Advanced HCC is associated with
wasting symptoms, such as ascites and weight loss (15). One of the biochemical
characteristics of HCC is AFP elevation (16–18).
Pre-neoplastic lesions, such as fatty change and nodular
hyperplasia, are frequently observed in HCC in both early and
advanced stages (19). DEN is an
established environmental hepatocarcinogen initially used to induce
liver cancer in animal models (20). It has been shown that on primary
metabolic activation, DEN produces the promutagenic adducts,
O6-ethyl deoxy guanosine and O4- and
O6-ethyl deoxy thymidine that causes DNA-chain damage
and miscode gene sequences, paving the way to initiation of liver
carcinogenesis (6,21). In DEN-treated hamsters, progressive
body weight loss, hepatomegaly, ascites and tumor formation are
noted (22). The DEN-induced
macronodular HCC was characterized by poor differentiation, AFP
immunoreativity, vessel invasion and a potential application for
primary cell culture and tumor transplantation. These findings
indicate that administration of DEN produces HCC in hamsters that
mimics human HCC (23–28).
Primary human HCC exhibits characteristics, such as
arterial phase hypervascularity with portal phase washout, and the
presence of fatty degeneration and portal venous invasion.
Intrahepatic biliary duct dilatation is well recognized with
cholangiocarcinoma and is frequently observed in metastatic HCC
(29). HCC is frequently
accompanied by dilatation of the intrahepatic bile ducts (30–37),
while focal bile duct hyperplasia often occurs in the adjacent
non-tumorous liver tissue of European patients (38). In this study, pre-neoplastic
lesions, including focal bile duct hyperplasia, fatty change and
clear cell hyperplasia, occurred in DEN-induced
hepatocarcinogenesis.
In the present study, lung neoplastic cells were
morphologically distinct from HCC and had negative AFP reactivity,
indicating that the lung tumors were more likely primary lesions
rather than metastases. This finding is consistent with an early
report that feeding of DEN induces tumors of the lung and liver in
rats (39). To our knowledge, this
is the first report of lung tumors in DEN-treated hamsters.
In this study, DEN-induced hepatocarcinogenesis
progressed over specific periods. A toxic reaction and hepatocyte
regeneration occurred in the early stage defined from the 1st to
15th week of treatment. This was followed by prominent nodular
hyperplasia and fatty changes from 16 to 24 weeks of
administration. From 25 to 50 weeks of induction, both micronodular
and macronodular HCC occurred. These serial events are consistent
with the development of human hepatocarcinogenesis (40), highlighting the potential
application of this animal model for HCC research.
In the present study, dose-response effects were not
tested, since long-term treatment with DEN results in the
substantial loss of animals. On the other hand, the greater
susceptibility of animals toward a carcinogenic effect seems well
documented, but the underlying mechanism remains undetermined. In
summary, DEN induces hepatocarcinogenesis in hamsters and may serve
as a new and dynamic animal model for studying the pathogenesis and
clinical treatment of hepatocellular carcinoma.
Acknowledgements
This study was in part supported by
the National Natural Science Foundation of China (no. 30760281), a
direct grant from the Guilin Medical College, and by a Guangxi
Medical Science Experimental Center open fund for special projects
(KFJJ2010-49).
References
|
1.
|
J FerlayHR ShinF BrayD FormanC MathersDM
ParkinEstimates of worldwide burden of cancer in 2008: Globocan
2008Int J Cancer12728932917201010.1002/ijc.2551621351269
|
|
2.
|
L HerszenyiZ TulassayEpidemiology of
gastrointestinal and liver tumorsEur Rev Med Pharmacol
Sci14249258201020496531
|
|
3.
|
M ShermanHepatocellular carcinoma:
epidemiology, risk factors, and screeningSemin Liver
Dis25143154200510.1055/s-2005-87119415918143
|
|
4.
|
PA FaraziRA DePinhoHepatocellular
carcinoma pathogenesis: from genes to environmentNat Rev
Cancer6674687200610.1038/nrc193416929323
|
|
5.
|
P JananiK SivakumariA GeethaB RavisankarC
ParthasarathyChemopreventive effect of bacoside a on
n-nitrosodiethylamine-induced hepatocarcinogenesis in ratsJ Cancer
Res Clin Oncol136759770201010.1007/s00432-009-0715-019916024
|
|
6.
|
L VernaJ WhysnerGM
WilliamsN-nitrosodiethylamine mechanistic data and risk assessment:
bioactivation, DNA-adduct formation, mutagenicity, and tumor
initiationPharmacol
Ther715781199610.1016/0163-7258(96)00062-98910949
|
|
7.
|
XE LiuS DewaeleV VanhoorenYD FanL WangJ
van HuysseH ZhuangR ContrerasC LibertCC ChenAlteration of n-glycome
in diethylnitrosamine-induced hepatocellular carcinoma mice: a
non-invasive monitoring tool for liver cancerLiver
Int3012211228201010.1111/j.1478-3231.2010.02279.x20524982
|
|
8.
|
AK MandalS DasM MitraRN ChakrabartiM
ChatterjeeN DasVesicular flavonoid in combating diethylnitrosamine
induced hepatocarcinoma in a rat modelJ Exp Ther
Oncol7123133200818771086
|
|
9.
|
VG BychkovLS TrukhanovaGG KrylovLN
KulikovaED Mal’tsevaEA SmirnovaVF
KondalenkoN-nitrosodiethylamine-induced changes in the liver of
syrian hamster with superinvasive opistorchiasisVopr
Onkol494764832003(In Russian).
|
|
10.
|
R SimonsenMA VirjiInterpreting the profile
of liver-function tests in pediatric liver transplantsClin
Chem301607161019846148160
|
|
11.
|
A Llombart BoschA Peydro
OlayaUltrastructure of human hepatic carcinomas compared with
ultrastructural findings in hepatomas induced by chemical agents in
Wistar rats and the Golden hamsterRev Esp Oncol302873021983(In
Spanish).
|
|
12.
|
AC FluckigerG MarcyM MarchandD NegreFL
CossetS MitalipovD WolfP SavatierC DehayCell cycle features of
primate embryonic stem cellsStem
Cells24547556200610.1634/stemcells.2005-019416239321
|
|
13.
|
M Di MaioE De MaioF PerroneS PignataB
DanieleHepatocellular carcinoma: systemic treatmentsJ Clin
Gastroenterol35S109S114200212394214
|
|
14.
|
AS YuEB KeeffeManagement of hepatocellular
carcinomaRev Gastroenterol Disord38242003
|
|
15.
|
MC KweTumors of the liverHepatology – A
Text Book of Liver DiseaseD ZakimTD
BoyerSaundersPhiladelphia151315481996
|
|
16.
|
JJ LokichDetermination of response in
treatment of hepatic neoplasiaSemin Oncol1022823719836867753
|
|
17.
|
F RosiA TabucchiF CarlucciP GalieniF
LauriaL ZanoniR GuerrantiE MarinelloR Pagani5′-nucleotidase
activity in lymphocytes from patients affected by B-cell chronic
lymphocytic leukemiaClin Biochem312692721998
|
|
18.
|
S SellFF BeckerAlpha-fetoproteinJ Natl
Cancer Inst6019261978
|
|
19.
|
R KutamiY NakashimaO NakashimaK ShiotaM
KojiroPathomorphologic study on the mechanism of fatty change in
small hepatocellular carcinoma of humansJ
Hepatol33282289200010.1016/S0168-8278(00)80369-410952246
|
|
20.
|
M KawabeC LinN KimotoM SanoM HiroseT
ShiraiModifying effects of propolis on MeiQx promotion of rat
hepatocarcinogenesis and in a female rat two-stage carcinogenesis
model after multiple carcinogen initiationNutr
Cancer37179186200010.1207/S15327914NC372_10
|
|
21.
|
MLZ DagliAgentes
antineoplasicosFarmacologia Aplicada á Medicina Veterinaria3rd
editionHS SpinosaSL GorniakMM BernardiEditora Guanabara-KooganSão
PauloBrasil5776082002
|
|
22.
|
HC PitotHA CampbellR MaronpotN BawaTA
RizviYH XuL SargentY DraganM PyronCritical parameters in the
quantitation of the stages of initiation, promotion, and
progression in one model of hepatocarcinogenesis in the ratToxicol
Pathol1759461219892697939
|
|
23.
|
T KalinskiA RoessnerHepatocellular
carcinoma: pathology and liver biopsyDig
Dis27102108200910.1159/00021834119546547
|
|
24.
|
SR PrasadH WangH RosasCO MeniasVR NarraWD
MiddletonJP HeikenFat-containing lesions of the liver:
radiologic-pathologic
correlationRadiographics25321331200510.1148/rg.25204508315798052
|
|
25.
|
T RoskamsM KojiroPathology of early
hepatocellular carcinoma: conventional and molecular diagnosisSemin
Liver Dis301725201010.1055/s-0030-124712920175030
|
|
26.
|
M SakamotoS HirohashiY ShimosatoEarly
stages of multistep hepatocarcinogenesis: adenomatous hyperplasia
and early hepatocellular carcinomaHum
Pathol22172178199110.1016/0046-8177(91)90039-R
|
|
27.
|
K ShirabeT MotomuraJ MutoT ToshimaR
MatonoY ManoK TakeishiH IjichiN HaradaH UchiyamaT YoshizumiA
TaketomiY MaeharaTumor-infiltrating lymphocytes and hepatocellular
carcinoma: pathology and clinical managementInt J Clin
Oncol15552558201010.1007/s10147-010-0131-020963618
|
|
28.
|
A VillanuevaY HoshidaC BattistonV TovarD
SiaC AlsinetH CornellaA LiberzonM KobayashiH KumadaSN ThungJ BruixP
NewellC AprilJB FanS RoayaieV MazzaferroME SchwartzJM
LlovetCombining clinical, pathology, and gene expression data to
predict recurrence of hepatocellular
carcinomaGastroenterology14015011512201110.1053/j.gastro.2011.02.00621320499
|
|
29.
|
KS JhaveriJ HalankarD AguirreM HaiderG
LockwoodM GuindiS FischerIntrahepatic bile duct dilatation due to
liver metastases from colorectal carcinomaAJR Am J
Roentgenol193752756200910.2214/AJR.08.218219696289
|
|
30.
|
M JinzakiA TanimotoK SuzukiT SekiY SatohK
HiramatsuM MukaiI NakanishiLiver metastases from colon cancer with
intra-bile duct tumor growth: Radiologic featuresJ Comput Assist
Tomogr21656660199710.1097/00004728-199707000-000279216779
|
|
31.
|
M KojiroK KawabataY KawanoF ShiraiN
TakemotoT NakashimaHepatocellular carcinoma presenting as intrabile
duct tumor growth: a clinicopathologic study of 24
casesCancer4921442147198210.1002/1097-0142(19820515)49:10%3C2144::AID-CNCR2820491026%3E3.0.CO;2-O6280834
|
|
32.
|
NW LeeKP WongKF SiuJ WongCholangiography
in hepatocellular carcinoma with obstructive jaundiceClin
Radiol35119123198410.1016/S0009-9260(84)80008-26321083
|
|
33.
|
K OkanoJ YamamotoY MoriyaT AkasuT KosugeM
SakamotoS HirohashiMacroscopic intrabiliary growth of liver
metastases from colorectal
cancerSurgery126829834199910.1016/S0039-6060(99)70022-X10568181
|
|
34.
|
K OkanoJ YamamotoT OkabayashiY SugawaraK
ShimadaT KosugeS YamasakiH FurukawaY MuramatsuCt imaging of
intrabiliary growth of colorectal liver metastases: a comparison of
pathological findings of resected specimensBr J
Radiol75497501200210.1259/bjr.75.894.75049712124235
|
|
35.
|
P SoyerA SibertJP LaissyIntrahepatic bile
duct dilatation secondary to hepatocellular carcinoma: Ct features
in 10 patientsAbdom
Imaging20114117199510.1007/BF002015167787711
|
|
36.
|
S TakamatsuK TeramotoT KawamuraA KudoN
NoguchiT IrieT OchiaiJ KumagaiM KoikeS AriiLiver metastasis from
rectal cancer with prominent intrabile duct growthPathol
Int54440445200410.1111/j.1440-1827.2004.01636.x15144404
|
|
37.
|
E van SonnenbergJT Ferrucci JrBile duct
obstruction in hepatocellular carcinoma (hepatoma) – clinical and
cholangiographic characteristics. Report of 6 cases and review of
the literatureRadiology1307131979
|
|
38.
|
Z SchaffCC HsiaI SarosiE
TaborOverexpression of transforming growth factor-alpha in
hepatocellular carcinoma and focal nodular hyperplasia from
European patientsHum
Pathol25644651199410.1016/0046-8177(94)90296-88026823
|
|
39.
|
KM HerroldLJ DunhamInduction of tumors in
the syrian hamster with diethylnitrosamine
(N-nitrosodiethylamine)Cancer Res23773777196313954107
|
|
40.
|
CY LeeYC HsuJY WangCC ChenJH
ChiuChemopreventive effect of selenium and chinese medicinal herbs
on n-nitrosobis(2-oxopropyl)amine-induced hepatocellular carcinoma
in syrian hamstersLiver
Int28841855200810.1111/j.1478-3231.2008.01698.x18346132
|