Introduction
Cervical carcinoma is the second leading cause of
cancer-related death in women worldwide, with over 500,000 new
cases reported each year in developing countries. High-risk human
papillomavirus (HPV) infection such as HPV16 and/or HPV18 is
regarded as the major etiological factor for cervical carcinoma
(1,2), however, genetic factors may play an
important role in its occurrence. The activation of oncogenes
and/or the inactivation of tumor-suppressor genes play an important
role in tumor development and progression (3,4).
Therefore, it is important to study the genes associated with
cervical carcinoma at the molecular level.
The bladder cancer-associated protein (BLCAP)
gene (GenBank accession no. NM006698) is located on chromosome 20
and is a novel tumor-suppression gene identified from human bladder
carcinoma. The mRNA level of BLCAP has been found to be
markedly downregulated in bladder carcinoma (5,6), as
well as human tongue carcinoma (7). In our previous study, BLCAP mRNA
expression was decreased in cervical tumor tissues (7) and overexpression of BLCAP was
found to inhibit cell growth and to induce apoptosis in the human
cervical cancer HeLa cell line (8). Since BLCAP may be a cervical
carcinoma-related suppressor gene, it is important to determine
protein expression levels in cervical carcinoma.
Here, we established a pET prokaryotic expression
system to express the His-tagged BLCAP fusion protein to immune
rabbits for preparing the polyclonal antibody. This purified BLCAP
polyclonal antibody was used to detect the BLCAP protein expression
level in 30 cervical carcinoma tissues and 30 normal cervical
tissues.
Materials and methods
Plasmid construction
The primer sequences of the BLCAP were designed with
Primer 5.0 software, and the coding region of the mature BLCAP
protein was amplified using polymerase chain reaction (PCR). Primer
1 (5′-GCA GAA TTC ATG TAT TGC CTC CAG TG-3′) and primer 2
(5′-GC AAG CTT TTA GGT GCC CAC AAC G-3′) were synthesized by
Invitrogen (Shanghai, China). Primer 1 was synthesized with an
EcoRI site (shown in bold) and primer 2 was synthesized with
a HindIII site (shown in bold). The amplification profile
included one initial hot-start denaturing step at 94°C for 5 min,
followed by 30 cycles of the following conditions: 94°C for 1 min,
58°C for 30 sec, and 72°C for 1 min, and a final extension at 72°C
for 10 min. The expression vector pET-32(a) was digested with
EcoRI and HindIII. The digested pET-32(a) was
purified by agarose gel and extracted using the QIAquick Gel
Extraction kit.
A recombinant plasmid was constructed by inserting
the PCR amplified fragment (also digested with EcoRI and
HindIII) into the pET-32(a) vector and transformed into the
E. coli strain Rosetta. The transformants [pET-32(a)-BLCAP]
were confirmed by PCR, restriction enzyme digestion and DNA
sequencing.
Prokaryotic expression and purification
of full length BLCAP
The prokaryotic expression vector pET-32a-BLCAP was
introduced into the bacterial host E. coli strain Rosetta
following standard protocol. Rosetta, the derivational strain of
BL21, contains the extra gene copies for coding rare tRNA, which
facilitates the prokaryotic expression of eukaryotic protein in
E. coli Rosetta. The transformants were cultured in LB
medium containing ampicillin (100 μg/ml) at 37°C in a shaking
incubator until an OD600 of 0.8 to 1.0 was attained. Recombinant
BLCAP expression was induced by adding 1 mM
isopropyl-1-thio-D-galactopyranoside (IPTG) and further incubation
at 37°C for 6 h. The cells were harvested by centrifugation at
5,000 × g for 15 min at 4°C, and the pellets were re-suspended in
lysis buffer [50 mM Tris (pH 8.9), 100 mM NaCl, 1 mM EDTA, 100
μg/ml lysozyme, 100 μg/ml phenylmethylsulfonyl fluoride (PMSF) and
1 μg/ml each of pepstatin, leupeptin and aprotinin], and further
incubated at 4°C for 1 h. Thereafter, the cells were sonicated and
centrifuged at 12,000 × g for 30 min. The clear supernatant was
collected and used for protein purification.
The recombinant protein was purified based on its
N-terminal His6-tag by affinity chromatography using a
Ni2+-NTA HiTrap chelating Sepharose column (Qiagen) that
was equilibrated with 20 ml buffer A containing 10 mM imidazole.
The cell lysate was applied to the column and allowed to bind using
a flow rate of 1.0 ml/min. The bound protein was eluted by applying
a gradient of 10–500 mM imidazole in buffer A using a flow rate of
1.5 ml/min. Peak fractions were collected between 200 and 300 mM
imidazole. The Bradford method was used to determine the protein
concentration, and the protein was confirmed by western blot
analysis using an anti-His monoclonal antibody.
Production and purification of polyclonal
antibody against BLCAP
New Zealand white rabbits received an intradermal
injection of BLCAP protein (500 μg/rabbit) mixed with CFA in a 1:1
ratio. After 4 week, the rabbits were boosted subsequently 3 times
at two-week intervals with the BLCAP protein (250 μg/rabbit) mixed
with IFA in a 1:1 ratio. Prior to immunization, blood samples were
taken from the marginal vein of the rabbit ear, and the sera were
obtained to determine the antibody titer by western blotting. The
polyclonal antibody was purified by following a standard protocol
for the purification of the antibody.
Western blot analysis and agar gel
precipitin test
Purified proteins were analyzed by western blot
analysis (12% SDS PAGE gel) following a standard procedure. After
an overnight blocking in 5% BSA the membrane was incubated with an
anti-BLCAP fusion protein polyclonal antibody for 1 h at room
temperature (RT) and then incubated in alkaline phosphatase
(Ap)-conjugated goat anti-rabbit IgG at a dilution of 1:1500 for 1
h at RT. The membrane was washed and the specific protein bands
were visualized using 3,3′-diaminobenzidine (DAB). To determine
antibody titer, the agar gel precipitin test was performed as
described previously with modification (9). Briefly, a cluster of six wells
surrounding a center well was cut into the solidified agar.
Antiserum (25 μl) with a series of dilution (1/2, 1/4, 1/8, 1/16,
1/32 and 1/64) was delivered to the outside well, and purified
BLCAP antigen was delivered to the center well. The plates were
incubated for 24 h and the precipitin reaction was determined.
Patient tissue specimens
A total of 60 cervical specimens consisting of 30
age-matched carcinoma tissues and 30 normal cervical tissues were
collected from the Pathology Department at Zhong Nan Hospital
(Wuhan University, China) between 2005 and 2006. The patients were
evaluated based on the AJCC TNM classification system (10) as: stage I–II (n=16), stage III–IV
(n=14); 13 patients had lymphatic metastasis; well-differentiated
tumors (n=16) and moderatedly/poorly differentiated tumors (n=14);
squamous cell (SCC) tumors (n=26) and adenosquamous (AC) tumors
(n=4). The average age of the patients was 43.3 years ranging from
33 to 63. None of the patients received radiochemotherapy prior to
surgery.
Immunohistochemistry
Sections (5-μm thick) from paraffin-embedded blocks
were mounted on slides. These sections were deparaffinized and then
rehydrated with gradient alcohols. Heat-induced antigen retrieval
was performed in citrate buffer (pH 6.0) by heating the slides in a
microwave oven (700 W for 15 min) and then processed for
immunohistochemistry. In brief, the tissue sections were washed
three times with phosphate-buffered saline (PBS) and incubated with
normal horse serum for 30 min at RT to block non-specific binding.
Endogenous peroxidase activity was quenched by incubating sections
in 3% H2O2 in PBS for 20 min. Sections were
then incubated with anti-BLCAP rabbit polyclonal antibody for 60
min at RT and washed with PBS-T containing 0.05% Tween-20 (3 × 5
min) before incubating with horseradish peroxidase-labeled
secondary antibody for 30 min. Slides were washed again (3 × 5 min)
with PBS-T. The reaction color was developed by incubating sections
with 3,3′-diaminobenzidine reagent (DAB) as per the manufacturer’s
instructions. The slides were washed with water, counterstained
with hematoxylin, dehydrated, mounted and examined under light
microscopy. A normal murine IgG was used for the negative
control.
Evaluation of immunohistochemistry (IHC)
and statistical analysis
IHC was performed as described previously.
Immunostaining was graded as negative (no cells stained), weak
(<10% cells stained), moderate (11–50% cells stained) and strong
(>51%). For statistical analysis, the staining results were
classified into two groups: group I was the ‘negative group’ (no
staining); and group II the ‘positive group’ (weak intensity,
moderate intensity and strong intensity). Statistical analysis for
group differences was performed with the χ2 test. For
all statistical tests, a P-value <0.05 was considered
statistically significant.
Results
Construction of expression plasmid
pET-32a-BLCAP
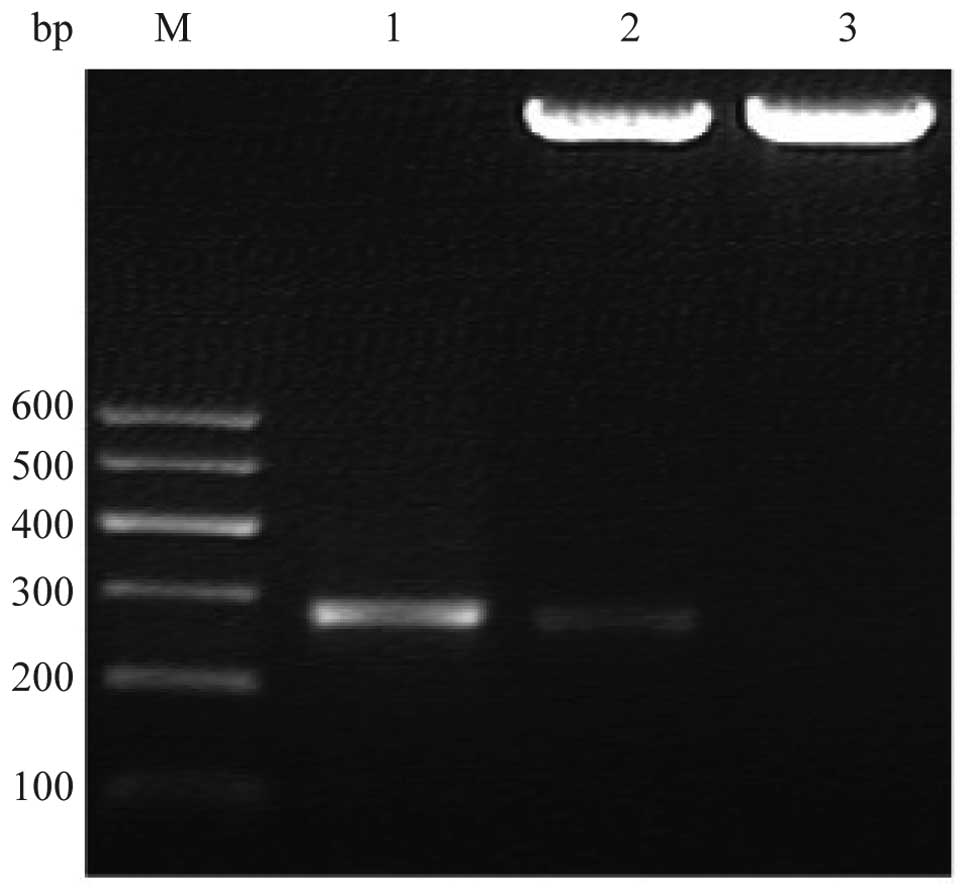
The 264 bp fragment of the BLCAP gene was amplified
by PCR. The product was cloned into pET-32a and confirmed by PCR
and restriction digestion (Fig.
1). DNA sequencing revealed that DNA was the reported sequence.
The recombinant plasmid DNA contained the BLCAP gene in-frame with
N-terminal Trx-tag and His-tag encoding the ∼28 kDa, 762-amino acid
fusion protein.
Expression, purification and analysis of
the protein
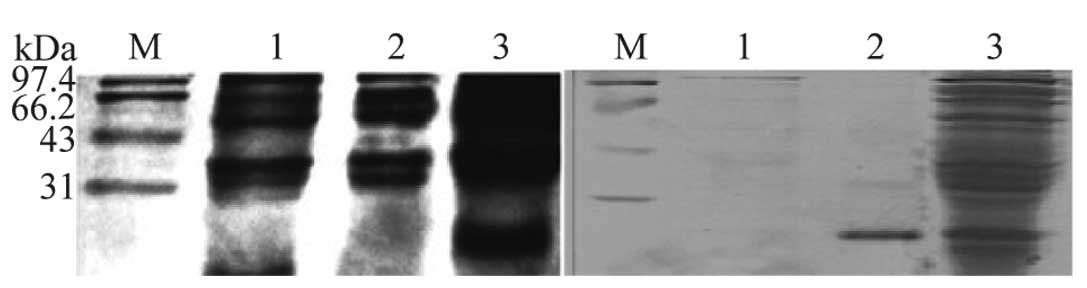
After induction with IPTG, E. coli Rosetta
transformed with pET-32a-BLCAP produced a 28-kDa protein shown in
Fig. 2a. The size of the protein
matched its theoretical molecular weight. To determine the optimal
induction period, we used different IPTG concentrations (0.1, 0.2,
0.4, 0.6, 0.8 and 1.0 mM), different temperatures (20, 25, 30 and
37°C), and varied induction times (1, 2, 3, 4, 5, 6, 8 and 12 h).
The results showed that the yield was increased with an incubation
time and reach a plateau after 6 h. The final concentration of IPTG
used in this experiment was 1 mmol/l, and the bacteria were
cultivated at 37°C for 6 h. Ni-NTA affinity chromatography was
applied for purification of the BLCAP fusion protein. The target
BLCAP appeared as a single band on SDS-PAGE (Fig. 2b, lane 2), which is in agreement
with the molecular weight reported.
Western blotting and titer
determination
A homogenized preparation containing a single
protein for BLCAP could be confidently used for immunizing the New
Zealand White rabbit. The antiserum was collected 15 weeks after
the initial injection. Three addition booster injections were given
and the antiserum was again collected after each booster. A 1:8,000
dilution of the 28-kDa His-tagged BLCAP protein was detected in
western blot analysis using IPTG-induced E. coli Rosetta
cell extract. There was no immune-reactivity observed in un-induced
cells (Fig. 3a). Agar gel
precipitin test demonstrated that distinct bands were observed
between the antigen and antiserum wells, as a result of the antigen
migrating through the agar matrix toward and interacting with
antiserum (Fig. 3b).
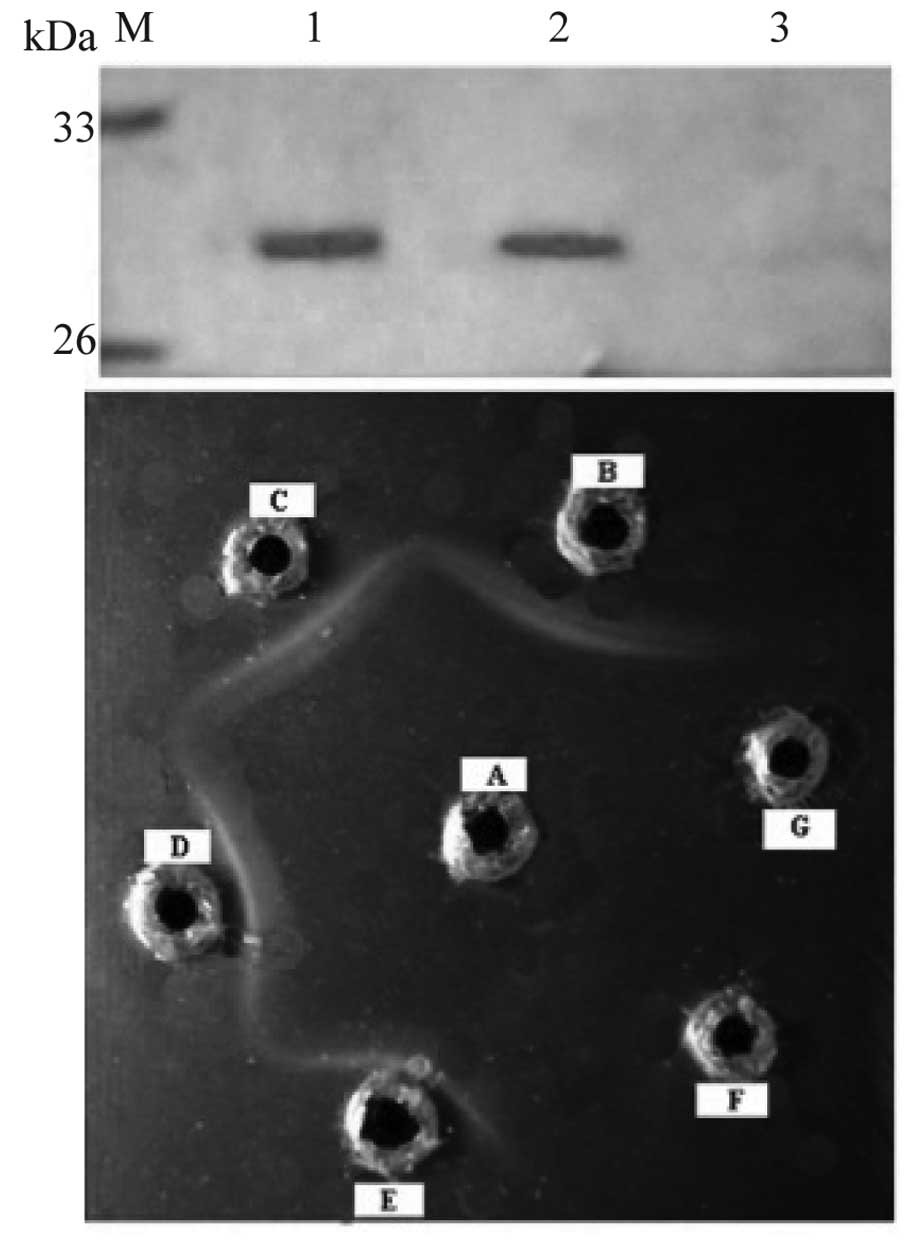 | Figure 3.(a) Western blot analysis of
recombinant protein using antiserum. Lanes 1 and 2, antiserum; lane
3, pre-immune serum. (b) Determination of antibody titer by agar
gel precipitin test, A, antigen, B–G, diluted antiserum, 1/2, 1/4,
1/8, 1/16, 1/32 and 1/64, respectively. |
Tissue immunohistochemistry
The purified polyclonal antibody was used to detect
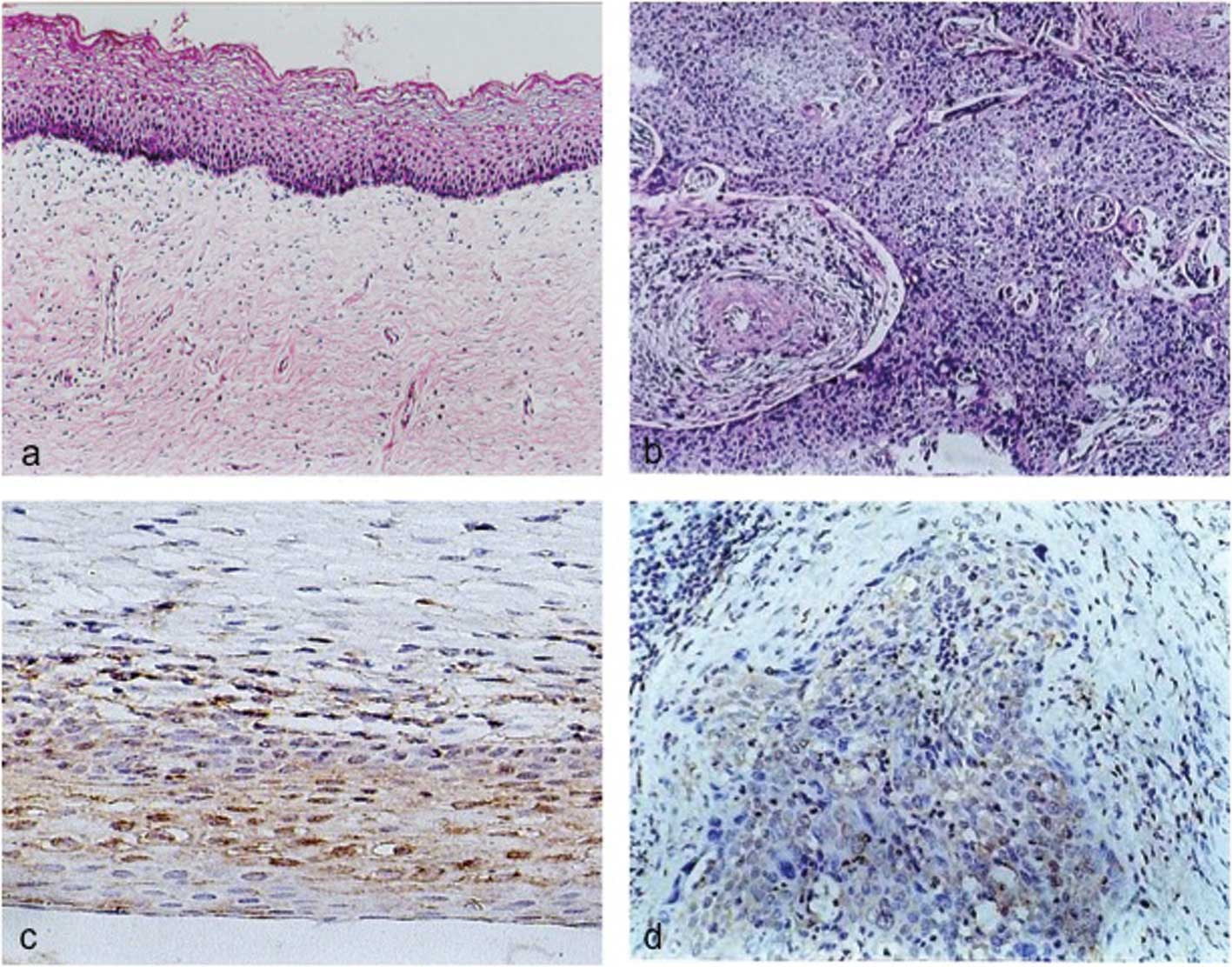
BLCAP protein levels in cervical tissues by immunohistochemistry.
This positive expression of BLCAP protein was detected in 27 of 30
(90%) normal cervical tissues with 15 of the 27 (55%) having
moderate to strong cytoplasmic staining. However, in cervical
carcinoma tissues 16 of 30 (53.33%) expressed BLCAP protein with 15
of the 16 (93.75%) having weak cytoplasmic staining (Table I). The expression level of BLCAP in
cervical carcinoma was significantly lower than that in the
corresponding normal tissues (P<0.05)(Fig. 4). Moreover, the expression level of
BLCAP in cervical carcinoma was significantly associated with
clinical degree and tumor differentiation of cervical carcinoma
(P<0.05). No significant difference was observed between SCC and
AC (Table II).
 | Table I.Immunohistochemical analysis of BLCAP
expression in cervical tissues. |
Table I.
Immunohistochemical analysis of BLCAP
expression in cervical tissues.
| Total | BLCAP expression
| Positive rate
(%) |
|---|
| − | + | ++ | +++ |
|---|
| Normal cervical
tissues | 30 | 3 | 8 | 15a | 4 | 90.00a |
| Cervical carcinoma
tissues | 30 | 14 | 15 | 1 | 0 | 53.33 |
 | Table II.Relationship between the expression
of BLCAP and the clinical and histopathological features of
cervical carcinoma. |
Table II.
Relationship between the expression
of BLCAP and the clinical and histopathological features of
cervical carcinoma.
| Clinical
pathological characteristic | n | BLCAP expression
| P-value |
|---|
| n (%) |
|---|
| Histological
type | | | |
| SCC | 26 | 14 (81.85) | >0.05 |
| AC | 4 | 2 (50) | |
| Stage | | | |
| I–II | 16 | 10 (62.5) | <0.05 |
| III–IV | 14 | 6 (42.86) | |
| Degree of
differentiation | | | |
| High | 10 | 7 (70) | <0.05 |
| Moderate/low | 20 | 9 (45) | |
| Lymphatic
metastasis | | | |
| Non-LM | 17 | 12 (70.59) | <0.05 |
| LM | 13 | 4 (30.77) | |
| Total | 30 | 16 (53.55) | |
Discussion
The pET-32(a) vector which contains the thioredoxin
(Trx) tag was used in this study to successfully produce a BLCAP
fusion protein with a sufficient quantum. The Trx tag increases the
molecular weight of the fusion protein. Athough this makes the
protein more soluble it does not affect its activity (11,12).
Western blot analysis revealed the molecular weight of the protein
to be approximately 28 kDa (10 kDa plus the 18 kDa Trx/His protein)
as predicted. To screen E. coli strains for expression, both
Rosetta and BL21 were used as host cells to perform prokaryotic
expression in the initial stage. The fusion proteins were expressed
in the Rosetta but not in BL21. The reason is that BL21 contains
rare tRNA codons in the coding sequence of BLCAP, which impedes the
expression of eukaryotic protein (13,14).
Using Rosetta as the host cell we optimized the culture and
induction parameters to get a more soluble fusion protein.
The expressed His-tagged protein was purified by
Ni2+ affinity chromatography column and monitored by
western blotting with an anti-hexahistidine tag antibody. The
purified recombinant proteins were found to be immunogenic in
rabbits and produced polyclonal antibodies. Western blot analysis
showed that this antibody was highly sensitive and specific.
The expression rate of BLCAP in cervical carcinoma
tissues was significantly lower than that in the normal tissues
(P<0.05). This suggests that decreased expression of BLCAP may
be directly related to the development of cervical carcinoma.
Meanwhile, the expression of BLCAP was related to clinical stage
and cell differentiation, which indicates that the loss of BLCAP
may be involved in tumor invasion and metastasis.
In conclusion, a high titer, highly specific BLCAP
polyclonal antibody was produced. The differential expression of
BLCAP at the protein level was consistent with that of mRNA levels.
BLCAP may be a cervical carcinoma-related suppressor gene, which is
the foundation for studying the function of BLCAP in clinical
applications as a tumor marker.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (nos. 81072123,
30571955 and 81102024).
References
|
1.
|
K SzokeT SapyZ KrasznaiZ HernadiG SzladekG
VeressJ DillnerL GergelyJ KonyaModerate variation of the oncogenic
potential among high-risk human papillomavirus types in gynecologic
patients with cervical abnormalitiesJ Med
Virol71585592200310.1002/jmv.10526
|
|
2.
|
J BultenWJ MelchersMM Kooy-SmitsPC de
WildePJ PoddigheJC RobbenMV MacvilleLF MassugerJM BakkersAG
HanselaarNumerical aberrations of chromosome 1 in cervical
intraepithelial neoplasia are strongly associated with infection
with high-risk human papillomavirus typesJ
Pathol198300309200210.1002/path.1222
|
|
3.
|
A SarasinAn overview of the mechanisms of
mutagenesis and carcinogenesisMutat
Res54499106200310.1016/j.mrrev.2003.06.02414644312
|
|
4.
|
J YokotaTumor progression and
metastasisCarcinogenesis21497503200010.1093/carcin/21.3.49710688870
|
|
5.
|
JM MoreiraG OhlssonP GromovR SimonG
SauterJE CelisI GromovaBladder cancer-associated protein, a
potential prognostic biomarker in human bladder cancerMol Cell
Proteomics9161177201010.1074/mcp.M900294-MCP20019783793
|
|
6.
|
I GromovaP GromovJE CelisBc10: a novel
human bladder cancer-associated protein with a conserved genomic
structure downregulated in invasive cancerInt J
Cancer98539546200210.1002/ijc.1024411920613
|
|
7.
|
Z ZuoM ZhaoJ LiuG GaoX WuFunctional
analysis of bladder cancer-related protein gene: a putative
cervical cancer tumor suppressor gene in cervical carcinomaTumour
Biol27221226200610.1159/00009305716675915
|
|
8.
|
ZH ZuoM ZhaoJ LiuY WeiXX WuInhibitory
effect of bladder cancer related protein gene on hela cell
proliferationAi Zheng258118172006(In Chinese).
|
|
9.
|
PS HoltHD StoneRK GastCR GreeneApplication
of the agar gel precipitin test to detect antibodies to
Salmonella enterica serovar enteritidis in serum and egg
yolks from infected hensPoult
Sci7912461250200010.1093/ps/79.9.124611020067
|
|
10.
|
B StephenDRB EdgeAJCC Cancer Staging
Manual7th editionSpringer2009
|
|
11.
|
G De WildeN MertensE BooneB De VreeseJ Van
BeeumenW FiersG HaegemanExpression in Escherichia coli of
the death domain of the human p55 tumor necrosis factor
receptorProtein Expr Purif232262322001
|
|
12.
|
LI LobelS PollakJ KleinJW
LustbaderHigh-level bacterial expression of a natively folded,
soluble extracellular domain fusion protein of the human
luteinizing hormone/chorionic gonadotropin receptor in the
cytoplasm of escherichia
coliEndocrine14205212200110.1385/ENDO:14:2:205
|
|
13.
|
T WakagiT OshimaH ImamuraH
MatsuzawaCloning of the gene for inorganic pyrophosphatase from a
thermoacidophilic archaeon, sulfolobus sp Strain 7, and
overproduction of the enzyme by coexpression of tRNA for arginine
rare codonBiosci Biotechnol
Biochem6224082414199810.1271/bbb.62.24089972267
|
|
14.
|
RA SpanjaardK ChenJR WalkerJ van
DuinFrameshift suppression at tandem AGA and AGG codons by cloned
tRNA genes: assigning a codon to argU tRNA and T4 tRNA (Arg)Nucleic
Acids Res1850315036199010.1093/nar/18.17.50312205835
|