Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies in the world, and is the third-leading cause of
mortality from cancer (1,2) and the fifth most prevalent malignancy
worldwide (3). Treatment options
for HCC include liver resection, radiofrequency ablation (RFA) and
molecular targeted therapies, such as sorafenib (4). In spite of the advances in the
treatment and early detection of HCC, the prognosis of patients
with HCC is still unsatisfactory and HCC remains an intractable
disease. Experimental animal models of HCC are feasible for
investigating novel chemopreventive remedies for patients with
chronic liver diseases who are at high risk of developing HCC.
Although a number of HCC models have been generated, including
hepatitis C virus (HCV) core transgenic mice (5), Pten-deficient mice (6) and activation-induced cytidine
deaminase (AID)-transgenic mice (7), these models require intricate genetic
manipulation.
Diethylnitrosamine (DEN) is present in tobacco
smoke, water, cured and fried meals, agricultural chemicals,
cosmetics and pharmaceutical agents (8) and is commercially available for
experimental use. DEN is an established powerful hepatocarcinogen
in rats, which possibly works by altering the DNA structure,
forming alkyl DNA adducts, and inducing chromosomal aberrations and
micronuclei in the liver (9,10).
It has also been reported that oxidative stress plays a pivotal
role during carcinogenesis (11).
Although a single injection of DEN followed by partial hepatectomy
coupled with 2-acetylaminofluorene (2-AAF) is an established
procedure for developing HCC in rodents (12), the sequential administration of DEN
for a number of weeks has also been employed for inducing HCC
(13,14). However, sequential changes in the
liver in DEN-based hepatocarcinogenesis have not been clarified. In
this study, we analyzed DEN-induced hepatocarcinogenesis in rats by
chronologically evaluating biological parameters and liver tissues
following treatment with DEN.
Materials and methods
Chemicals
DEN and an anti-β-actin antibody were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Pentobarbital was purchased
from Dainippon Sumitomo Pharma Co., Ltd. (Osaka, Japan). Antibodies
against proliferating cell nuclear antigen (PCNA) and glutathione
S-transferase placental type (GST-P) were purchased from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA) and Assay Designs, Inc.
(Ann Arbor, MI, USA), respectively. Secondary anti-mouse and
anti-rabbit horseradish peroxidase (HRP) antibodies for western
blot analysis were obtained from GE Healthcare Ltd.
(Buckinghamshire, UK). All other chemicals and solvents used in
this study were of analytical grade.
Animals, treatments and tissue
collection
Male Wistar rats weighing ~200 g were purchased from
Japan SLC, Inc. (Hamamatsu, Shizuoka, Japan). All animals received
humane care and the experimental protocols were approved by the
Tottori University Animal Ethics Committee. The animals were housed
two per cage with rice husks for bedding in an air-ventilated room
under a 12-h light/dark cycle with a constant temperature (22°C)
and humidity (55%). The animals were allowed access to food and tap
water ad libitum during the experiment. The rats were
randomly divided into two groups and intraperitoneally injected
with DEN (40 mg/kg body weight) in phosphate-buffered saline (PBS)
(DEN groups, 4 rats were assigned to each treatment week) or PBS
(control groups, 2 rats were assigned to each treatment week)
weekly for 4, 6, 8, 10, 12 and 14 weeks (Fig. 1). Body weights were monitored
weekly throughout the experimental period. One week following the
last treatment, the rats were sacrificed under anesthesia by
pentobarbital. Blood samples were collected via cardiac puncture
and serum samples were stored at −30°C until analysis. Immediately
after the livers were excised, they were weighed and divided into
two sections for histological examination in 10% neutral buffered
formalin and for protein extraction at −80°C.
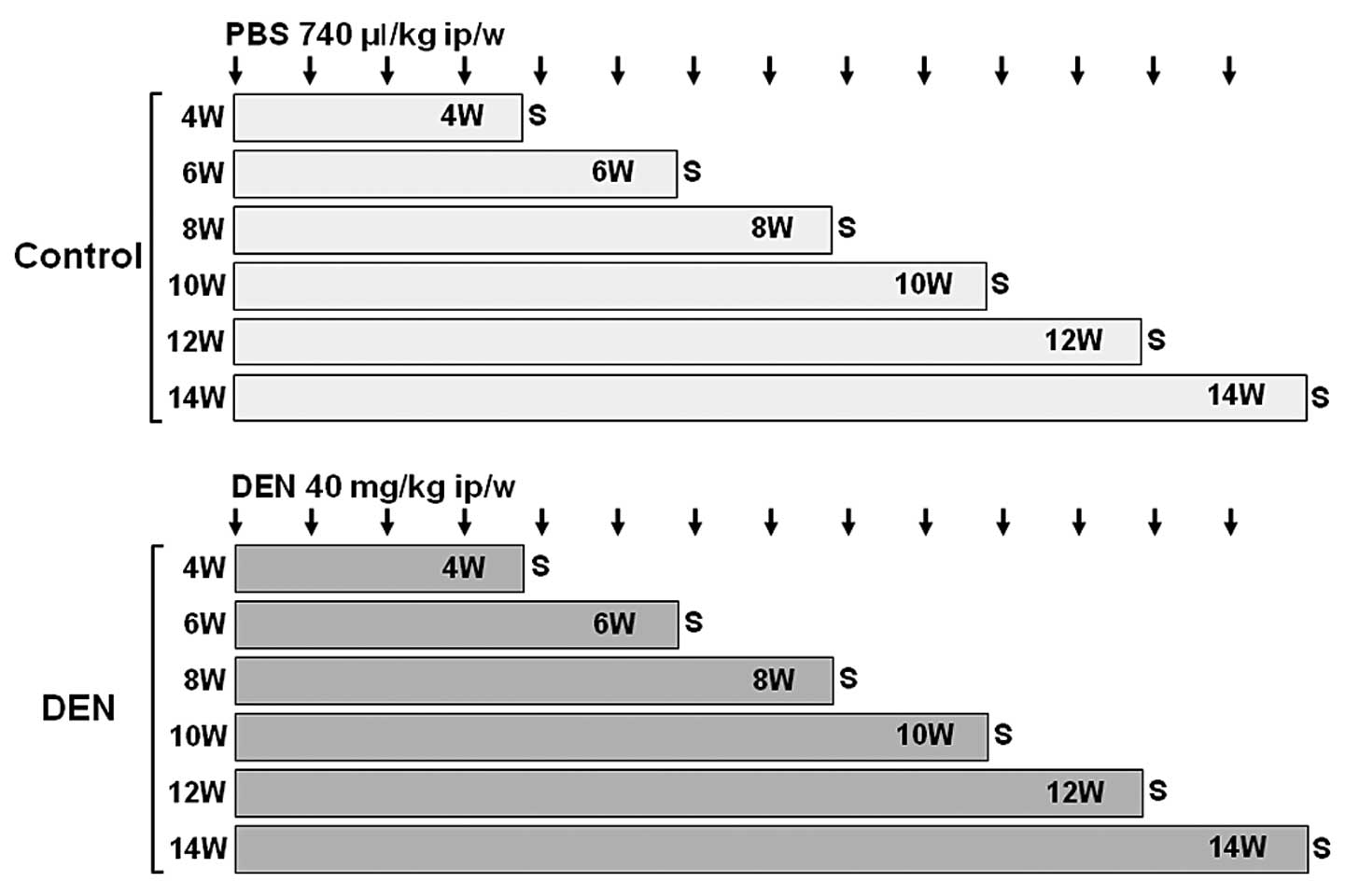 | Figure 1.Experimental schedules of Wistar
rats. Male Wistar rats were randomly divided into two groups: DEN
and control groups. Rats in the DEN group were intraperitoneally
injected with 40 mg/kg body weight of DEN dissolved in PBS for 4,
6, 8, 10, 12 and 14 weeks. Four rats were assigned to each
treatment week. Rats in the control group were intraperitoneally
injected with 740 μl/kg body weight of PBS for 4, 6, 8, 10, 12 and
14 weeks. Two rats were assigned to each treatment week. DEN,
diethylnitrosamine; ip, intraperitoneal; PBS, phosphate-buffered
saline; S, sacrifice; W, weeks. |
Measurement of serum transaminase and
total bilirubin
Serum aspartate aminotransferase (AST), alanine
aminotransferase (ALT) and total bilirubin levels were measured at
SRL, Inc. (Tokyo, Japan).
Total protein preparation and western
blotting
The liver samples were mashed with a BioMasher
(Nippi Inc., Tokyo, Japan) and lysed in radioimmune precipitation
(RIPA) buffer (Millipore Corp., Bedford, MA, USA) supplemented with
1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride
(PMSF) and a protease inhibitor mixture tablet (Roche Diagnostics,
Basel, Switzerland) for 10 min on ice. Total protein samples (5 μg)
were separated on a sodium lauryl sulfate (SDS)-polyacrylamide gel
(PAGE) (SuperSep, Wako Pure Chemical Industries, Ltd., Osaka,
Japan) and transferred to a polyvinylidene difluoride (PVDF)
membrane (Immobilon-P, Millipore Corp.). After the membranes were
blocked in 5% non-fat milk (Santa Cruz Biotechnology Inc.) in TBST
(10 mM Tris, 150 mM NaCl, pH 8.0, and 0.1% Tween-20) for 1 h at
room temperature, they were probed with primary antibodies
overnight at 4°C, washed three times in TBST, and incubated with
anti-mouse or anti-rabbit HRP antibody in TBST for 1 h at room
temperature. After the signals were developed with a
chemiluminescence solution (ECL, GE Healthcare Ltd.), they were
visualized and quantified using an image analyzer (LAS-3000 mini,
Fujifilm Co., Tokyo, Japan).
Histology and immunohistochemistry
The rat liver tissues were fixed in 10% neutral
buffered formalin and paraffin embedded. For histologic analysis,
serial sections (5 μm) were stained with hematoxylin and eosin
(H&E). Neoplastic nodules and HCC were classified on the basis
of Japanese criteria (15).
Degenerated hepatocytes, oval cells, renewed hepatocytes and
hyperplastic nodules were quantified as follows: grade 1 when
<5%, grade 2 when 5–50%, and grade 3 when >50% in the field.
For immunohistochemistry with the PCNA and GST-P antibodies,
Histofine® Simple Stain Rat MAX PO was employed
(Nichirei Biosciences Inc., Tokyo, Japan). Briefly, after routine
dewaxing with xylene and hydration through a graded ethanol series,
the sections were incubated with 3% hydrogen peroxide solution for
15 min at room temperature to quench endogenous peroxidase
activity. After washing in gently-running tap water, the sections
were rinsed with PBS, and incubated with primary antibodies
overnight at 4°C. After rinsing with PBS, the sections were
incubated with biotinylated secondary antibody for 30 min at room
temperature. The peroxidase activity was developed with DAB
solution (Vector Laboratories, Inc., Burlingame, CA, USA).
Counterstaining was performed with hematoxylin. The PCNA labeling
indices were represented as the percentage of positively stained
nuclei by counting 1,000 cells in the field at x400 magnification.
The GST-P-positive area was measured on images captured by a charge
coupled device (CCD) camera on a Windows® computer.
Statistical analysis
All data were expressed as the means ± standard
deviation (SD). Statistical analysis was performed by the unrelated
t-test or the Mann-Whitney U test. A p-value <0.05 was
considered to indicate a statistically significant difference.
Results
Relative liver weight and biological
parameters
All rats survived throughout the experimental
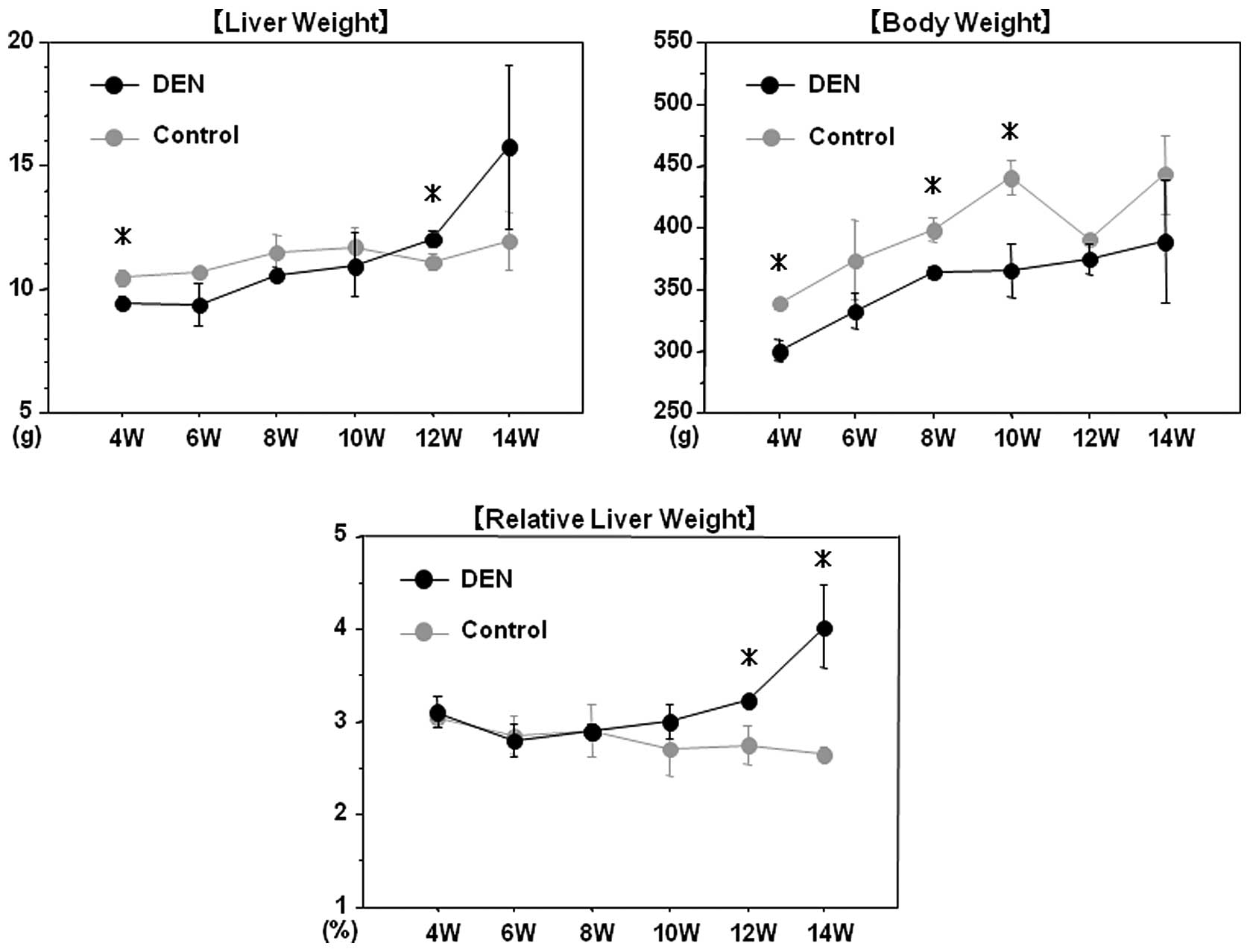
period. Chronological changes in the liver and body weights are
demonstrated in Fig. 2. After 12
and 14 weeks of the treatment, the relative liver weight (liver
weight/body weight) in the DEN group was significantly higher than
that in the control group, presumably due to the development of
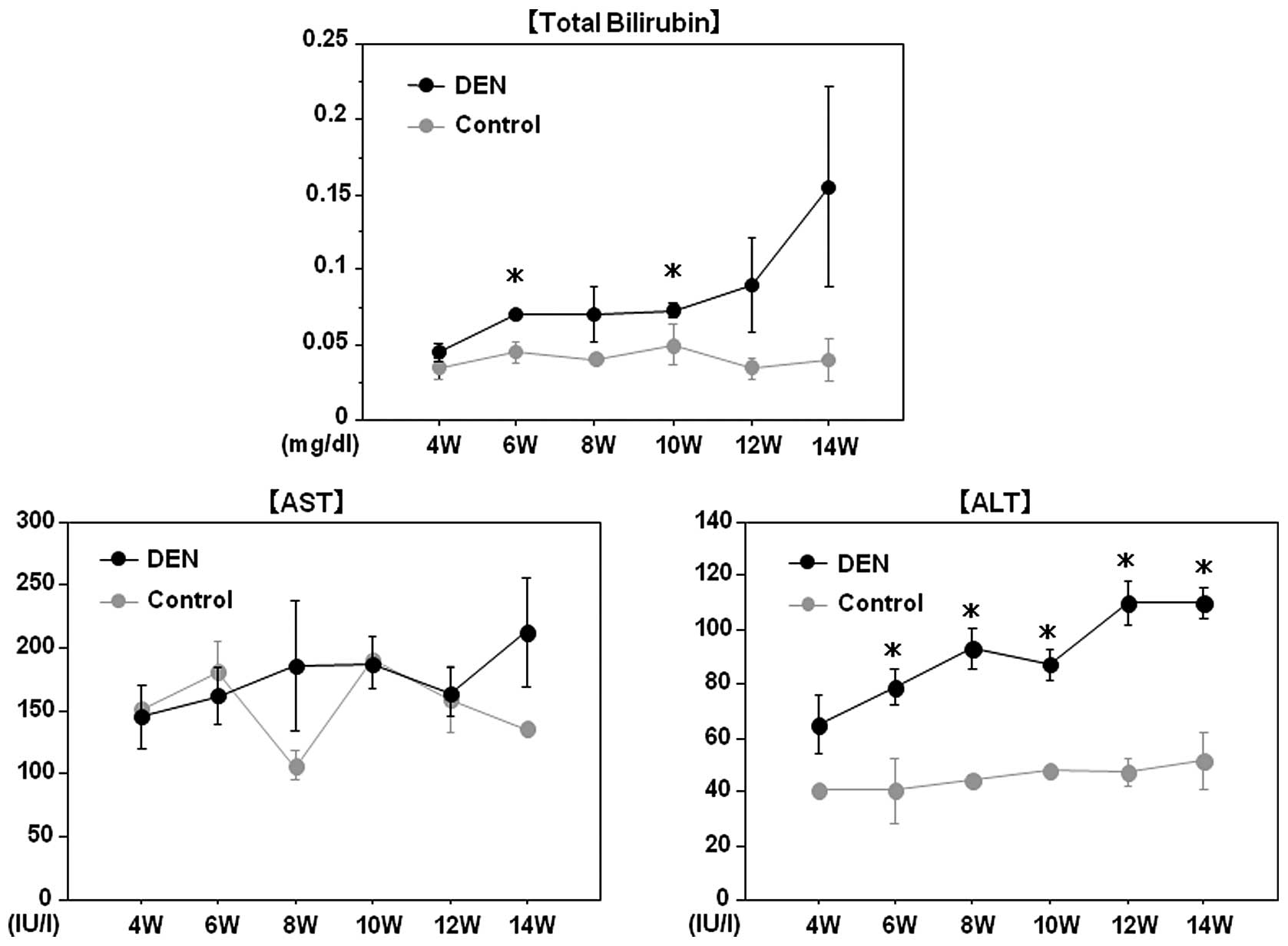
liver tumors in the DEN group. Although serum AST levels did not
differ between the two groups, total bilirubin and ALT levels were
significantly higher in the DEN group than in the control group
throughout the experimental period, possibly reflecting liver
injury induced by DEN (Fig.
3).
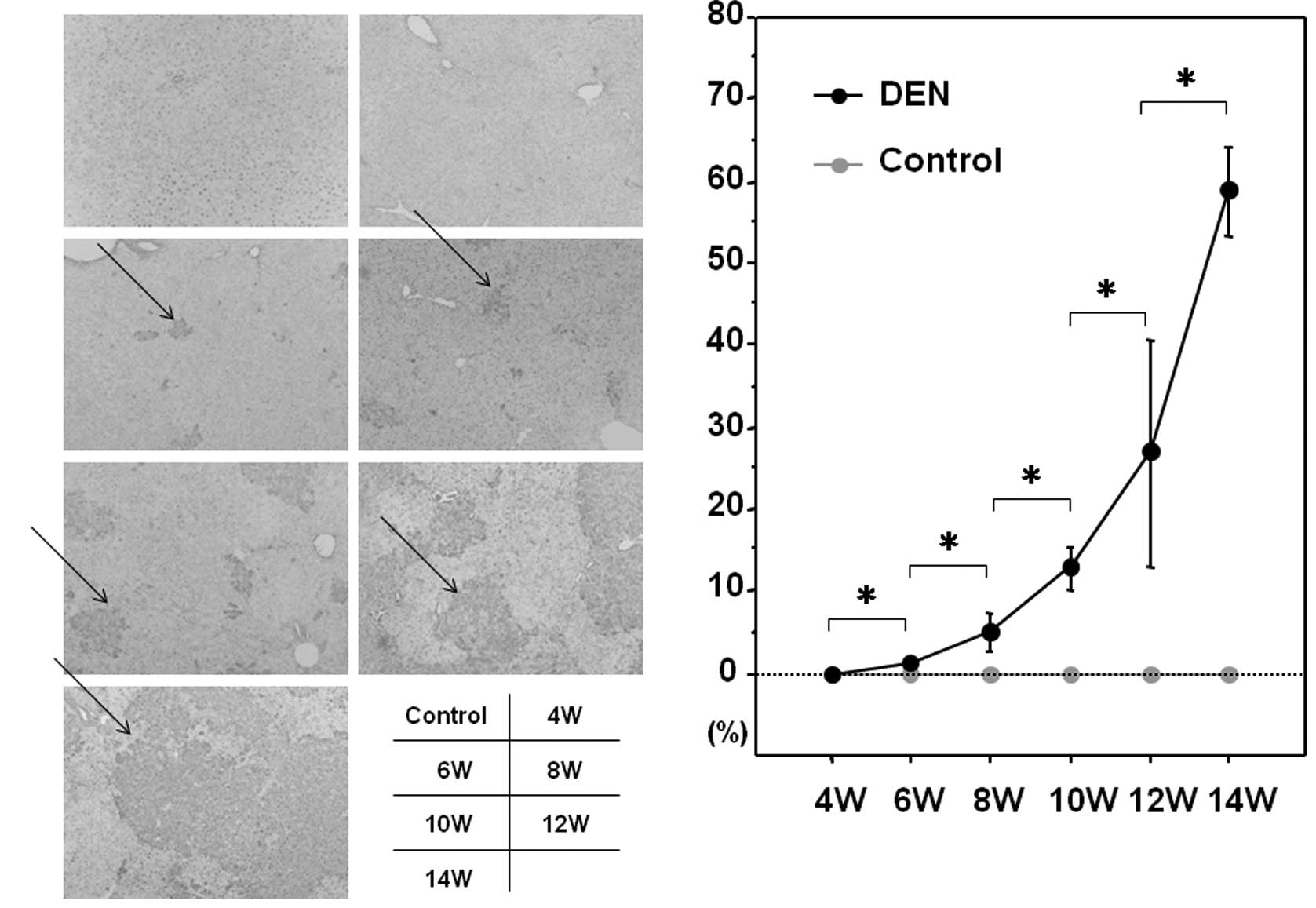
Histological examinations
Macroscopic and microscopic features of the liver
were chronologically examined. In the control group, as expected,
no liver tumors were observed throughout the experimental period
(Fig. 4A). In the DEN group, no
significant gross lesions were observed following 8 weeks of the
treatment. However, following 10 weeks of the treatment, white
nodules were macroscopically noted, the number of which increased
thereafter (Fig. 4B–D).
Microscopic analysis revealed that renewed hepatocytes started to
appear after 4 weeks of the treatment, and degenerated hepatocytes,
oval cells and fibrotic changes were observed after 6 weeks of the
treatment (Fig. 4E). After 12
weeks of the DEN treatment, hyperplastic nodules developed
(Fig. 4D). However, no definite
HCC was observed throughout the experimental period. Quantified
histological changes following the DEN treatment are summarized in
Table I.
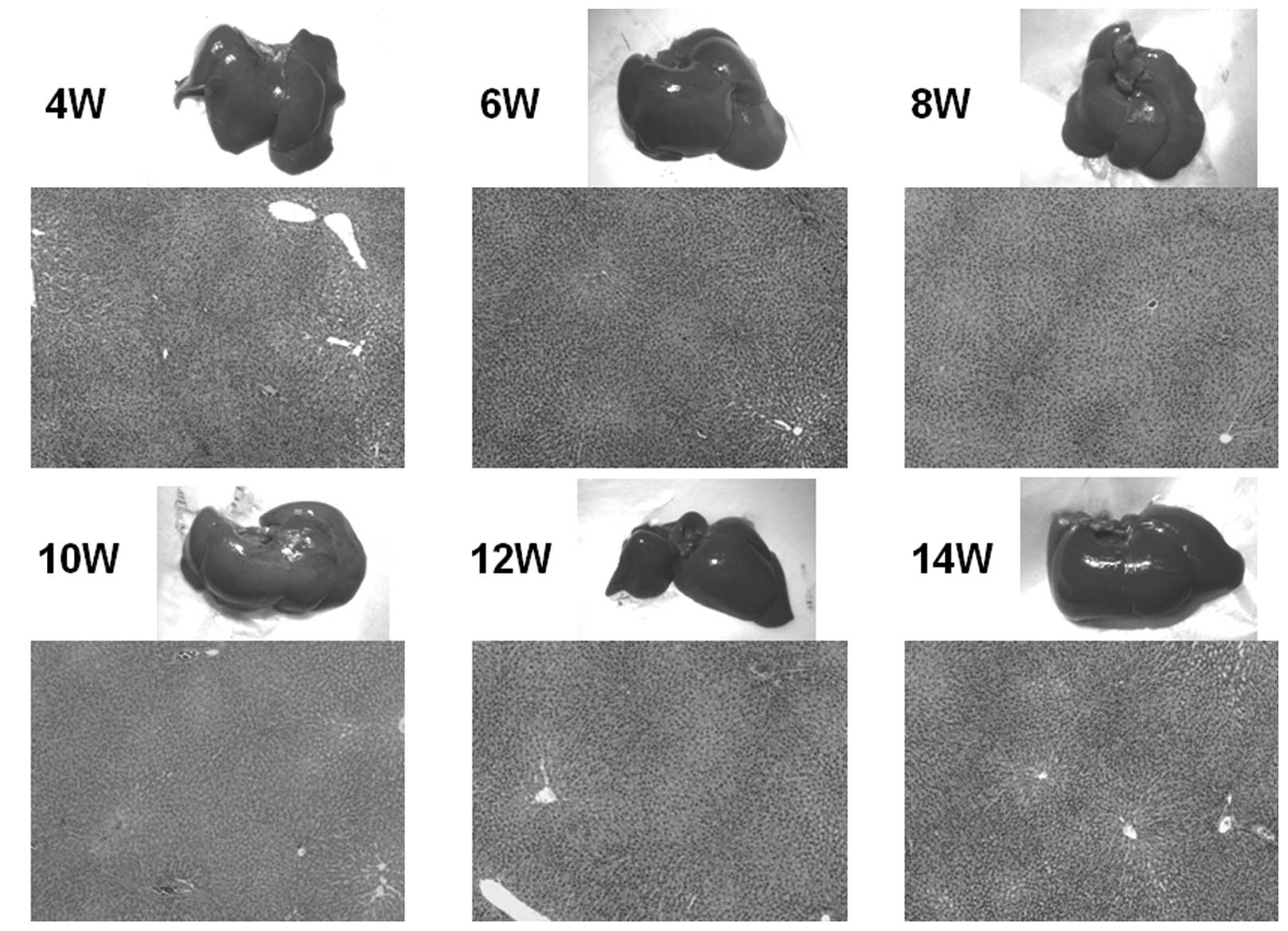 | Figure 4.Macroscopic and microscopic features
of the liver. Macroscopic and microscopic features of the liver in
(A) the control group and (B–E) the DEN group are chronologically
demonstrated. (A) Livers in the control group appeared normal
throughout the experimental period. Original maginification, x100.
(B and C) After 10 weeks of the DEN treatment, white nodules
started to appear. Renewed hepatocytes appeared after 4 weeks, and
degenerated hepatocytes, oval cells and fibrotic changes appeared
after 6 weeks of the treatment (original maginification, x100). (D)
After 12 weeks of the DEN treatment, a number of white nodules were
observed. Histological analysis revealed that these were
hyperplastic nodules (original maginification, x100). (E)
Regenerated hepatocytes (single arrow) and renewed hepatocytes
(double arrows) observed after 6 weeks of the DEN treatment are
demonstrated in the top image (original maginification, x400). Oval
cells observed after 10 weeks of the DEN treatment are demonstrated
in the bottom image (original maginification, x400). DEN,
diethylnitrosamine; W, weeks. |
 | Table I.Summary of quantified histological
changes after the DEN treatment. |
Table I.
Summary of quantified histological
changes after the DEN treatment.
| Group | Weeks | No. | Degenerated
hepatocytes | Oval cells | Renewed
hepatocytes | Hyperplastic
nodules | HCC | Fibrosis |
|---|
| Control | | | | | | | | |
| 4 | 301 | 0 | 0 | 0 | 0 | 0 | 0 |
| 302 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 303 | 0 | 0 | 0 | 0 | 0 | 0 |
| 304 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 305 | 0 | 0 | 0 | 0 | 0 | 0 |
| 306 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 307 | 0 | 0 | 0 | 0 | 0 | 0 |
| 308 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 309 | 0 | 0 | 0 | 0 | 0 | 0 |
| 310 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 311 | 0 | 0 | 0 | 0 | 0 | 0 |
| 312 | 0 | 0 | 0 | 0 | 0 | 0 |
| DEN | | | | | | | | |
| 4 | 501 | 0 | 0 | 0 | 0 | 0 | 0 |
| 502 | 0 | 0 | 1 | 0 | 0 | 0 |
| 503 | 0 | 0 | 0 | 0 | 0 | 0 |
| 504 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 505 | 1 | 0 | 1 | 0 | 0 | 0 |
| 506 | 1 | 0 | 1 | 0 | 0 | 1 |
| 507 | 1 | 1 | 1 | 0 | 0 | 1 |
| 508 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 509 | 2 | 0 | 1 | 0 | 0 | 1 |
| 510 | 2 | 1 | 1 | 0 | 0 | 1 |
| 511 | 2 | 1 | 1 | 0 | 0 | 1 |
| 512 | 2 | 1 | 1 | 0 | 0 | 1 |
| 10 | 513 | 2 | 1 | 1 | 0 | 0 | 1 |
| 514 | 2 | 1 | 1 | 0 | 0 | 1 |
| 515 | 2 | 1 | 1 | 0 | 0 | 1 |
| 516 | 2 | 1 | 1 | 0 | 0 | 1 |
| 12 | 517 | 1 | 1 | 2 | 1 | 0 | 2 |
| 518 | 1 | 1 | 2 | 2 | 0 | 2 |
| 519 | 1 | 1 | 2 | 1 | 0 | 1 |
| 520 | 1 | 2 | 2 | 1 | 0 | 1 |
| 14 | 521 | 1 | 2 | 2 | 2 | 0 | 1 |
| 522 | 2 | 2 | 2 | 1 | 0 | 2 |
| 523 | 1 | 2 | 2 | 1 | 0 | 2 |
| 524 | 2 | 2 | 3 | 2 | 0 | 2 |
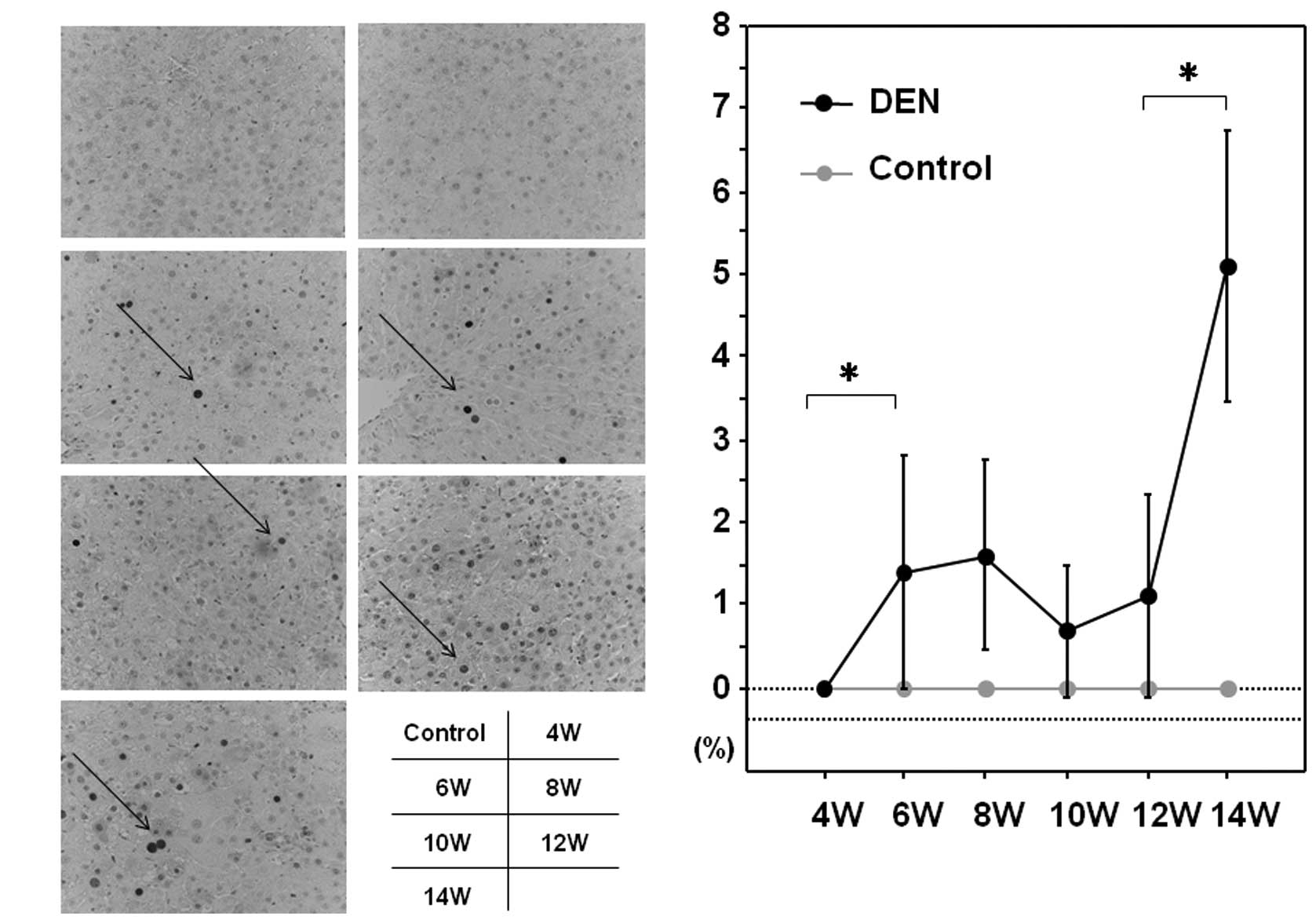
Expression levels of GST-P and PCNA
Among the GSTs, a family of detoxification enzymes
that catalyze the conjugation of glutathione with a large number of
carcinogens, GST-P has been used as a reliable tumor marker for
experimental hepatocarcinogenesis in the rat (16). PCNA is an essential regulator of
the cell cycle, whose expression has been a useful tool for
studying cell proliferation, including cell proliferation in the
liver (17). We sought to
investigate the expression levels of GST-P and PCNA during
hepatocarcinogenesis induced by DEN. As expected, the control liver
did not express a significant amount of GST-P when evaluated by
immunohistochemical or western blot analysis (Fig. 5A and B). GST-P-positive foci
started to appear after 6 weeks of the DEN treatment and the
expression levels of GST-P were significantly increased thereafter
throughout the experimental period (Fig. 5A and B). The expression levels of
PCNA were sequentially increased after the treatment with DEN when
analyzed by immunohistochemical and western blot analysis (Fig. 6A and B).
Discussion
DEN-based HCC models have been utilized for
investigating the beneficial effects of anti-carcinogenic compounds
in vivo. Since the sequential changes in the liver following
the administration of DEN have not been clarified, we evaluated
them by sequentially examining biological parameters and liver
tissues. After 14 weeks of DEN treatment, hyperplastic nodules
developed as a consequence of the appearance of renewed
hepatocytes, degenerated hepatocytes, oval cells and fibrotic
changes. Unfortunately, we did not observe the rats beyond 14
weeks. It is plausible that longer treatment with DEN could lead to
the development of HCC. In addition, since we did not investigate
the molecular mechanisms involved in the histological changes
following treatment with DEN in this study, future intensive
studies are necessary to unveil these issues.
Compounds which have potential chemopreventive
effects on the liver include acyclic retinoid (ACR), caffeine,
capsaicin, cinnamaldehyde, curcumin, diallyl sulfide (DAS),
eicosapentaenoic acid (EPA), epigallocatechin-3-gallate (EGCG),
genistein, lycopene, resveratrol, silymarin and sulforaphane (SFN)
(18). Since the
anti-hepatocarcinogenic effects of these compounds have become
known mainly from in vitro experimental studies and
epidemiology, hepatocarcinogenic models in rats would be useful for
testing these compounds in vivo. As shown in the present
study, the sequential analysis of DEN-induced hepatocarcinogenesis
may be valuable for investigating the effects of compounds at
variable stages of hepatocarcinogenesis.
Abbreviations:
|
ALT,
|
alanine aminotransferase;
|
|
AST,
|
aspartate aminotransferase;
|
|
DEN,
|
diethylnitrosamine;
|
|
GST,
|
glutathione S-transferase;
|
|
H&E,
|
hematoxylin and eosin;
|
|
HCC,
|
hepatocellular carcinoma;
|
|
HCV,
|
hepatitis C virus;
|
|
PBS,
|
phosphate-buffered saline;
|
|
PCNA,
|
proliferating cell nuclear antigen
|
Acknowledgements
We would like to thank Mr. Yujirou
Ikuta for his technical assistance.
References
|
1.
|
FX BoschJ RibesJ BorrasEpidemiology of
primary liver cancerSemin Liver
Dis19271285199910.1055/s-2007-100711710518307
|
|
2.
|
HB El-SeragKL RudolphHepatocellular
carcinoma: epidemiology and molecular
carcinogenesisGastroenterology13225572576200710.1053/j.gastro.2007.04.06117570226
|
|
3.
|
PA FaraziRA DePinhoHepatocellular
carcinoma pathogenesis: from genes to environmentNat Rev
Cancer6674687200610.1038/nrc193416929323
|
|
4.
|
YC ShenC HsuAL ChengMolecular targeted
therapy for advanced hepatocellular carcinoma: current status and
future perspectivesJ
Gastroenterol45794807201010.1007/s00535-010-0270-020567987
|
|
5.
|
K MoriyaH FujieY ShintaniThe core protein
of hepatitis C virus induces hepatocellular carcinoma in transgenic
miceNat Med410651067199810.1038/20539734402
|
|
6.
|
Y HorieA SuzukiE
KataokaHepatocyte-specific Pten deficiency results in
steatohepatitis and hepatocellular carcinomasJ Clin
Invest11317741783200410.1172/JCI2051315199412
|
|
7.
|
A TakaiT ToyoshimaM UemuraA novel mouse
model of hepatocarcinogenesis triggered by AID causing deleterious
p53 mutationsOncogene28469478200910.1038/onc.2008.41518997814
|
|
8.
|
M El-ShahatS El-AbdM AlkafafyG
El-KhatibPotential chemoprevention of diethylnitrosamine-induced
hepatocarcinogenesis in rats: Myrrh (Commiphora molmol) vs.
turmeric (Curcuma longa)Acta HistochemAug252011(E-pub ahead
of print)
|
|
9.
|
SS Al-RejaieAM AleisaAA
Al-YahyaProgression of diethylnitrosamine-induced hepatic
carcinogenesis in carnitine-depleted ratsWorld J
Gastroenterol1513731380200910.3748/wjg.15.137319294768
|
|
10.
|
L VernaJ WhysnerGM
WilliamsN-nitrosodiethylamine mechanistic data and risk assessment:
bioactivation, DNA-adduct formation, mutagenicity, and tumor
initiationPharmacol
Ther715781199610.1016/0163-7258(96)00062-98910949
|
|
11.
|
S JayakumarA MadankumarS
AsokkumarPotential preventive effect of carvacrol against
diethylnitrosamine-induced hepatocellular carcinoma in ratsMol Cell
BiochemAug312011(E-pub ahead of print)
|
|
12.
|
DB SoltE CayamaH TsudaK EnomotoG LeeE
FarberPromotion of liver cancer development by brief exposure to
dietary 2-acetylaminofluorene plus partial hepatectomy or carbon
tetrachlorideCancer Res4318819119836291753
|
|
13.
|
S-E ChuangA-L ChengJ-K LinM-L
KuoInhibition by curcumin of diethylnitrosamine-induced hepatic
hyperplasia, inflammation, cellular gene products and
cell-cycle-related proteins in ratsFood Chem
Toxicol38991995200010.1016/S0278-6915(00)00101-011038236
|
|
14.
|
G ShiotaK HaradaM IshidaInhibition of
hepatocellular carcinoma by glycyrrhizin in
diethylnitrosamine-treated
miceCarcinogenesis205963199910.1093/carcin/20.1.599934850
|
|
15.
|
RA SquireMH LevittReport of a workshop on
classification of specific hepatocellular lesions in ratsCancer
Res35321432231975171067
|
|
16.
|
M SakaiM MuramatsuRegulation of
glutathione transferase P: a tumor marker of
hepatocarcinogenesisBiochem Biophys Res
Commun357575578200710.1016/j.bbrc.2007.03.17417434454
|
|
17.
|
FQ AlenziEM El-NasharSS
Al-GhamdiInvestigation of Bcl-2 and PCNA in hepatocellular
carcinoma: relation to chronic HCVJ Egypt Natl Canc
Inst228794201021503011
|
|
18.
|
J OkanoY FujiseR AbeR ImamotoY
MurawakiChemo-prevention against hepatocellular carcinomaClin J
Gastroenterol4185197201110.1007/s12328-011-0227-8
|