Introduction
Ameloblastoma is the most frequently encountered
benign, locally invasive tumor and the second most common
odontogenic tumor (1). It may
arise from rests of dental lamina, from a developing enamel organ
or from basal cells of the oral mucosa. Ameloblastomas tend to
infiltrate between intact cancellous bones at the periphery of the
lesion before bone resorption becomes radiographically evident. As
a result, the actual margin of the tumor often extends beyond its
apparent radiographic or clinical margin. Attempts to remove the
tumor often leave small islands of tumor, which later result in
recurrence in 50–90% of cases (2).
This has raised questions regarding the tumor cell populations that
are responsible for tumor growth and recurrence.
In the past few years, it has been hypothesized that
tumors are most likely initiated in normal stem cells or their
immediate descendants, and then are perpetuated by a minority of
these cells, known as cancer stem cells (CSCs) or tumor-initiating
cells (TICs) (3). According to the
American Association for Cancer Research (AACR), ‘a cell within a
tumor that possesses the capacity to self-renew and to cause
heterogeneous lineages of cancer cells that comprise the tumor is
known as a cancer stem cell’. This observation implies that within
a given tumor, there exists a small population of cells with the
capacity to behave like stem cells (4). The difficulty in eradicating tumors
may be due to the fact that conventional treatments target the bulk
of the tumor cells leaving unaffected the CSCs, which like their
normal counterparts, maintain tumor tissue. According to this
hypothesis, identifying and exterminating CSCs may be an effective
treatment modality (5).
Recently, several CD markers have been identified as
solid CSC markers. CD133, also known as PROML1 or prominin, is a
stem cell surface antigen that has been recently identified as a
potential CSC marker in brain, colon, hepatocellular and prostate
cancer (6–10). CD44, also known as homing cell
adhesion molecule, is a cell surface glycoprotein expressed on
lymphocytes, monocytes and granulocytes, which has been identified
as a stem cell marker for breast, prostate, pancreatic and head and
neck cancer (6,11–13).
The ABCG2 transporter is a member of the ATP binding cassette
transporter family (14)
responsible for the side population phenotype in various human
cancers and the corresponding non-malignant tissues, and is widely
used to detect and isolate somatic stem/progenitor cells (7). Xu et al for the first time
demonstrated that stem-like cells are present in benign tumors.
They isolated self-renewable and multipotent stem-like cells from
various pituitary adenomas. Implanted into immune compromised mice,
these cells initiated transplantable pituitary tumors that
resembled the primary tumors (15).
Ameloblastoma is a benign tumor with two
histologically distinct cell types: peripheral ameloblast-like
cells and central stellate reticulum-like cells. The presence of
cancer stem-like cells in ameloblastoma remains undetermined.
However, if cancer stem-like cells are present in ameloblastoma, it
is important to identify which type of cell possesses these cancer
stem-like characteristics and is responsible for ameloblastoma
progression and recurrence. Therefore, in this study we analyzed
the protein expression of the three most putative candidate stem
cell markers, CD133, CD44 and ABCG2, in ameloblastoma.
Materials and methods
Tissue sample selection
Twenty-three blocks from 17 cases embedded in
paraffin were selected from the Surgical Pathology Unit of the
Department of Oral Pathology and Medicine, Graduate School of
Medicine, Dentistry and Pharmaceutical Science of Okayama
University, Japan. These samples were fixed in 10% neutral buffered
formalin and routinely processed and embedded in paraffin.
Histological diagnosis was carried out by routine H&E,
according to WHO histological typing of odontogenic tumors.
Clinical characteristics and histological types of the cases are
shown in Table I.
 | Table I.Clinical and histopathological data
of the patients with ameloblastoma. |
Table I.
Clinical and histopathological data
of the patients with ameloblastoma.
| Case no. | Gender | Age (years) | Location | Histological
type |
|---|
| 1 | Female | 41 | Mandible | Solid multilocular
(Plexiform) |
| 2 | Male | 60 | Mandible | Solid multilocular
(Mixed) |
| 3 | Male | 58 | Mandible | Solid multilocular
(Mixed) |
| 4 | Male | 31 | Mandible | Solid multilocular
(Plexiform)a |
| 5 | Male | 69 | Mandible | Solid multilocular
(Mixed) |
| 6 | Male | 71 | Mandible | Solid multilocular
(Mixed)a |
| 7 | Male | 39 | Maxilla | Solid multilocular
(Mixed)a |
| 8 | Male | 25 | Maxiila | Solid multilocular
(Plexiform) |
| 9 | Male | 25 | Maxilla | Solid multilocular
(Mixed)a |
| 10 | Male | 21 | Maxilla | Solid multilocular
(Mixed) |
| 11 | Female | 46 | Mandible | Solid multilocular
(Mixed)a |
| 12 | Female | 42 | Mandible | Solid multilocular
(Mixed) |
| 13 | Female | 30 | Maxilla | Solid multilocular
(Plexiform)a |
| 14 | Female | 27 | Maxilla | Solid multilocular
(Plexiform) |
| 15 | Male | 45 | Mandible | Solid multilocular
(Follicular)a |
| 16 | Male | 44 | Maxilla | Solid multilocular
(Mixed) |
| 17 | Male | 50 | Mandible | Solid multilocular
(Follicular) |
| 18 | Male | 53 | Mandible | Solid multilocular
(Mixed)a |
| 19 | Male | 55 | Mandible | Solid multilocular
(Mixed)a |
| 20 | Male | 55 | Mandible | Solid multilocular
(Mixed)a |
| 21 | Female | 83 | Maxilla | Solid multilocular
(Plexiform)a |
| 22 | Female | 81 | Maxilla | Solid multilocular
(Mixed) |
| 23 | Female | 37 | Mandible | Solid multilocular
(Follicular) |
Immunohistochemistry
Sections (3-μm) mounted on salinized slides were
used for immunohistochemical staining. Briefly, sections were
deparaffinized in a series of xylene for 15 min and rehydrated in
graded ethanol solutions. Endogenous peroxidase activity was
blocked by incubating the sections in 0.3%
H2O2 in methanol for 30 min. Antigen
retrieval was achieved by heat treatment using 10 mM citrate buffer
solution pH 6.0 (CD133, CD44, ABCG2 and Ki-67). After treatment
with normal serum, the sections with primary antibodies were
incubated at 4°C overnight. The tagging of the primary antibody was
achieved by subsequent application of anti-goat/mouse IgG and
avidin-biotin complexes (Mouse ABC kit; Vector Laboratories, Inc.,
Burlingame, CA, USA) or Envision peroxidase detecting reagent
(Dako, Carpinteria, CA, USA). Visualization of the
immunohistochemical reaction was performed by developing the enzyme
complex with DAB/H2O2 solution (Histofine DAB
substrate; Nichirei, Japan) and counterstaining with Mayer's
hematoxylin. The antibodies used are listed in Table II.
 | Table II.Details of the antibodies used. |
Table II.
Details of the antibodies used.
| Antibody | Clonality | Supplier | Dilution | Treatment |
|---|
| CD133 | Rabbit
polyclonal | Abcam, Cambridge,
MA, USA | 1:300 | Heat |
| CD44 | Rabbit
polyclonal | Abcam, Cambridge,
MA, USA | 1:200 | Heat |
| ABCG2 | Mouse
monoclonal | Abcam, Cambridge,
MA, USA | 1:50 | Heat |
| Ki-67 | Mouse
monoclonal | Dako, Denmark | 1:50 | Heat |
Ki-67-positive cell count
For analysis of the percentage of Ki-67-positive
cells, in each tissue section, several fields were chosen randomly
and the fields that complied with the requirements were further
selected. The requirements were that the well-preserved fields were
stained by anti-Ki-67 antibodies. Clear brown nuclei were regarded
as positive cells, and at least three to five fields in each
section were counted using an eyepiece micrometer and counted at
x40 magnification. The process was repeated three times to decrease
the operator error. The percent labeling index (LI) (Number of
positive cells/total cells x 100) was calculated for each
field.
Scoring for protein expression
Immunoreactivity with each of the antibodies was
graded in each histopathological compartment of ameloblastoma: low
expression (0–30%) and high expression (≥31%).
Statistical analysis
The LI of Ki-67 was counted; the mean and the
standard deviation (SD) were calculated. The Student's t-test was
used, and P-values <0.05 were considered to denote statistical
significance. Clinicopathological data and low and high expression
of different CSC markers were statistically compared using the
t-test and Fisher's exact test, and P-values <0.05 were
considered to denote statistical significance. All computations
were computer-based using Stat View for Windows version 5.0
statistical program, SAS Inc.
Results
Immunohistochemistry
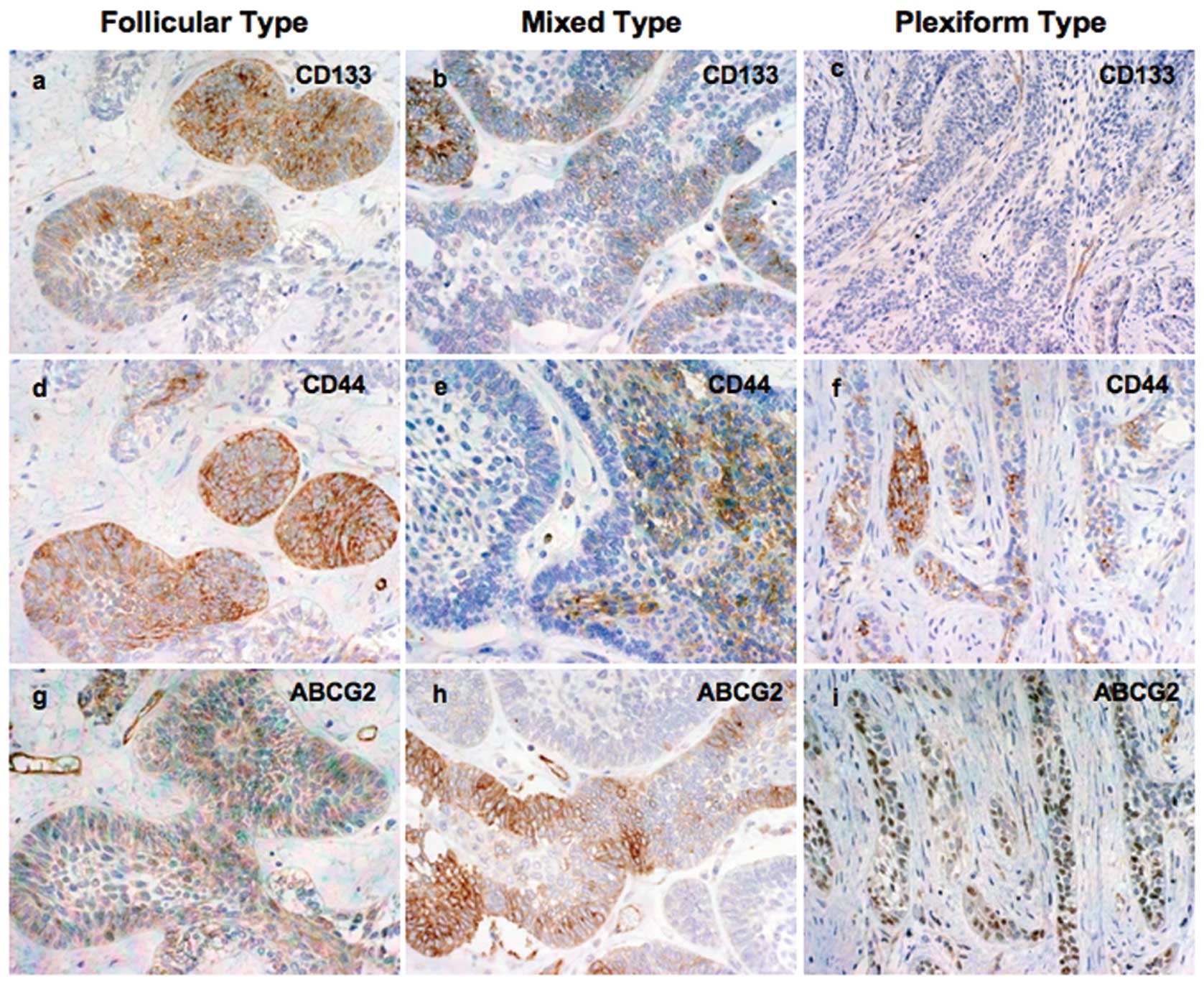
Representative immunohistochemical staining for the
three CSC proteins investigated in this study are shown in Fig. 1.
CD133
The location of staining was cytoplasmic. Intense
immunostaining was observed in small tumor follicles and peripheral
ameloblast-like cells. In plexiform type, several cases showed a
positive immunoreaction with peripheral columnar cells, with few
central cells at the close vicinity of the peripheral ameloblast.
Certain cases showed a weak or negative reaction. Nine (39.13%)
cases showed high and 14 cases (60.87%) showed low CD133 expression
(Fig. 1a–c).
CD44
The location of staining was cytoplasmic. In several
cases, CD44 expression was strong in small tumor follicles.
However, plexiform type ameloblastoma showed intense immunoreaction
in center stellate reticulum-like cells. Twelve of 23 cases
(52.17%) showed high and 11 cases (47.83%) showed low CD44
expression (Fig. 1d–f).
ABCG2
The location of staining of these molecules was also
cytoplasmic. Diffuse and intense staining was observed in
peripheral ameloblast-like cells, the budding area and central
stellate reticulum-like cells of the tumors. Small tumor follicles
showed weak immunoexpression. High ABCG2 expression was observed in
12 (52.17%) out of 23 cases and 11 cases (47.83%) showed low
expression (Fig. 1g–i).
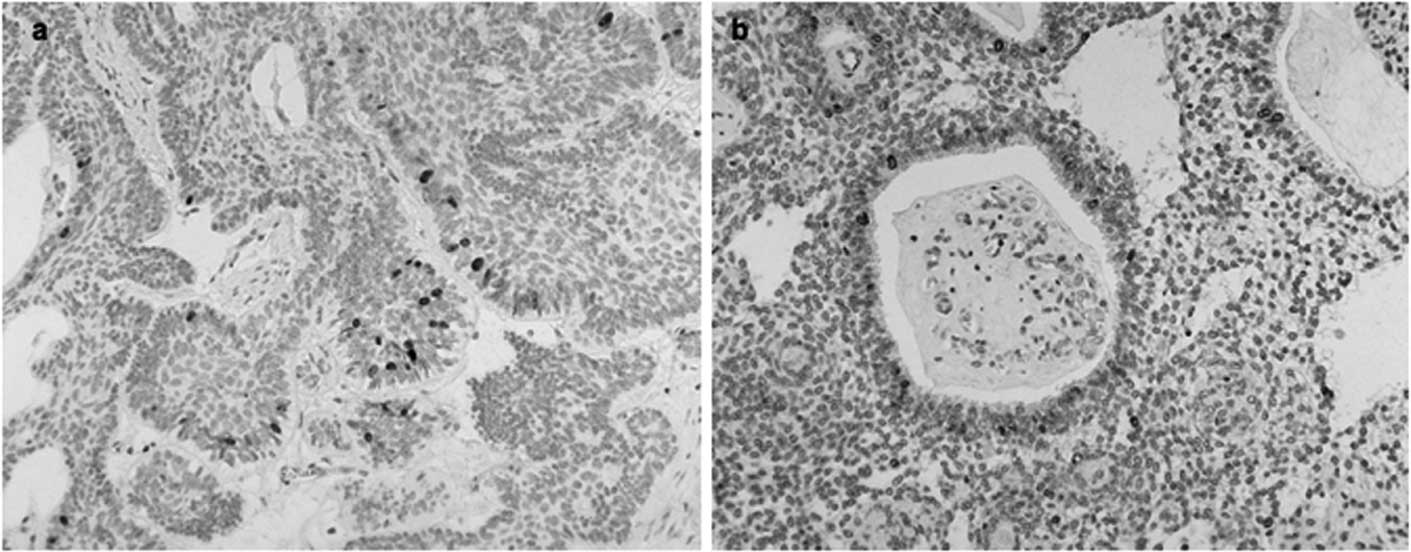
Ki-67 expression and LI
Ki-67 antigen was expressed in 82.6% of
ameloblastoma cases and the positive cells present in the different
histological types were counted. Ki-67 expression was mainly
observed in the nuclei of the peripheral ameloblast-like cells, and
only a few positive cells were observed in the central stellate
reticulum-like cells (Fig. 2).
Most of the tissue sections did not contain positive central cells,
and peripheral cells are known to reflect the growth activity of
ameloblastoma (16). The mean and
standard deviation of Ki-67 LI was 23.13 and 13.9,
respectively.
Correlation between CSC expression and
clinicopathological factors
As shown in Table
III, only CD44 expression was correlated with tumor recurrence
(0.0391) and ABCG2 was correlated with the tumor location (0.0361).
However, there was no significant association between CD133
expression and clinicopathological features.
 | Table III.Correlation between CSC markers
expression and clinicopathological factors in ameloblastoma. |
Table III.
Correlation between CSC markers
expression and clinicopathological factors in ameloblastoma.
| CD133 expression
|
| Total [n=23
(%)] | High (n=9;
39.13%) | Low (n=14;
60.87%) | P-value |
|
| Age (years; mean ±
SD)a | 47.33±9.73 | 43.71±21.83 | 0.1874 | |
| Genderb | | | | 0.0858 |
| Male | 15 (65.22) | 8 (53.33) | 7 (46.67) | |
| Female | 8 (34.78) | 1 (12.5) | 7 (87.5) | |
| Locationb | | | | 0.2283 |
| Mandible | 14 (60.87) | 7 (50.0) | 7 (50.0) | |
| Maxilla | 9 (39.13) | 2 (22.22) | 7 (77.78) | |
| Recurrenceb | | | | 0.6802 |
| Yes | 11 (47.83) | 5 (45.45) | 6 (54.55) | |
| No | 12 (52.17) | 4 (33.33) | 8 (66.67) | |
| Ki-67 (mean ±
SD)a | 27.80±13.84 | 19.63±13.44 | 0.1892 | |
|
| CD44 expression
|
| Total [n=23
(%)] | High (n=12;
52.17%) | Low (n=11;
47.83%) | P-value |
|
| Age (years; mean ±
SD)a | 48.58±16.31 | 41.36±19.48 | 0.1988 | |
| Genderb | | | | 0.2203 |
| Male | 12 (52.17) | 8 (66.67) | 4 (33.33) | |
| Female | 11 (47.83) | 4 (36.36) | 7 (63.64) | |
| Locationb | | | | 0.0995 |
| Mandible | 13 (56.52) | 9 (69.23) | 4 (30.77) | |
| Maxilla | 10 (43.48) | 3 (30.0) | 7 (70.0) | |
| Recurrenceb | | | | 0.0391c |
| Yes | 11 (47.83) | 3 (27.27) | 8 (72.73) | |
| No | 12 (52.17) | 9 (75.0) | 3 (25.0) | |
| Ki-67 (mean ±
SD)a | 22.21±15.35 | 24.15±12.86 | 0.7579 | |
|
| ABCG2 expression
|
| Total [n=23
(%)] | High (n=12;
52.17%) | Low (n=11;
47.83%) | P-value |
|
| Age (years; mean ±
SD)a | 44.00±14.23 | 46.17±21.26 | 0.9039 | |
| Genderb | | | | 0.1930 |
| Male | 16 (69.57) | 10 (62.5) | 6 (37.5) | |
| Female | 7 (30.43) | 2 (28.57) | 5 (71.43) | |
| Locationb | | | | 0.0361c |
| Mandible | 14 (60.87) | 10 (71.43) | 4 (28.57) | |
| Maxilla | 9 (39.13) | 2 (22.22) | 7 (77.78) | |
| Recurrenceb | | | | 0.6843 |
| Yes | 11 (47.83) | 5 (45.45) | 6 (54.55) | |
| No | 12 (52.17) | 7 (58.33) | 5 (41.67) | |
| Ki-67 (mean ±
SD)a | 25.83±15.67 | 21.10±12.74 | 0.4543 | |
Correlation between CSC expression and
Ki-67-positive cells
Next, we analyzed the possible correlation between
the high and low expression of three CSC markers and Ki-67
expression in all ameloblastoma cases using the Student's t-test.
No significant association was observed between Ki-67 expression
and expression of all three CSC markers (Table III).
Discussion
Tumor recurrence after curative surgery remains a
major obstacle for improving overall cancer survival. Recurrence
may be in part due to the existence of CSCs. Growing evidence
suggests that human cancers are stem cell diseases, and only a
small subpopulation of cancer cells, endowed with stem cell-like
features, may be responsible for tumor initiation and progression
(17). Novel strategies for
successful tumor treatment should focus on the elimination of CSCs.
In this study, we analyzed immunohistochemical staining to detect
the expression of three different CSC markers in relation to the
proliferation activity of the tumor cells in ameloblastoma, a
highly apoptosis-resistant and invasive tumor with a high
recurrence rate and possesing different histological patterns
(18–21).
Increasing evidence highlights the role of CD133 as
a marker of CSCs in various human tumors as well as in
ameloblastoma (22,23). CD44 is one of the cell surface
markers currently used to identify CSCs in various solid tumors
(6,11,12).
CD133 was found to be expressed in combination with
CD44+ in prostate tumors. These cells were found to be
capable of self-renewal, proliferation and multi-lineage
differentiation in vitro to recapitulate the original tumor
phenotype, consistent with CSC properties (6). In the present study, CD133, the most
important CSC marker, was stained fairly well in 39.13% of the
cases. CD44 is another important candidate stem cell marker and
demonstrated positive expression in 52.17% of the cases. The
samples positive for both CD133 and CD44 showed that the two
markers present a similar expression pattern and are mainly located
in peripheral cells. This co-expression suggests that these two
molecules may be potential biomarkers for the initiation,
progression and cell differentiation in ameloblastoma (24).
It has already been reported that CD44+
cells have the capability to initiate tumor recurrence upon
completion of treatment (25). In
the present study, statistical data revealed that CD44 expression
was significantly associated with tumor recurrence. This suggests
that, in ameloblastoma, CD44 may play a central role in tumor
recurrence. ABCG2 is a member of the ATP binding cassette
transporter family, is widely expressed in stem cells, and is
recognized as a universal marker of stem cells. Recent studies
strongly suggest that ABCG2 expression in tumors may contribute to
tumor growth initiation, invasiveness and relapse (26–30).
In this study, diffuse expression of ABCG2 was observed in
ameloblastoma, suggesting that ABCG2 may enhance the proliferation
and invasion of tumors by maintaining the existing cancer stem-like
cells in ameloblastoma. This also supports other studies regarding
ABCG2 expression in ameloblastoma (22).
In ameloblastoma, even though different types of
histological variations are present, two distinct cell types are
observed in all subtypes: peripheral columnar epithelium or
ameloblast-like cells and central stellate reticulum-like cells
(1). Peripheral cells are always
situated at the invasive front, but no morphological
differentiation can be observed. By contrast, in many cases,
central stellate reticulum-like cells show cellular differentiation
and morphological change (1). In
the present experiment, it was observed that most of the peripheral
cells positive for CSC markers were also positive for the
proliferative marker Ki-67. However, cell proliferation marker
Ki-67-positive cells have a higher degree of differentiation and
are, therefore, less likely to contain cancer stem-like cells
(31). Therefore, in these cells,
it is possible that CSC markers only maintain cellular
proliferation and tumor progression. In contrast, few CSC
marker-positive central stellate reticulum-like cells situated at
the close vicinity of the peripheral cells were devoid of Ki-67
expression. Thus, these cells may have the potential to be cancer
stem-like cells. In accordance with this hypothesis, these central
stellate reticulum-like cells may change their morphology and
differentiate into different types of cellular patterns, for
example, granular, squamous and acanthomatous type (1).
The presence of candidate CSCs in ameloblastoma
supports the findings of a previous study which revealed that it is
possible that CSCs are present not only in malignant lesions, but
also in benign lesions (15).
In conclusion, immunohistochemical results indicate
that all three candidate CSC markers are expressed in ameloblastoma
and are possibly involved in cell proliferation, tumor progression
and recurrence. Among the CSC marker-positive cells, central
stellate reticulum-like cells, situated at the close vicinity of
the peripheral ameloblast-like cells, may be candidate cancer
stem-like cells in ameloblastoma. Understanding the biological
function of the expression of CSC markers in ameloblastoma may aid
in elucidating their role in tumor pathogenesis, and continued
research may lead to the development of more effective therapeutic
approaches.
Acknowledgements
This study was supported by a
Grant-in-Aid for Research (C) (no. 21592326) from the Japan Society
for the Promotion of Science (JSPS), and a Grant-in-Aid for Young
Scientists (B) (nos. 22791977, 20791337 and 22791766) from the
Japanese Ministry of Education, Culture, Sports, Science and
Technology (MEXT).
References
|
1.
|
DG GardnerK HeikinheimoM ShearHP
PhilipsonH ColemanAmeloblastomasWorld Health Organization
Classification of Tumours. Pathology and Genetics of Head and Neck
TumoursL BarnesJW EvesonP ReichartD SidranskyIARC
PressLyon2963002005
|
|
2.
|
Tumors of odontogenic epitheliumOral and
Maxillofacial PathologyBW NevilleDD DammCM AllenJE BouquotSaunders
ElsevierSt. Louis, Missouri7027182009
|
|
3.
|
C WuBA AlmanSide population cells in human
cancersCancer Lett26819200810.1016/j.canlet.2008.03.048
|
|
4.
|
MF ClarkeJE DickPB DirksCancer stem cells
– perspectives on current status and future directions: AACR
Workshop on Cancer Stem CellsCancer Res66933993442006
|
|
5.
|
J BurkertNA WrightMR AlisonStem cells and
cancer: an intimate relationshipJ
Pathol209287297200610.1002/path.201616770755
|
|
6.
|
AT CollinsPA BerryC HydeMJ StowerNJ
MaitlandProspective identification of tumorigenic prostate cancer
stem cellsCancer
Res651094610951200510.1158/0008-5472.CAN-05-201816322242
|
|
7.
|
M OlempskaPA EisenachO AmmerpohlH
UngefrorenF FandrichH KalthoffDetection of tumor stem cell markers
in pancreatic carcinoma cell linesHepatobiliary Pancreat Dis
Int69297200717287174
|
|
8.
|
CA O'BrienA PollettS GallingerJE DickA
human colon cancer cell capable of initiating tumour growth in
immunodeficient miceNature445106110200717122772
|
|
9.
|
A SuetsuguM NagakiH AokiT MotohashiT
KunisadaH MoriwakiChlaracterization of CD133+ hepatocellular
carcinoma cells as cancer stem/progenitor cellsBiochem Biophys Res
Commun3518208242006
|
|
10.
|
SK SinghID ClarkeM TerasakiIdentification
of a cancer stem cell in human brain tumorsCancer
Res6358215828200314522905
|
|
11.
|
M Al-HajjMS WichaA Benito-HernandezSJ
MorrisonMF ClarkeProspective identification of tumorigenic breast
cancer cellsProc Natl Acad Sci
USA10039833988200310.1073/pnas.053029110012629218
|
|
12.
|
C LiDG HeidtP DalerbaIdentification of
pancreatic cancer stem cellsCancer
Res6710301037200710.1158/0008-5472.CAN-06-203017283135
|
|
13.
|
ME PrinceR SivanandanA
KaczorowskiIdentification of a subpopulation of cells with cancer
stem cell properties in head and neck squamous cell carcinomaProc
Natl Acad Sci USA104973978200710.1073/pnas.061011710417210912
|
|
14.
|
S ZhouJD SchuetzKD BuntingThe ABC
transporter Bcrp1/ABCG2 is expressed in a wide variety of stem
cells and is a molecular determinant of the side-population
phenotypeNat Med710281034200110.1038/nm0901-102811533706
|
|
15.
|
Q XuX YuanP TuniciIsolation of tumour
stem-like cells from benign tumoursBr J
Cancer101303311200910.1038/sj.bjc.660514219568241
|
|
16.
|
T MitsuyasuH HaradaY HiguchiK KimuraN
NakamuraT KatsukiImmunohistochemical demonstration of bcl-2 protein
in ameloblastomaJ Oral Pathol
Med26345348199710.1111/j.1600-0714.1997.tb00228.x9379422
|
|
17.
|
SS ZekiTA GrahamNA WrightStem cells and
their implications for colorectal cancerNat Rev Gastroenterol
Hepatol890100201110.1038/nrgastro.2010.21121293509
|
|
18.
|
GS SathiH NagatsukaR TamamuraStromal cells
promote bone invasion by suppressing bone formation in
ameloblastomaHistopathology53458467200810.1111/j.1365-2559.2008.03127.x18983611
|
|
19.
|
GA SathiM InoueH HaradaSecreted frizzled
related protein (sFRP)-2 inhibits bone formation and promotes cell
proliferation in ameloblastomaOral
Oncol45856860200910.1016/j.oraloncology.2009.02.00119362047
|
|
20.
|
BS SiriwardenaY KudoI OgawaWM TilakaratneT
TakataAberrant beta-catenin expression and adenomatous polyposis
coli gene mutation in ameloblastoma and odontogenic carcinomaOral
Oncol45103108200910.1016/j.oraloncology.2008.03.00818486530
|
|
21.
|
GS SathiM FujiiR TamamuraJuxta-epithelial
hyalinization inhibits tumor growth and invasion in ameloblastomaJ
Hard Tissue Biol176368200810.2485/jhtb.17.63
|
|
22.
|
H KumamotoK OhkiDetection of CD133, Bmi-1,
and ABCG2 in ameloblastic tumorsJ Oral Pathol
Med398793201010.1111/j.1600-0714.2009.00807.x19659474
|
|
23.
|
J NeuzilM StanticR
ZobalovaTumour-initiating cells vs. cancer ‘stem’ cells and CD133:
what's in a name?Biochem Biophys Res
Commun355855859200717307142
|
|
24.
|
X HuangY ShengM GuanCo-expression of stem
cell genes CD133 and CD44 in colorectal cancers with early liver
metastasisSurg OncolJuly142011(Epub ahead of print)
|
|
25.
|
KD SteffensenAB AlveroY YangPrevalence of
epithelial ovarian cancer stem cells correlates with recurrence in
early-stage ovarian cancerJ Oncol2011620523201121904548
|
|
26.
|
AM BleauJT HuseEC HollandThe ABCG2
resistance network of glioblastomaCell
Cycle1529362944200919713741
|
|
27.
|
Z ChenF LiuQ RenSuppression of ABCG2
inhibits cancer cell proliferationInt J
Cancer126841851201019642144
|
|
28.
|
SS KarhadkarGS BovaN AbdallahHedgehog
signalling in prostate regeneration, neoplasia and
metastasisNature431707712200410.1038/nature0296215361885
|
|
29.
|
X LiaoMK SiuCW AuAberrant activation of
hedgehog signaling pathway in ovarian cancers: effect on prognosis,
cell invasion and
differentiationCarcinogenesis30131140200910.1093/carcin/bgn23019028702
|
|
30.
|
JE KimRR SinghJH Cho-VegaSonic hedgehog
signaling proteins and ATP-binding cassette G2 are aberrantly
expressed in diffuse large B-cell lymphomaMod
Pathol2213121320200910.1038/modpathol.2009.9819593328
|
|
31.
|
O FelthausT EttlM GosauCancer stem
cell-like cells from a single cell of oral squamous carcinoma cell
linesBiochem Biophys Res
Commun4072833201110.1016/j.bbrc.2011.02.08421342656
|