Introduction
Human cutaneous squamous cell carcinoma (SCC) is the
sixth most common cancer in the world. It arises in the
keratinocytes of the epidermis or its appendages. Skin SCC may
arise de novo or develop from precursor lesions, including
actinic keratosis (AK) or Bowen’s disease. The majority of primary
skin SCCs have a good prognosis and are usually curable in
comparison with other cancer types; however, the potential for
invasion and metastases significantly contributes to mortality.
Based on the degree of tumor differentiation, SCC can be graded
into three histological categories; well-differentiated,
moderately-differentiated and poorly-differentiated.
Poorly-differentiated SCCs usually exhibit a higher risk of
recurrence and metastasis. However, biological behaviors can be
difficult to predict from the status of differentiation of the
tumor cells. Therefore, a novel molecular biomarker that reveals
associations between the aggressive, invasive and metastatic
behaviors of cutaneous SCC needs to be identified.
The translationally controlled tumor protein (TCTP),
a 172-amino acid anti-apoptotic polypeptide, was originally
identified in the Ehrlich ascites tumor cell line (1). It is a cell growth-associated protein
that is ubiquitously present in a wide variety of organisms and is
pivotal in the development of various organisms (2–4). It
is present extra- and intracellularly and has been implicated in a
number of cellular functions related to cell growth and apoptosis
(5). The TCTP gene is
significantly downregulated in revertant tumor cells, and it was
therefore suggested that inhibiting the expression of TCTP could
revert cancer cells back into normal phenotypes (6). Certain stimuli, including dioxin,
heavy metals, growth factors and vitamin D, can regulate the
expression of TCTP (7–9). TCTP also interacts with numerous
cellular proteins, including tubulin (6), translation elongation factor IA
(eEF1A) and associated guanine nucleotide exchange factor (eEF1B-b)
(10), Mcl-1 (11), TSAP6 (12), Na,K-ATPase (13), and Bcl-XL (14). Recently, the anti-apoptotic
activity of TCTP has been reported, which could be related to its
interaction with Mcl-1 and/or Bcl-XL (11,15).
TCTP is highly expressed in several cancer types; however, the
expression of TCTP has not been investigated in cutaneous SCC. Its
roles in skin carcinogenesis also need to be elucidated.
In this study, we investigated the expression of
TCTP in cutaneous SCC and its function in skin carcinogenesis by
using a small interfering RNA (siRNA) gene silencing approach.
Materials and methods
Specimens
From a total of 65 cases of cutaneous SCC,
paraffin-embedded samples were retrieved from the Department of
Pathology at The First Affiliated Hospital of Nanjing Medical
University, China. All hematoxylin and eosin-stained slides
diagnosed as SCC were reviewed by two independent pathologists
using the accepted histopathological criteria. Five healthy
individuals without skin disease were used as controls.
The histological grading of the SCC was reevaluated
for this study according to the following classification;
well-differentiated, moderately-differentiated and
poorly-differentiated SCCs, which were recognized as grade I, II
and III, respectively. There were 24 cases of grade I, 23 cases of
grade II and 18 cases of grade III. Of the total 65 SCC cases, nine
patients had metastatic SCC.
Immunohistochemical analysis
Paraffin-embedded blocks were resected as 5-μm
sections and mounted onto positively charged Superfrost slides. One
section from each sample was stained with hematoxylin and eosin to
facilitate histological assessment. The immunostaining was
performed according to a standard protocol. Briefly, paraffin
sections from the SCC specimens and normal patients were
deparaffinized in xylene and rehydrated through a graded series of
ethanol. Following blocking samples with 3% hydrogen peroxide for
10 min, the samples were subjected to microwave antigen retrieval
using in 0.01 M citrate buffer for 15 min. The slides were then
washed twice with PBS buffer and incubated with primary polyclonal
anti-TCTP antibody (1:100 dilutions; bs-3578R; Beijing Biosynthesis
Biotechnology Co. Ltd, Beijing, China) at room temperature for 30
min. Then the sections were incubated with goat anti-mouse
horseradish peroxidase (HRP)-streptavidin immunoglobulin (Abcam,
Cambridge, UK) at room temperature for 30 min.
Following counterstaining the slides with
hematoxylin and mounting on cover slips, the slides were analyzed
by Image Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA)
for measurement of the optical density. Any evidence of cytoplasmic
or membrane staining for TCTP was considered as positive. The
immunostaining results were evaluated by defining a threshold of
positive staining for all sections prior to automated processing.
The intensity was averaged from ten fields of view. All images
analyzed with IPP 6.0 were counter-checked by an experienced
histopathologist.
Cell culture
The epidermoid carcinoma cell lines, A431 and SCL-1,
and the keratinocyte cell line, HaCaT, were gifts kindly provided
by Dr Gu at the Department of Dermatology of Changhai Hospital,
Shanghai, China. Cells were cultured in either 25 cm2
tissue culture flasks, 6- or 96-well plates or tissue culture glass
slides at 37°C in a 5% CO2 environment. DMEM medium with
10% fetal bovine serum (FBS) was used for cell growth.
Western blot analysis
Protein extracts were prepared from the cell lines
using lysis buffer containing 50 mM Tris-HCl (pH 7.4), 1% NP-40
(Sigma, St. Louis, MO, USA), 0.25% sodium deoxycholate, 150 mM
NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1
μg/ml leupeptin and 1 μg/ml pepstatin. The protein concentrations
were determined using the Bradford assay. The protein lysate (50
μg) was separated on a 10% SDS-PAGE gel and transferred onto
polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA)
for western blot analysis detection. The blot was blocked with 5%
non-fat dry milk in a buffer containing 10 mM Tris (pH 7.5), 100 mM
NaCl and 0.1% Tween-20 (Sigma). The blot was washed and incubated
with primary polyclonal anti-TCTP antibody (1:1000 dilution) for 1
h and then incubated for 30 min with secondary goat anti-rabbit
antibody conjugated with horseradish peroxidase (1:3000 dilution).
Immunoreactive protein signals were visualized by an enhanced
chemiluminescence kit (Thermo Scientific, Amersham, USA).
siRNA treatment
The TCTP protein is encoded by the TPT1 gene.
The siRNAs for the TPT1 gene were 21 base pairs of
oligonucletides synthesized at Biomics Biotechnologies Company Ltd.
(Nantong, China). Three specific siRNAs were designed to start at
various nucleotide sites on the TPT1 gene cDNA:
TPT1-272-siRNA (sense siRNA, 5′-GGUACCG AAAGCACAGUAAdTdT-3′;
antisense siRNA, 5′-UUACUGU GCUUUCGGUACCdTdT-3′);
TPT1-316-siRNA (sense siRNA, 5′-CCAUCACCUGCAGGAAACAdTdT-3′;
antisense siR NA, 5′-UGUUUCCUGCAGGUGAUGGdTdT-3′);
TPT1-351-siRNA (sense siRNA, 5′-ACCAUCACCUGCAGG AAACdTdT-3′;
antisense siRNA, 5′-GUUUCCUGCAGG UGAUGGUdTdT-3′). A negative
control siRNA (siRNA-NC) that shared a poor homology with the human
genome sequence was designed as the negative control (sense siRNA,
5′-CCGA ACGUGUCACGUUCdTdT-3′; antisense siRNA, 5′-GAACG
UGACACGUUCGGdTdT-3′). We also designed a FAM-labeled siRNA-NC probe
to examine the transfection efficiency.
All procedures were performed in an RNAse-free
environment using RNAse-free water. Cells were then transfected
with an approximate final concentration of 100 nM of siRNA duplexes
using Lipofectamine (Invitrogen Life Technologies, Carlsbad, CA,
USA). Cells were collected at 24, 48 and 72 h following the
transfection and used for cell viability, fluorescence microscopy
studies and RT-PCR analysis.
RT-PCR analysis
mRNA was isolated from mock-transfected and
TPT1-siRNA transfected A431 cells using TRIzol reagent
according to the manufacturer’s instructions (Invitrogen). The mRNA
was then converted to cDNA using a RT-PCR kit (Ambion, Kyoto,
Japan). Expression of TPT1 in the cDNA samples was
determined using PCR using gene-specific TPT1 primers (sense
primer 5′-TCTATCACCTGTCATCATAACTG-3′; antisense primer
5′-CCACTCCAAATAAATCACAGTC-3′), which were designed by Biomics
Biotechnologies. PCR parameters were as follows: 30 sec of
denaturation at 95°C, 30 sec of primer annealing at 55°C and 20 sec
of primer extension at 72°C; the cycle was repeated 40 times. A
final extension of 5 min at 72°C was performed prior to storing the
samples at 4°C. Following PCR, the products were analyzed by 1%
agarose gel electrophoresis.
Cell viability assay
A431 cells (2x103 cells/ml) were cultured
in 96-well plates until they reached 50% confluency. Cells were
then transfected with a final concentration of 100 nM TCTP siRNA. A
total of 72 h following siRNA transfection, cell viability was
determined by a Cell Counting kit-8 (CCK-8; Beyotime Institute of
Biotechnology, Jiangsu, China). Briefly, 10 μl of water-soluble
formazan dye was added to the mock-transfected and
siRNA-transfected cells, and plates were incubated for 2 h in 5%
CO2. The developed color was measured at 450 nm using an
ELISA plate reader.
Hoechst staining
A431 cells were cultured in 6-well tissue culture
plates until they were transfected with TCTP siRNA. Following 72-h
incubation, the cells were stained with Annexin V-FITC and
propidium iodide. Stained cells were visualized with a fluorescence
microscope (Nikon Eclipse ET2000-E, Japan).
Cell apoptosis detection
A431 cells were harvested by trypsinization 72 h
following transfection and centrifugation x 1000 g for 5 min. Cells
were fixed with 70% ethanol overnight at 4°C. Following a wash in
PBS, the cells were incubated with 1 mg/ml RNase A (Sigma) for 30
min at 37°C and then with 100 ug/ml propidium iodide (Sigma) for 20
min at 4°C. The apoptosis of cells was evaluated by flow cytometry
on a FACSort flow cytometer (BD Biosciences FACSCalibur). A total
of 20,000 events were determined by the forward side scatter (FSC)
signal to exclude cell aggregates. The data were analyzed using the
CellQuest software.
Statistical analysis
Statistical analysis was performed using
SPSS® for Windows version 16.0 (SPSS, Chicago, IL, USA).
The statistical analysis for the TCTP immunostaining in different
groups was performed using a nonpaired t-test.
Results and Discussion
Although TCTP appears to be ubiquitous and
multifunctional, its expression and role in cutaneous SCC have not
been investigated. In this study, we first examined TCTP expression
in skin SCCs using immunohistochemistry. Then we further
investigated its functions in epidermoid carcinoma cell lines using
a siRNA gene silencing approach.
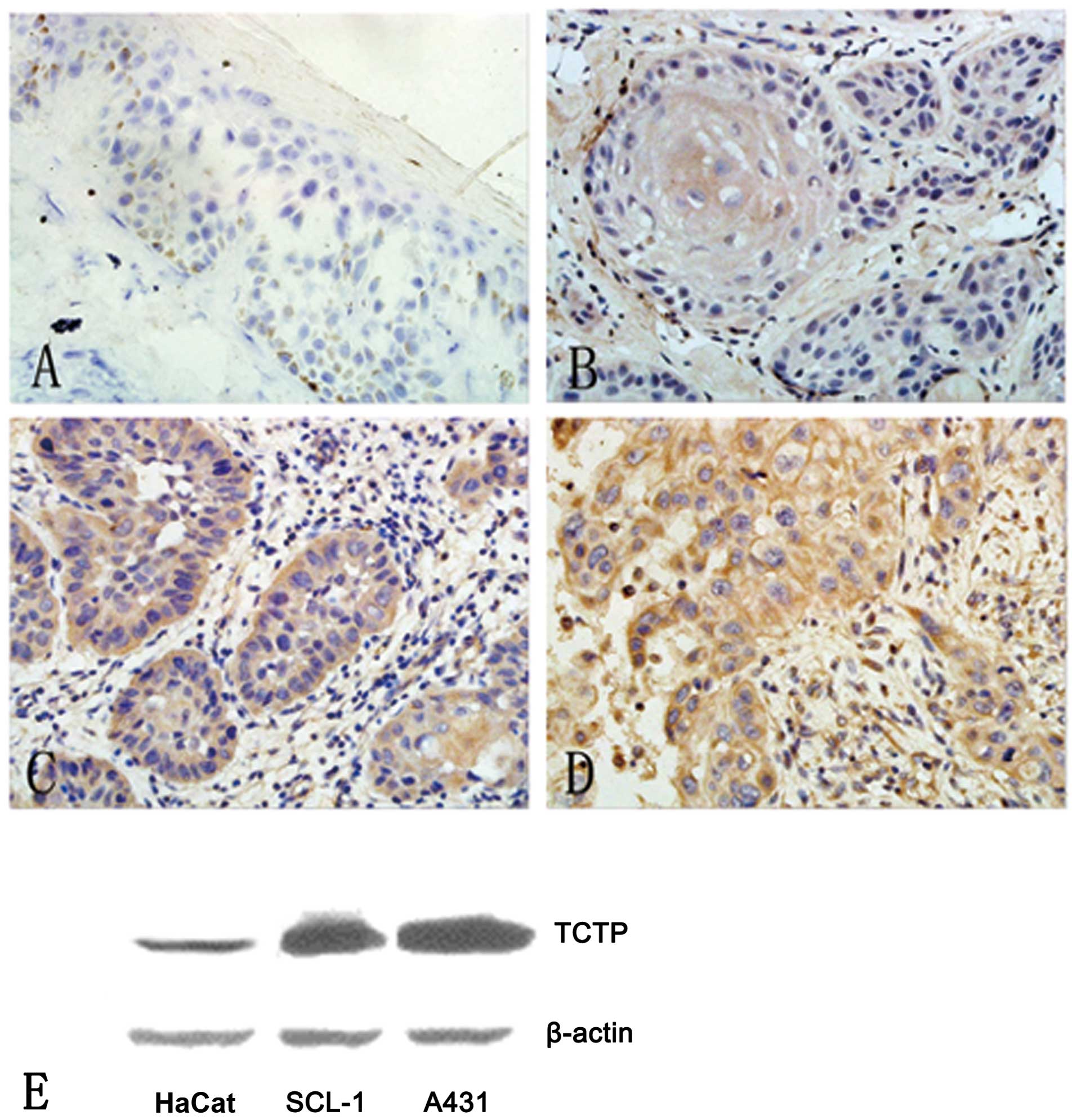
Five normal cases exhibited slight immunostaining of
TCTP in skin keratinocytes majorly located in the basal layers
(Fig. 1A). However, all skin SCC
samples displayed typical diffused cytoplasmic staining of TCTP.
More importantly, the density of immunostaining was significantly
increased with the grade of malignancy. Well-differentiated SCCs
(grade I) exhibited relatively weak cytoplasmic staining of the
tumor cells, with an average optical density (OD) of 0.155±0.061,
but a horny pearl was evident as a typical feature (Fig. 1B). Moderately-differentiated SCCs
(grade II) were observed with moderate cytoplasmic staining, with
an average OD of 0.255±0.031 (Fig.
1C). Poorly-differentiated SCC (grade III) displayed robust
cytoplasmic staining with an average OD of 0.341±0.135 (Fig. 1D). The expression of the TCTP
protein revealed a statistically significant difference between
grade II and grade I SCCs (p<0.01). The expression of TCTP in
grade III SCCs was also significantly higher than in grade I and II
SCC samples (p<0.01; p<0.05, respectively). TCTP was
initially reported to be a cytoplasmic protein (16). However, other studies later
reported the nuclear localization of TCTP by immunohistochemical
analysis (11). Based on our
results that revealed TCTP is a cytoplasmic protein in skin SCC
tumor cells, we predict that TCTP may shuttle between the cytoplasm
and the nucleus, depending on the cell cycle phase, differentiation
state or environmental conditions. We presume that upregulation of
TCTP in skin SCC may contribute to tumor growth through the
promotion of cell growth and inhibition of apoptosis in tumor
cells, and eventually result in increasing metastasis. In our
study, among the 65 cases of skin SCCs, there were nine patients
with lymph node metastatic SCC. However, there is no statistically
significant difference in TCTP expression between the secondary and
primary cutaneous SCCs (OD: 0.290±0.201 vs. 0.236±0.110,
p>0.05). Thus, the expression of TCTP appeared to be not
significantly associated with lymph node metastasis in cutaneous
SCC, which was consistent with an existing study (17).
TCTP has been found to be expressed in several
healthy and tumor cells, including erythrocytes, hepatocytes,
macrophages, platelets, keratinocytes, erythroleukemia cells,
gliomas, melanomas, hepatoblastomas and lymphomas, but it is not
detected in renal cell carcinoma (16). Using immunohistochemical analysis,
our study indicates that the expression of TCTP is upregulated in
skin SCCs. More importantly, the level of TCTP expression is
significantly associated with the grade of cutaneous SCCs. The
poorly-differentiated SCC had a higher level of TCTP expression.
Next, using western blot analysis, we further demonstrated that
TCTP had a higher expression level in the two epidermoid SCC cell
lines in comparison with normal keratinocytes. Western blot
analysis results revealed that the skin SCC cell lines, A431 and
SCL-1, expressed fold higher levels of TCTP than the normal
keratinocyte cell line, HaCaT (Fig.
1E). Between these two SCC cell lines, A431 cells express
slightly higher TCTP than SCL-1 cells (Fig. 1E). Therefore, we selected the SCC
cell line, A431, to further investigate the role of TCTP in skin
SCC using RNA interference.
Previously, siRNA techniques that efficiently block
specific target gene expression have been demonstrated. Thus, we
selected this method to observe the effect of TCTP in A431 SCC
cells when TCTP expression was downregulated by specific siRNAs.
For this purpose, we designed three TPT1-siRNAs from various
nucleotide sites initiating from the start code in the TPT1
cDNA, which were named TPT1-272-siRNA, TPT1-316-siRNA
and TPT1-351-siRNA. Since the tumor cells with the highest
level of endogenous protein are likely to get the most prominent
inhibitory effect from the antisense oligonucleotides (18), we selected the A431 cell line with
the highest level of TCTP expression for the siRNA gene silencing
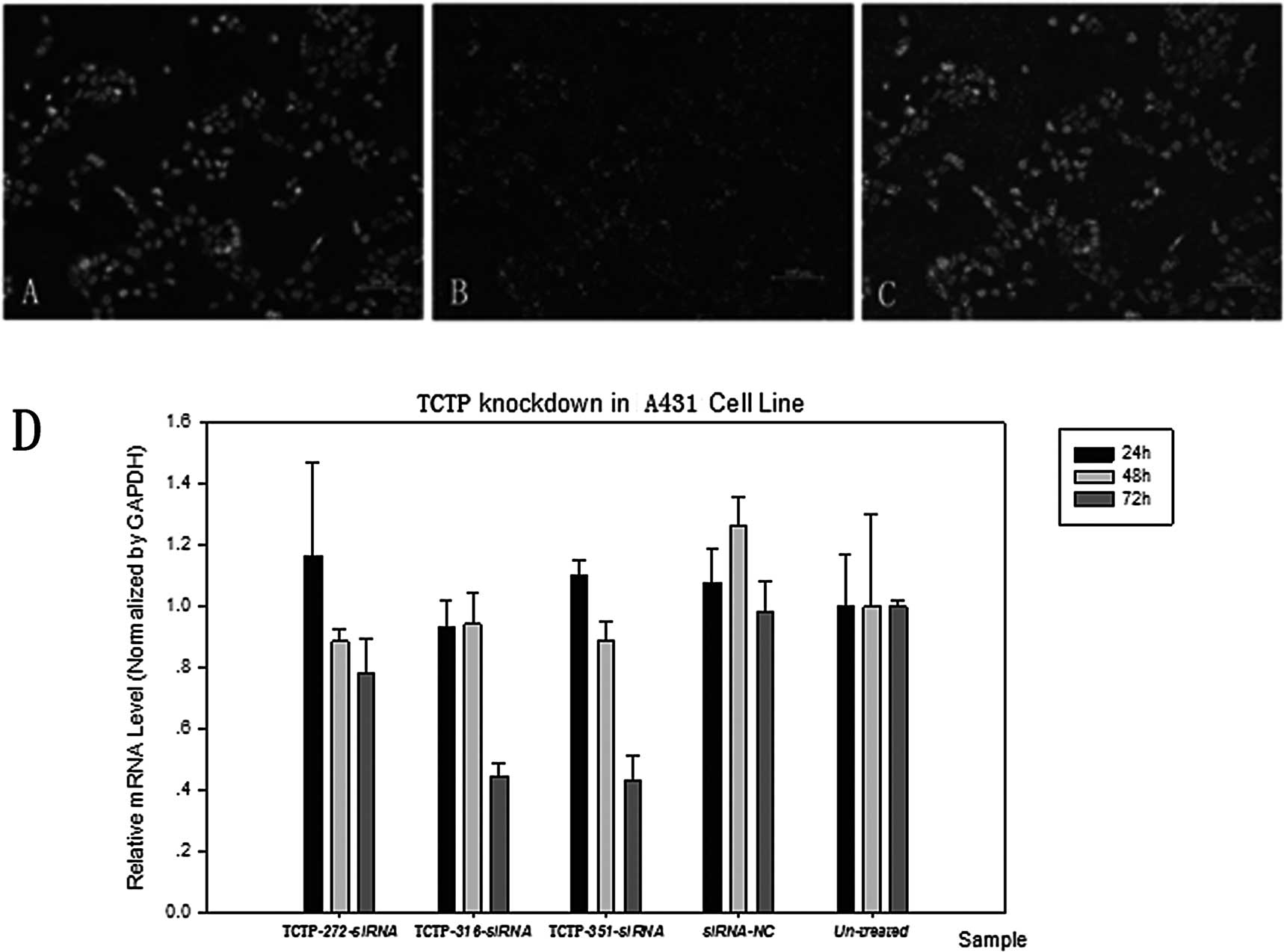
investigation. A431 cells were first transfected with siRNA-NC to
examine the transfection efficiency. Varying siRNA concentrations
and siRNA transfection reagents were compared by observing the
levels of green fluorescence to obtain the optimal transfection
efficiency. The highest transfection efficiency reached 98%
(Fig. 2B and C). Hoechst staining
was used to confirm the location of the cells (Fig. 2A and C). Cells were then
transfected with three types of TPT1-siRNAs:
TPT1-272-siRNA, TPT1-316-siRNA and
TPT1-351-siRNA. The silencing of the target gene was
confirmed by RT-PCR. Time course analyses revealed that 72 h
following transfection with TPT1-351-siRNA, there was a
significant decrease in the level of TCTP mRNA transcripts
in the transfected cells, whereas TPT1-272-siRNA and
TPT1-316-siRNA induced a significant reduction in TCTP
transcript, but a relatively lower reduction compared to
TPT1-351-siRNA (Fig. 2D).
These results indicate that TPT1-351-siRNA most effectively
and specifically downregulated the expression of the TCTP protein
in the A431 cell line. We therefore selected the
TPT1-351-siRNA and the time of 72 h for the next function
studies.
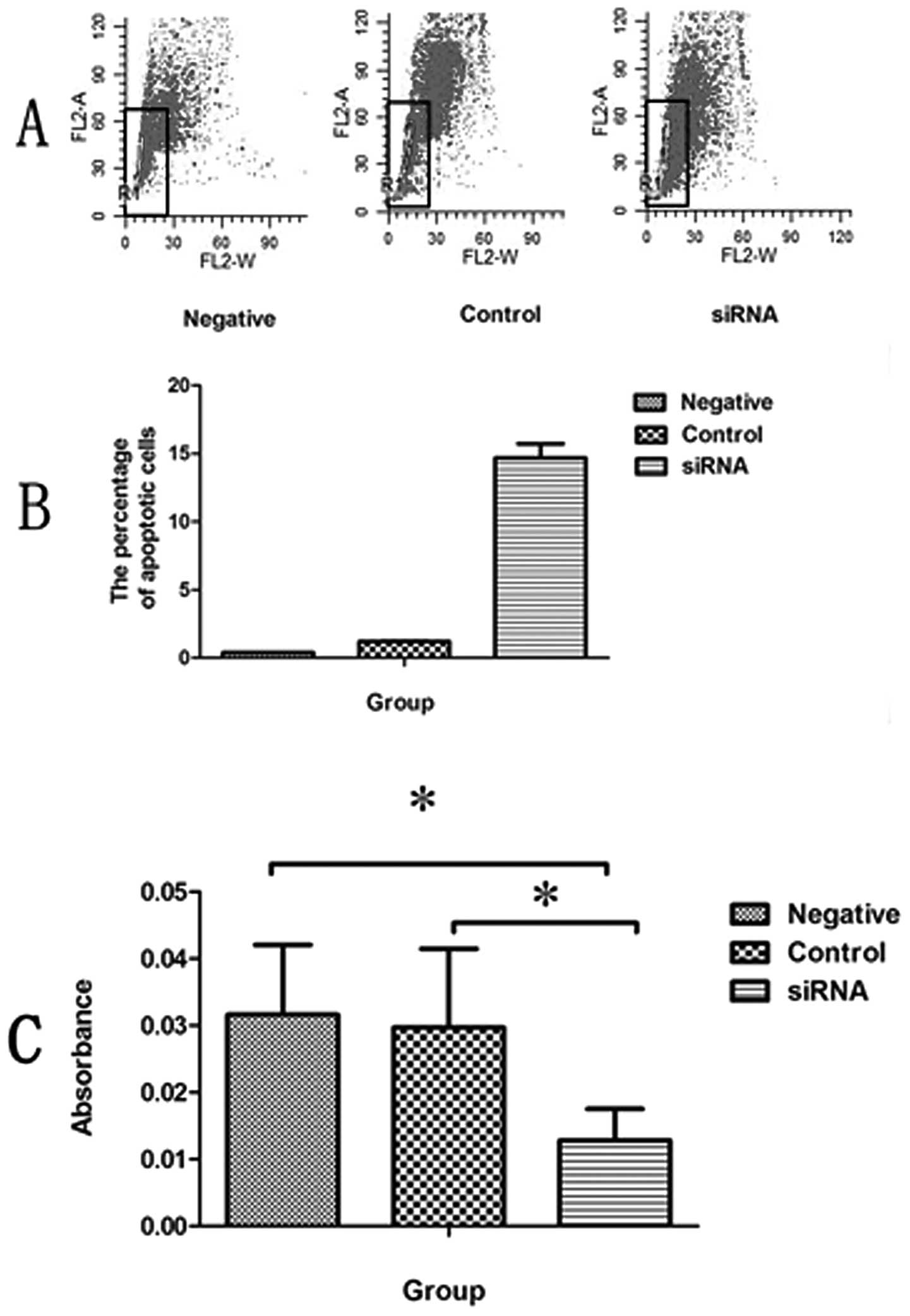
Flow cytometry was used to determine the
anti-apoptotic effect of TCTP on the SCC cell line, A431.
Transfection with TPT1-351-siRNA resulted in producing
significantly more apoptotic cells in the SCC cell line A431
(Fig. 3A). The proportion of
apoptotic cells out of the total A431 cells transfected with
TPT1-351-siRNA was 14.69±1.03%, whereas the proportion of
apoptotic cells in the control group treated with Lipofectamine
alone was 0.4±0.03%. The percentage of apoptotic cells in the
negative control group treated with siRNA-NC was 1.21±0.055%
(Fig. 3B). Our results revealed
that the A431 cells transfected with TPT1-351-siRNA
underwent considerably more apoptosis compared to the cells
transfected with control siRNA-NC or Lipofectamine alone (Fig. 3A and B).
Cells that were transfected with
TPT1-351-siRNA for 72 h were further selected for the cell
proliferation assay, which demonstrated a significant decrease in
cell proliferation in the TPT1-351-siRNA-transfected A431
cells using the CCK-8 assay. These studies revealed that knocking
down the TCTP gene resulted in decreased cell proliferation
in the A431 cells compared to cells that were transfected with a
control siRNA-NC or Lipofectamine alone (p<0.05; Fig. 3C).
Li et al were the first to demonstrate the
anti-apoptotic property of TCTP. Another study then revealed
similar results that altered levels of TCTP affect the cell
proliferation and apoptotic states in hepatoma cells (11). Furthermore, a recent study has also
demonstrated that TCTP acts as a negative regulator of apoptosis in
lung cancer. TCTP prevents apoptosis by destabilizing p53, and the
overexpression of TCTP promotes the degradation of p53 (19). In this study, we provided evidence
to support the theory that overexpression of TCTP in skin SCCs
leads to increased tumor cell proliferation through an
anti-apoptotic function. Several anti-apoptotic mechanisms have
been proposed for TCTP (17,20).
However, the molecular mechanisms concerning how TCTP acts as an
anti-apoptotic protein in skin tumors, including SCC, need to be
further elucidated. Additional studies are in progress in our
laboratory to elucidate the anti-apoptotic mechanisms of TCTP, as
well as the siRNA delivery route and its therapeutic potential
against SCC in animal models.
In conclusion, TCTP is highly expressed in human
skin squamous cell carcinoma. The level of TCTP expression
increases with the grade of cutaneous SCCs. Downregulation of TCTP
in the SCC cell line by a siRNA gene silencing approach leads to
decreased cell viability and increased apoptosis. Our findings
indicate that TCTP could be a candidate gene for the
development of diagnostic and therapeutic strategies for skin
SCC.
Acknowledgements
The study was supported by the
National Natural Science Foundation of China (grant no. 30771946
and grant no. 81000700).
References
|
1.
|
H BohmR BenndorfM GaestelB GrossP
NurnbergR KraftA OttoH BielkaThe growth-related protein P23 of the
Ehrlich ascites tumor: translational control, cloning and primary
structureBiochem Int1927728619892479380
|
|
2.
|
C BonnetE PerretX DumontA PicardD CaputG
LenaersIdentification and transcription control of fission yeast
genes repressed by an ammonium starvation growth
arrestYeast162333200010.1002/(SICI)1097-0061(20000115)16:1%3C23::AID-YEA503%3E3.0.CO;2-A10620772
|
|
3.
|
SM MacDonaldT RafnarJ LangdonLM
LichtensteinMolecular identification of an IgE-dependent
histamine-releasing
factorScience269688690199510.1126/science.75428037542803
|
|
4.
|
SH ChenPS WuCH ChouYT YanH LiuSY WengA
knockout mouse approach reveals that TCTP functions as an essential
factor for cell proliferation and survival in a tissue- or cell
type-specific mannerMol Biol
Cell1825252532200710.1091/mbc.E07-02-018817475776
|
|
5.
|
A TelermanR AmsonThe molecular programme
of tumour reversion: the steps beyond malignant transformationNat
Rev Cancer9206216200910.1038/nrc258919180095
|
|
6.
|
M TuynderL SusiniS PrieurS BesseG FiucciR
AmsonA TelermanBiological models and genes of tumor reversion:
cellular reprogramming through tpt1/TCTP and SIAH-1Proc Natl Acad
Sci USA991497614981200210.1073/pnas.22247079912399545
|
|
7.
|
K OikawaT OhbayashiJ MimuraY
Fujii-KuriyamaS TeshimaK RokutanK MukaiM KurodaDioxin stimulates
synthesis and secretion of IgE-dependent histamine-releasing
factorBiochem Biophys Res
Commun290984987200210.1006/bbrc.2001.630211798171
|
|
8.
|
SR SturzenbaumP KilleAJ
MorganIdentification of heavy metal induced changes in the
expression patterns of the translationally controlled tumour
protein (TCTP) in the earthworm Lumbricus rubellus1Biochim
Biophys Acta1398294304199810.1016/S0167-4781(98)00077-39655922
|
|
9.
|
AS Vercoutter-EdouartX CzeszakM CrepinJ
LemoineB BoillyX Le BourhisJP PeyratH HondermarckProteomic
detection of changes in protein synthesis induced by fibroblast
growth factor-2 in MCF-7 human breast cancer cellsExp Cell
Res2625968200110.1006/excr.2000.5066
|
|
10.
|
JM LangdonBM VonakisSM
MacDonaldIdentification of the interaction between the human
recombinant histamine releasing factor/translationally controlled
tumor protein and elongation factor-1 delta (also known as
eElongation factor-1B beta)Biochim Biophys
Acta1688232236200410.1016/j.bbadis.2003.12.007
|
|
11.
|
F LiD ZhangK FujiseCharacterization of
fortilin, a novel antiapoptotic proteinJ Biol
Chem2764754247549200110.1074/jbc.M10895420011598139
|
|
12.
|
N AmzallagBJ PasserD AllanicE SeguraC
TheryB GoudR AmsonA TelermanTSAP6 facilitates the secretion of
translationally controlled tumor protein/histamine-releasing factor
via a nonclassical pathwayJ Biol
Chem27946104461012200410.1074/jbc.M40485020015319436
|
|
13.
|
J JungM KimMJ KimJ KimJ MoonJS LimM KimK
LeeTranslationally controlled tumor protein interacts with the
third cytoplasmic domain of Na,K-ATPase alpha subunit and inhibits
the pump activity in HeLa cellsJ Biol
Chem2794986849875200410.1074/jbc.M40089520015383549
|
|
14.
|
Y YangF YangZ XiongY YanX WangM NishinoAn
N-terminal region of translationally controlled tumor protein is
required for its antiapoptotic
activityOncogene2447784788200510.1038/sj.onc.120866615870695
|
|
15.
|
H LiuHW PengYS ChengHS YuanHF
Yang-YenStabilization and enhancement of the antiapoptotic activity
of mcl-1 by TCTPMol Cell
Biol2531173126200510.1128/MCB.25.8.3117-3126.200515798198
|
|
16.
|
JC SanchezD SchallerF RavierO GolazS
JaccoudM BeletTranslationally controlled tumor protein: a protein
identified in several nontumoral cells including
erythrocytesElectrophoresis18150155199710.1002/elps.1150180127
|
|
17.
|
L SusiniS BesseD DuflautA LespagnolC
BeekmanG FiucciTCTP protects from apoptotic cell death by
antagonizing bax functionCell Death
Differ1512111220200810.1038/cdd.2008.1818274553
|
|
18.
|
TH HuCC HuangLF LiuPR LinSY LiuHW
ChangExpression of hepatoma-derived growth factor in hepatocellular
carcinomaCancer9814441456200310.1002/cncr.1165314508832
|
|
19.
|
SB RhoJH LeeMS ParkHJ ByunS KangSS
SeoAnti-apoptotic protein TCTP controls the stability of the tumor
suppressor p53FEBS
Lett5852935201110.1016/j.febslet.2010.11.01421081126
|
|
20.
|
P GraidistM YazawaM TonganuntA NakatomiCC
LinJY ChangFortilin binds Ca2+ and blocks
Ca2+-dependent apoptosis in vivoBiochem
J408181191200717705784
|