Introduction
Colorectal cancer is the third leading cause of
cancer-related mortality in Taiwan (1) and the second leading cause of
cancer-related mortality in Western countries (2,3).
Traditional Chinese herbal medicines are widely accepted as an
option for the treatment of colorectal cancers and there are
intense efforts under way to identify new herbs and bioactive pure
compounds (4,5). Danshen (Salviae miltiorrhizae
Radix) is widely prescribed in traditional Chinese medicine for
cardiovascular diseases (6,7).
Tanshinone IIA (Tan-IIA; C19H18O3)
is extracted from Danshen (8,9), and
possesses anti-inflammatory (10,11)
and antioxidant properties (12,13).
Our previous studies have shown that Tan-IIA inhibits growth and
induces apoptosis in Colo205 human colon cancer cells (14) and MDA-MB-231 breast cancer cells
in vitro (15).
5-Fluorouracil (5-FU) is one of the chemotherapeutic medicines for
colon cancer, but has low efficacy (16). To increase its therapeutic
potential, there is interest in combining it with another medicine.
The efficacy of Tan-IIA plus 5-FU in human colon cancer cells has
not been established. Accordingly, we investigated the effects of
Tan-IIA and 5-FU in a Colo205 human colon cancer cell xenograft
model.
Materials and methods
Materials
Tan-IIA (purity >96%; HPLC) was purchased from
Herbasin Co. (China). Aprotinin, antipain, sodiumdeoxycholate,
leupeptin, sodium orthovanadate, Triton X-100 and Tris-HCl were
obtained from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl
sulfoxide (DMSO), potassium phosphate and TE buffer were purchased
from Merck KGaA (Darmstadt, Germany). RPMI-1640 medium and fetal
bovine serum (FBS) were obtained from Gibco BRL (Grand Island, NY,
USA). Antibodies to P53, P21, VEGF, MMP-2 and -7, Topoisomerase I,
Erb-B2, P-glycoprotein (P-gp), LC-3II, Bcl-2 and β-actin were
obtained from Sigma-Aldrich. Penicillin-streptomycin, Trypsin-EDTA
and glutamine were obtained from Gibco BRL. Sodium dodecylsulfate
polyacrylamide gel and electrophoresis (SDS-PAGE) running buffer
(10X), Tris, Tween-20, SDS and 5X TBE buffer were obtained from
Amresco (St. Louis, MO, USA). BioMax film was obtained from
Kodak.
Cell cultures
The Colo205 human colon cancer cell line was
obtained from the Food Industry Research and Development Institute
(Hsin-chu, Taiwan). The cells were placed into tissue culture
flasks (75 cm2, 250 ml) and grown at 37°C in humidified
5% CO2 and 95% air atmosphere in RPMI-1640 medium
containing 10% heat-inactivated FBS, 1% HEPES, 1% sodium pyruvate,
1% glutamine and 2% penicillin-streptomycin (10,000 U/ml
penicillin; 10 mg/ml streptomycin).
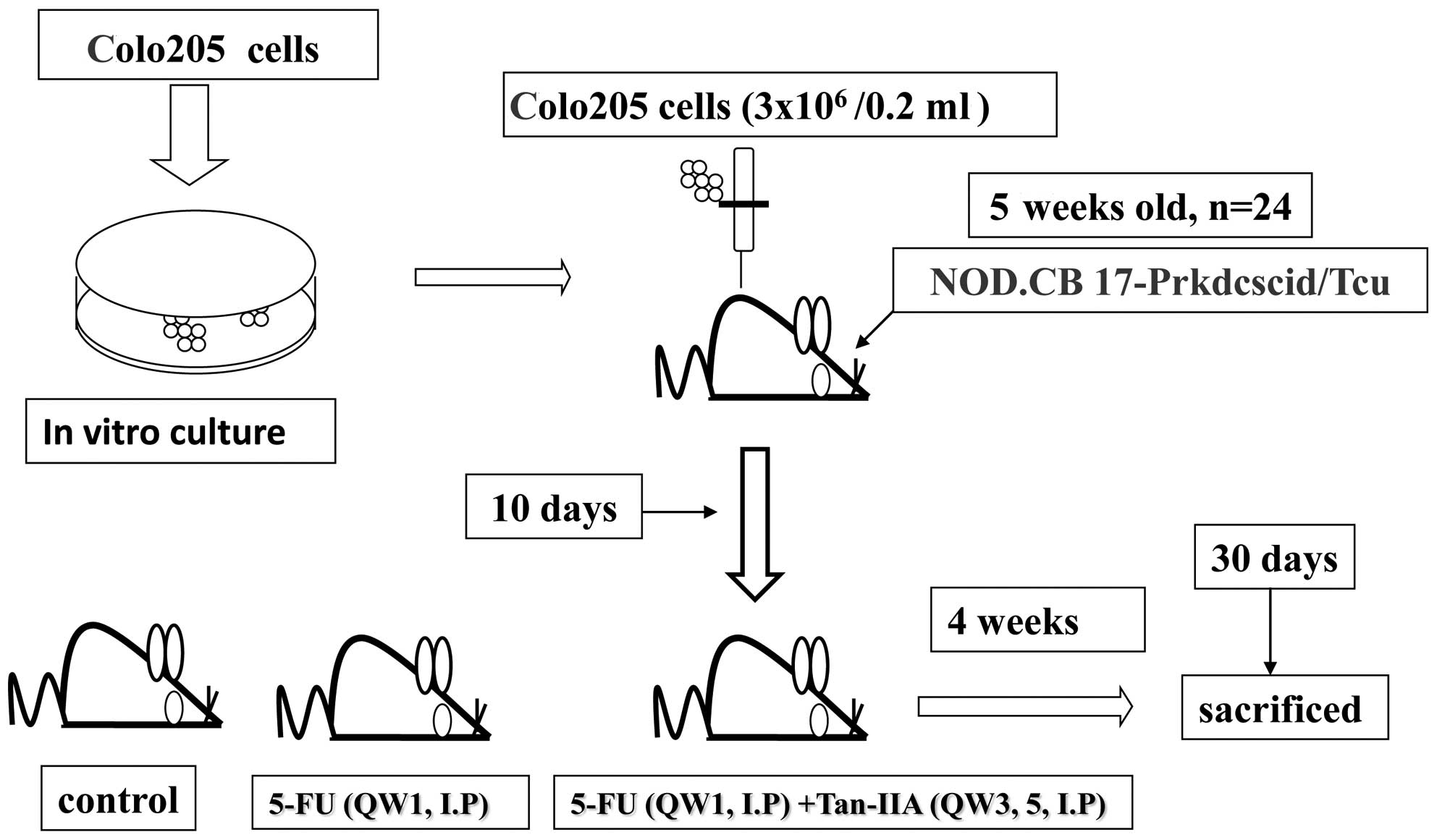
In vivo tumor xenograft study
In the present study, 5-week-old male nude SCID mice
(24 in total) were xenografted with Colo205 colon cancer cells
(3×106/0.2 ml) and maintained in a pathogen-free
environment (Laboratory Animal Center of Tzu Chi University,
Hualien, Taiwan). From day 10, SCID mice bearing Colo205 human
colon cancer cell engrafts were divided randomly into 3 groups and
then treated with 5-FU (20 mg/kg, every week on day 1) plus Tan-IIA
(20 mg/kg, every week on days 3 and 5), 5-FU (20 mg/kg, every week
on day 1) plus corn oil (every week on days 3 and 5) or the vehicle
alone (normal saline, every week on day 1 and corn oil every week
on days 3 and 5) (Fig. 1).
Following xenograft transplantation, mice exhibiting tumors were
monitored and tumor size was measured once every 3 days using
calipers. The tumor volume in each animal was estimated according
to the formula: tumor volume (mm3) = L × W2/2 (where L
is the length and W is the width) with the final measurement taken
4 weeks after tumor cell inoculation. At the end of the 4-week
dosing schedule, the SCID mice were sacrificed with CO2
inhalation, xenograft tumors were dissected and total protein
extracted for western blot analysis. The animal use protocol has
been reviewed and approved by the Institutional Animal Care and Use
Comittee (IACUC) Board, TZU-CHI Hospital (IACUC Approval No:
97-21).
Synergistic effects of Tan-IIA and 5-FU
on the protein expression of p53, p21, VEGF, MMP-2 and -7,
topoisomerase I, Erb-B2, P-gp, LC-3II, Bcl-2 and β-actin in Colo205
cell xenograft tumors
Protein preparation. Total proteins were
extracted as previously described (17). Briefly, the xenograft tumors were
broken into pieces and the resulting thick liquid was then
suspended in modified PRO-PREP™ buffer (iNtRON Biotechnology Inc.,
Korea) for 40 min at 4°C. Lysates were immediately centrifuged at
13,000 × g for 20 min at 4°C, and the supernatant was collected,
aliquated (20 μl/ tube) and stored at −80°C until assay. The
extracted protein concentrations were measured using the Bradford
method (18).
Western blot analysis
All protein samples were separated by 8–15% SDS-PAGE
as previously described (17). The
SDS-separated proteins were equilibrated in transfer buffer (25 mM
Tris, pH 8.5, 0.2 M glycine and 20% methanol) and transferred onto
a PVDF membrane (Millipore, Bedford, MA, USA). The membranes were
incubated with 5% non-fat dry milk in Tris-buffered saline
containing 0.1% Tween-20 for 1 h. These membranes were then washed
and incubated with appropriate dilutions of specific antibodies at
4°C overnight [including p53 (1:2000, MAB 1355; R&D Systems),
p21 (1:2000, MAB 1047; R&D Systems), VEGF (1:500, V4758;
Sigma), MMP-2 (1:200, MAB 902; R&D Systems) and MMP-7 (1:500,
MAB 9071; R&D Systems), topoisomerase I (1:1000, T8573; Sigma),
Erb-B2 (1:500, MAB 1129; R&D Systems), P-gp (1:500, P7965;
Sigma), microtubule-associated protein light chain 3 (LC3)-II
(1:1500, L7543; Sigma), Bcl-2 (1:500; R&D Systems) and β-actin
(1:15000, A5441, Sigma)]. Following incubation with anti-mouse
peroxidase-conjugated antibody (1:15000) (Sigma-Aldrich) the
immunoreactive bands were visualized with an enhanced
chemiluminescence (ECL, Millipore) detection kit. The detection of
β-actin was used as an internal control in all of the data for
western blot analysis. Immunoreactive bands were scanned (GS-800;
Bio-Rad Life Science Products, Hercules, CA, USA) and analyzed
using a digital scanning densitometer (Quantity One, v4.4.0;
Bio-Rad Life Science Products).
Statistical analysis
Values were presented as the means ± SD. The
Student's t-test was used to analyze statistical significance.
P<0.05 was considered to indicate statistically significant
differences for all tests.
Results
Synergistic effects of Tan-IIA and
5-FU in the Colo205 cell xenograft tumor model
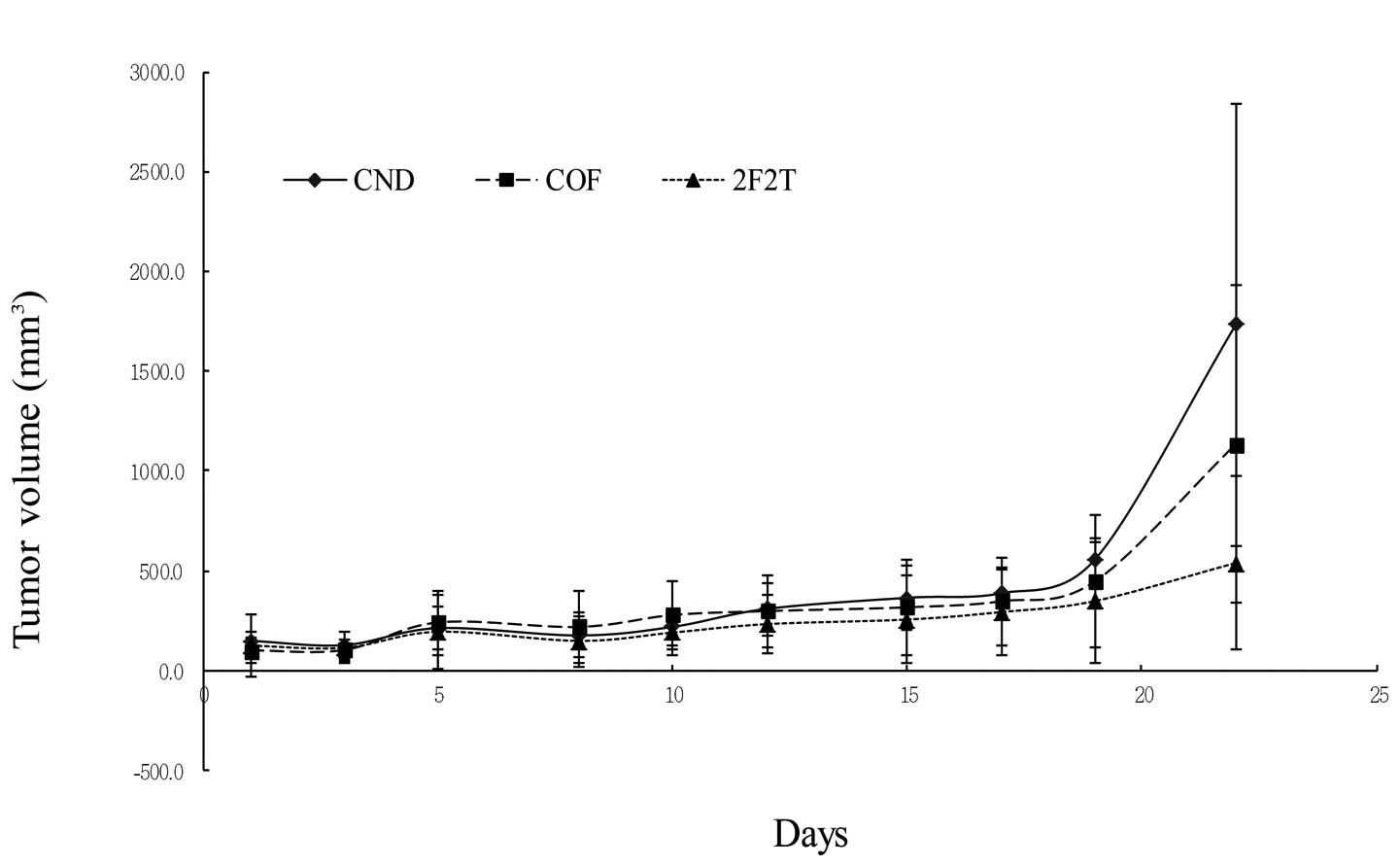
SCID mice bearing Colo205 human colon cancer cell
xenografts were divided randomly into 3 groups and then treated
with 5-FU plus Tan-IIA, 5-FU only, or the vehicle alone. The tumor
volumes and SCID mouse body weights were measured every 3 days. The
xenograft tumor volumes were measured. The tumor volumes were
1,733.20±1,113.68, 1,134±795.95 and 537.42±437.36 mm3
for the control, 5-FU, and 5-FU plus Tan-IIA groups, respectively.
The tumor volume enlarged ratios were 11.82±0.64, 11.5±0.7 and
4.38±0.31 for the control, 5-FU, and 5-FU plus Tan-IIA groups,
respectively. The results showed that Tan-IIA could potentiate the
effect of 5-FU in a colon cancer nude mouse model (Fig. 2A and B).
Effects of Tan-IIA and 5-FU on the
protein expressions of p53, p21, VEGF, MMP-2 and -7, topoisomerase
I, Erb-B2, P-gp, LC-3II, Bcl-2 and β-actin in Colo205 cell
xenograft tumors
SCID mice bearing Colo205 human colon cancer cell
xenograft tumors were divided randomly into 3 groups and then
treated with 5-FU plus Tan-IIA, 5-FU only, or the vehicle alone. At
the end of the 4-week dosing schedule, the SCID mice were
sacrificed by CO2 inhalation and xenograft tumors
dissected with total protein extracted for western blot analysis.
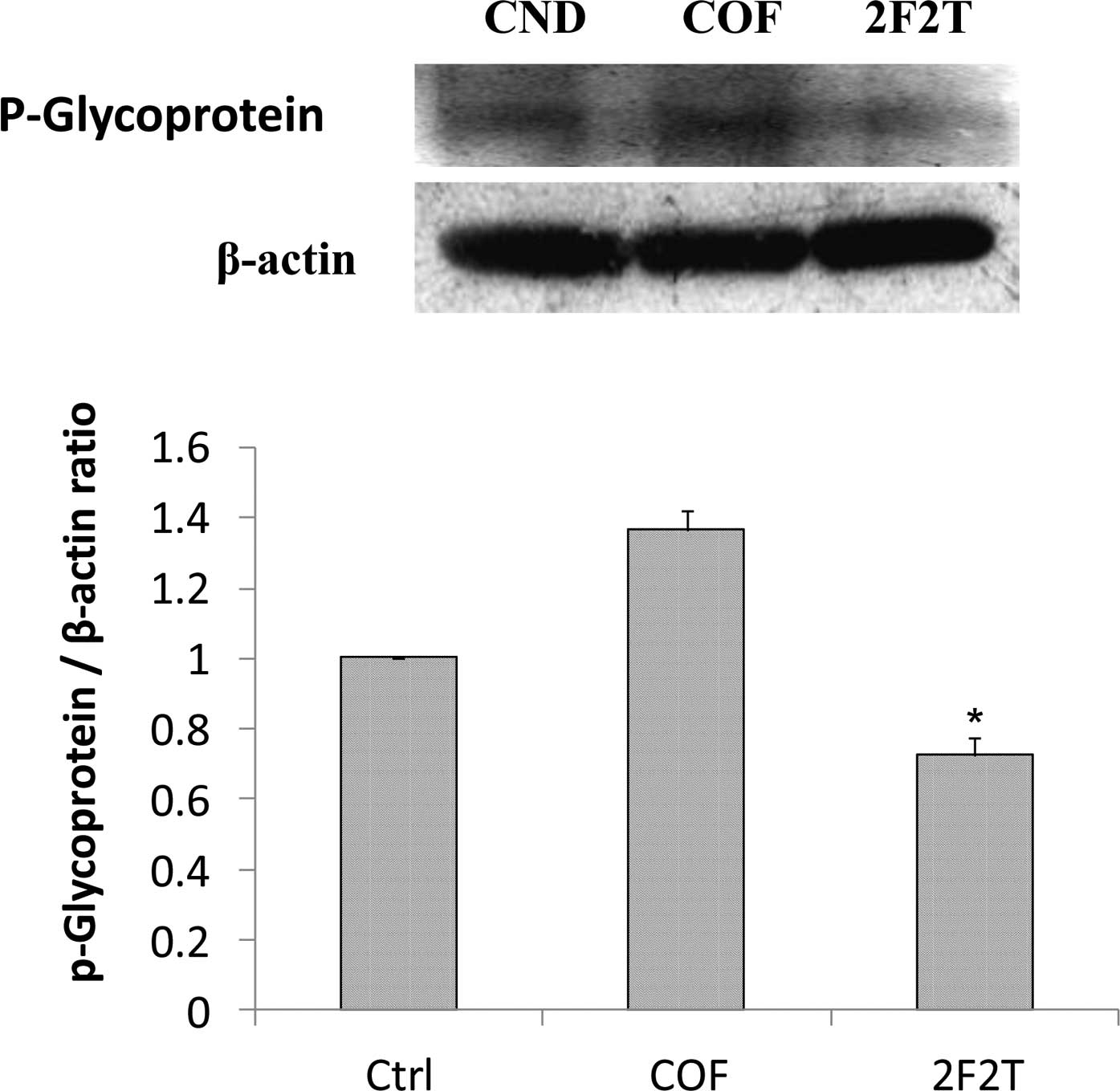
The results showed that SCID mice with Colo205 cell xenograft
tumors treated with 5-FU [20 mg/kg intraperitoneally (i.p.), every
week on day 1] plus Tan-IIA (20 mg/kg i.p., every week on days 3
and day 5) had downregulated P-gp (Fig. 3A), LC-3II (Fig. 3B), VEGF (Fig. 3C), NF-κB p65 (Fig. 3D) and MMP-7 (Fig. 3E) expression when compared to the
group treated only with 5-FU.
Discussion
P-gp is a cell membrane-associated protein, with
high expression in cancer tissue, which functions as a drug export
pump that decreases intracellular concentrations of
chemotherapeutic agent (19,20).
Our results showed that 5-FU plus Tan-IIA decreased the protein
expression of P-gp when compared to the 5-FU group. It was
documented that simultaneous and continuous exposure of colon
cancer cells to low concentrations of 5-FU for 144 h showed a
strong antagonism in vitro and that 14-days of continuous
infusion of 5-FU significantly stimulated angiogenesis in
vivo (21,22) Our results also showed that 5-FU
increased the protein expression of VEGF when compared to the
control group. The 5-FU plus Tan-IIA group decreased the protein
expression of VEGF when compared to the 5-FU group. It has been
well documented that LC3-II can be used as a marker of autophagy
and can be detected using western blot analysis (23,24).
Our results showed that the combination treatment of Tan-IIA plus
5-FU decreased the protein expression of LC3-II when compared to
the 5-FU group. The combination treatment of autophagy inhibitor
plus 5-FU significantly increased apoptotic cell death.
5-FU-induced apoptosis in colon cancer cells can be enhanced by the
inhibition of autophagy (21).
Elevated MMP-7 protein expression was a predictor of colon cancer
recurrence and liver metastasis. High expression of MMP-7 was
related to decreased survival (25). It has been well documented that the
combination of gambogic acid and celastrol has a synergistic
antitumor effect. The effect can be attributed to apoptosis induced
by a decrease in NF-κB pathway activation, therefore, NF-κB p65
protein may be involved (26,27).
The inhibition of NF-κB augments sensitivity to 5-FU/folinic acid
in colon cancer (28). Our results
showed that Tan-IIA plus 5-FU decreased the protein expression of
MMP-7 and NF-κB p65 when compared to the 5-FU group. Tan-IIA plus
5-FU has a synergistic effect on Colo205 cell xenograft tumors. The
effect can be primarily attributed to a decrease in MMP-7 and NF-κB
p65 expression. These observations suggest that Tan-IIA potentiates
the efficacy of 5-FU in a colon cancer nude SCID mouse model
through downregulating P-gp, LC3-II, VEGF, MMP-7 and NF-κB p65
protein expression. This is the first report of 5-FU plus Tan-IIA
downregulating P-gp, LC3-II, VEGF, MMP-7 and NF-κB p65 protein
expression in vivo. The use of Tan-IIA may be a promising
strategy for the adjuvant chemotherapy of colon cancer.
Acknowledgements
This study was supported by a grant
(CCMP97-RD-041) from the Committee on Chinese Medicine and
Pharmacy, Department of Health, Executive Yuan, Taiwan, R.O.C.
References
|
1.
|
Department of Health, Executive Yuan,
Taipei, Taiwan R.O.C: Statistics of Causes of Death, 2007. p33,
2008.
|
|
2.
|
A JemalRC TiwariT MurrayCancer statistics,
2004CA Cancer J Clin54829200410.3322/canjclin.54.1.8
|
|
3.
|
SC WeiYN SuJJ Tsai-WuGenetic analysis of
the APC gene in Taiwanese familial adenomatous polyposisJ Biomed
Sci11260265200410.1007/BF0225656914966376
|
|
4.
|
MJ VerhoefLG BalneavesHS BoonA
VroegindeweyReasons for and characteristics associated with
complementary and alternative medicine use among adult cancer
patients: a systematic reviewIntegr Cancer
Ther4274286200510.1177/153473540528236116282504
|
|
5.
|
H BoonJ WongBotanical medicine and cancer:
a review of the safety and efficacyExpert Opin
Pharmacother524852501200410.1517/14656566.5.12.248515571467
|
|
6.
|
JM FishDR WelchonsYS KimSH LeeWK HoC
AntzelevitchDimethyl lithospermate B, an extract of Danshen,
suppresses arrhythmogenesis associated with the Brugada
syndromeCirculation11313931400200610.1161/CIRCULATIONAHA.105.60169016534004
|
|
7.
|
PN ChangJC MaoSH HuangAnalysis of
cardio-protective effects using purified Salvia miltiorrhiza
extract on isolated rat heartsJ Pharmacol
Sci101245249200610.1254/jphs.FPJ05034X
|
|
8.
|
AJ CheJY ZhangCH LiXF ChenZD HuXG
ChenSeparation and determination of active components in Radix
Salviae miltiorrhizae and its medicinal preparations by
nonaqueous capillary electrophoresisJ Sep
Sci27569575200415335042
|
|
9.
|
L ZhouZ ZuoMS ChowDanshen: an overview of
its chemistry, pharmacology, pharmacokinetics, and clinical useJ
Clin Pharmacol4513451359200510.1177/009127000528263016291709
|
|
10.
|
SI JangHJ KimYJ KimSI JeongYO
YouTanshinone IIA inhibits LPS induced NF-kappaB activation in RAW
264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and
JNK pathwaysEur J
Pharmacology54217200610.1016/j.ejphar.2006.04.04416797002
|
|
11.
|
W LiJ LiM AshokR WuA cardiovascular drug
rescues mice from lethal sepsis by selectively attenuating a
late-acting pro-inflammatory mediator, high mobility group box 1J
Immunology7838563864200710.4049/jimmunol.178.6.385617339485
|
|
12.
|
R LinWR WangJT LiuGD YangCJ HanProtective
effect of Tanshinone IIA on human umbilical vein endothelial cell
injured by hydrogen peroxide and its mechanismJ
Ethnopharmacol108217222200610.1016/j.jep.2006.05.00416797899
|
|
13.
|
AM WangSH ShaW LesniakJ SchachtTanshinone
(Salviae miltiorrhizae extract) preparations attenuate
aminoglycoside-induced free radical formation in vitro and
ototoxicity in vivoAntimicrob Agents Chemother47183618412003
|
|
14.
|
CC SuGW ChenJC KangMH ChanGrowth
inhibition and apoptosis induction by tanshinone IIA in human colon
adenocarcinoma cellsPlanta
Med7413571362200810.1055/s-2008-108129918622903
|
|
15.
|
CC SuYH LinTanshinone IIA inhibits human
breast cancer cells through increased Bax to Bcl-xL ratiosInt J Mol
Med22357361200818698495
|
|
16.
|
I ChauAR NormanD CunninghamA randomised
comparison between 6 months of bolus fluorouracil/leucovorin and 12
weeks of protracted venous infusion fluorouracil as adjuvant
treatment in colorectal cancerAnn
Oncol16549557200510.1093/annonc/mdi116
|
|
17.
|
HC ChenWT HsiehWC ChangJG ChungAloe-emodin
induced in vitro G2/M arrest of cell cycle in human promyelocytic
leukemia HL-60 cellsFood Chem
Toxicol4212511257200410.1016/j.fct.2004.03.00215207375
|
|
18.
|
MM BradfordA rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye bindingAnal
Biochem72248254197610.1016/0003-2697(76)90527-3942051
|
|
19.
|
MM GottesmanI PastanSV
AmbudkarP-glycoprotein and multidrug resistanceCurr Opin Genet
Dev6610617199610.1016/S0959-437X(96)80091-88939727
|
|
20.
|
BL LumMP GoslandS KaubischBI
SikicMolecular targets in oncology: implications of the multidrug
resistance genePharmacotherapy138810919938097038
|
|
21.
|
J LiN HouA FariedS TsutsumiT TakeuchiH
KuwanoInhibition of autophagy by 3-MA enhances the effect of
5-FU-induced apoptosis in colon cancer cellsAnn Surg
Oncol16761771200910.1245/s10434-008-0260-019116755
|
|
22.
|
P AlbertssonB LennernäsK NorrbyLow-dose
continuous 5-fluorouracil infusion stimulates VEGF-A-mediated
angiogenesisActa
Oncol48418425200910.1080/0284186080240951218932044
|
|
23.
|
K KirkegaardMP TaylorWT JacksonCellular
autophagy: Surrender, avoidance and subversion by microorganismsNat
Rev Microbiol2301314200410.1038/nrmicro86515031729
|
|
24.
|
EL EskelinenAR PrescottJ CooperInhibition
of autophagy in mitotic animal
cellsTraffic3878893200210.1034/j.1600-0854.2002.31204.x12453151
|
|
25.
|
YJ FangZH LuGQ WangZZ PanZW ZhouJP YunMF
ZhangDS WanElevated expressions of MMP7, TROP2, and survivin are
associated with survival, disease recurrence, and liver metastasis
of colon cancerInt J Colorectal
Dis24875884200910.1007/s00384-009-0725-z19421758
|
|
26.
|
WL XuJR LiuHK LiuGY QiXR SunWG SunBQ
ChenInhibition of proliferation and induction of apoptosis by
gamma-tocotrienol in human colon carcinoma HT-29
cellsNutrition25555566200910.1016/j.nut.2008.10.01919121919
|
|
27.
|
D HeQ XuM YanP ZhangX ZhouZ ZhangW DuanL
ZhongD YeW ChenThe NF-kappa B inhibitor, celastrol, could enhance
the anti-cancer effect of gambogic acid on oral squamous cell
carcinomaBMC Cancer9343200910.1186/1471-2407-9-34319778460
|
|
28.
|
R VoborilSN HochwJ LiA BrankJ WeberovaF
WesselsLL MoldawerER CampSL MacKayInhibition of NF-kappa B augments
sensitivity to 5-fluorouracil/folinic acid in colon cancerJ Surg
Res120178188200410.1016/j.jss.2003.11.02315234211
|