Introduction
Recent epidemiological studies have revealed that
colorectal cancer (CRC) ranks among the leading malignancies in
incidence in Western countries and Japan (1–3). It
is well known that the lung is the most common extra-abdominal site
of metastasis from primary CRC (4). Approximately 10–20% of surgical CRC
cases develop lung metastases (5).
In consideration of the vast prevalence of CRC and of the marked
progress in diagnostic and treatment options, we anticipate more
opportunities to detect lung metastases; however, there is still a
paucity of detailed data on these issues in the literature.
In this study, we retrospectively investigated the
features of metastasized lung tumors observed in over 2,000
surgical cases of sporadic CRC in a representative university
hospital in Japan. Moreover, we assessed the risk factors for lung
metastases in CRC patients and analyzed recent trends in frequency
and survival.
Patients and methods
Patients
Patients with primary CRC who underwent surgery in
our hospital between January 1990 and December 2009 were enrolled
in this study. The subjects included those with i) inflammatory
bowel disease-associated cancer, ii) synchronous multiple cancers
of the colorectum, iii) cancer with simultaneous malignancies in
other organs and iv) patients who had undergone palliative surgery
for primary CRC. The exclusion criteria were i) cancer arising in
patients diagnosed with familial adenomatous polyposis or Lynch
syndrome, ii) anal fistula-associated cancer, iii) locally
recurrent tumor alone in previously resected CRC, and iv)
metastasized tumors in the colorectum originating from other
primary organs. For analyses of metachronous lung tumors, only
patients who had undergone curative surgery (R0) for primary CRC
were assembled. Written, informed consent was obtained from each
patient participating in this study.
Synchronous tumors
Synchronous tumors were defined as those cases in
which lesions were observed prior to or within 3 months after the
surgery for CRC, and were otherwise judged as metachronous tumors.
There is no definite consensus regarding synchronous and
metachronous metastases; however, we set this cut-off time-point,
since the first opportunity for follow-up image study occurred at 3
postoperative months, as mentioned below, in our institution.
Patients with lower rectal cancer were often treated with
preoperative chemoradiation therapy, once there was no clear
distant metastasis. Certain CRC patients with massive liver
metastases received systemic chemotherapy prior to surgery.
Pulmonary tumors revealed during such neoadjuvant therapies, for
example, were defined as synchronous tumors.
Patient examination
In our hospital, patients were routinely examined by
chest radiography, abdomino-pelvic and chest CT scans with contrast
in order to identify potential liver, lung, lymph node, or other
organ metastases prior to CRC surgery. Following surgery, patients
were surveyed primarily by CT scans and chest X-ray, first within
3–6 months, then every 6 months for 2 years, and thereafter every
12 months. Other image studies such as magnetic resonance imaging
and positron emission tomography were additionally performed at the
doctors' discretion.
Clinical data
Clinical data such as gender, age, ECOG performance
status (PS), family history of CRC, i.e. CRC cases in first-degree
relative(s), and smoking habits, histopathological parameters of
primary CRC, parameters of lung metastases such as the number,
tumor laterality and size at diagnosis, and survival time were
obtained from medical records. The pathological description of CRC
was essentially based on the TNM classification, 7th edition
(6). The study was conducted with
the approval of the ethics committee of our hospital.
Statistical analysis
Comparisons between groups were performed using the
Student's t-test, Welch's t-test, Fisher's exact test or the
Chi-square test, applying Yates' correction when needed.
Multivariate analysis by logistic regression was used to identify
independent predictors. The survival curve was estimated using the
Kaplan-Meier method and analyzed using the log-rank test. A p-value
of <0.05 was considered indicative of statistical
significance.
Results
Profile of surgical CRC patients
A total of 2,286 patients (1,448 males and 838
females; age, 22–94 years; average, 64.2 years) with primary CRC
were eligible for analysis of synchronous metastases in this study.
Other clinical and pathological data are shown in Table I. Among them, 2,082 patients
underwent curative surgery and were subjected to analyses of
metachronous lung metastases. Non-curative CRC cases showed
markedly higher percentages of poor PS (PS ≥1, 32%),
undifferentiated histology (16%), larger T (T4, 11%) and N numbers
(N+, 59%) and M1/UICC stage IV (94%), and larger tumor diameter
(mean, 57.4 mm) than curative cases (PS ≥1, 9%; undifferentiated
histology, 6%; T4, 4%; and N+, 34%; M1, 8% and mean diameter, 42.1
mm, respectively). In addition, female patients, a right-sided
colon, and a positive family history of CRC were more frequent in
non-curative than in curative cases.
 | Table I.Clinicopathological profile of CRC
patients. |
Table I.
Clinicopathological profile of CRC
patients.
| Variable | All (n=2,286)
(%) | Curative (n=2,082)
(%) | Non-curative
(n=204) (%) | P-value |
|---|
| Gender | | | | |
| Male | 1,448 (63) | 1,337 (64) | 111 (54) | 0.006 |
| Female | 838 (37) | 745 (36) | 93 (46) | |
| Age (years) | | | | |
| Mean ± SD | 64.2±11.2 | 64.2±11.2 | 64.2±11.3 | 0.99 |
| ECOG performance
status | | | | |
| 0 | 2,031 (88) | 1,894 (91) | 137 (67) | <0.0001 |
| 1 | 181 (8) | 143 (7) | 38 (19) | |
| 2 | 48 (2) | 31 (2) | 17 (8) | |
| 3 | 13 (1) | 7 (0) | 6 (3) | |
| 4 | 11 (1) | 7 (0) | 4 (2) | |
| Unknown | 2 (0) | 0 (0) | 2 (1) | |
| Location of primary
cancera | | | | |
| Right-sided
colon | 551 (24) | 489 (23) | 62 (30) | 0.008 |
| Left-sided
colon | 769 (34) | 719 (35) | 50 (25) | |
| Rectum | 966 (42) | 874 (42) | 92 (45) | |
| Histological
type | | | | |
|
Differentiated | 2,101 (92) | 1,944 (93) | 157 (76) | <0.0001 |
|
Undifferentiated | 161 (7) | 129 (6) | 32 (16) | |
| Unknown | 26 (1) | 9 (1) | 17 (8) | |
| Maximum tumor
diameter (mm)b | | | | |
| Mean ± SD | 43.1±23.7 | 42.1±23.4 | 57.4±23.6 | <0.0001 |
| Depth | | | | |
| T0c | 89 (4) | 87 (4) | 2 (1) | <0.0001 |
| T1 | 316 (14) | 316 (15) | 0 (0) | |
| T2 | 334 (15) | 329 (16) | 5 (2) | |
| T3 | 1,397 (61) | 1,274 (61) | 123 (60) | |
| T4 | 99 (4) | 76 (4) | 23 (11) | |
| Unknown | 51 (2) | 2 (0) | 49 (24) | |
| Regional lymph node
metastasis | | | | |
| N0 | 1,353 (59) | 1,325 (64) | 28 (13) | <0.0001 |
| N1 | 567 (25) | 507 (24) | 60 (29) | |
| N2 | 215 (9) | 176 (8) | 39 (19) | |
| N3 | 59 (2) | 37 (2) | 22 (11) | |
| Unknown | 92 (4) | 37 (2) | 55 (27) | |
| Distant
metastasis | | | | |
| M0 | 1,922 (84) | 1,915 (92) | 7 (3) | <0.0001 |
| M1 | 358 (16) | 167 (8) | 191 (94) | |
| Unknown | 6 (0) | 0 (0) | 6 (3) | |
| Initial UICC
stage | | | | |
| 0c | 86 (4) | 85 (4) | 1 (0) | <0.0001 |
| I | 542 (24) | 542 (26) | 0 (0) | |
| II | 689 (30) | 689 (33) | 0 (0) | |
| III | 600 (26) | 599 (29) | 1 (0) | |
| IV | 358 (16) | 167 (8) | 191 (94) | |
| Unknown | 11 (0) | 0 (0) | 11 (6) | |
| Family history of
primary CRCb | | | | |
| Absent | 2,007 (88) | 1,920 (92) | 87 (43) | <0.0001 |
| Present | 271 (12) | 154 (7) | 117 (57) | |
| Unknown | 8 (0) | 8 (0) | 0 (0) | |
| Smoking habit | | | | |
| Never smoked | 1,021 (45) | 9,243 (45) | 97 (48) | 0.40 |
| Current or
ex-smoker | 1,258 (55) | 1,152 (55) | 107 (52) | |
| Unknown | 7 (0) | 7 (0) | 0 (0) | |
Frequencies of synchronous and
metachronous lung metastases in CRC patients between 1990–1999 and
2000–2009
Table II summarizes
the frequencies of metastasized lung tumors observed in CRC
patients. Lung metastases were identified in 64 (2.8%) of 2,286
surgical cases at the time of colorectal surgery. Among them, 18
patients (28%) underwent curative resection for both primary and
metastasized tumors. Of the 2,082 curatively operated CRC cases,
212 (10.2%) developed metastasized tumors in the lung
metachronously.
 | Table II.Frequencies of lung metastases in (A)
all surgical CRC cases and (B) the curatively resected cases
according to the time period. |
Table II.
Frequencies of lung metastases in (A)
all surgical CRC cases and (B) the curatively resected cases
according to the time period.
| A, All cases. |
|
| Cases with lung
metastasis
| |
| Total (n=2,286)
(%) | 1990–1999 (n=919)
(%) | 2000–2009 (n=1,367)
(%) | P-value |
|
| Synchronous | 64 (2.8) | 13 (1.4) | 51 (3.7) | 0.001 |
|
| B, Curative
cases. |
|
| Cases with lung
metastasis
| |
| Total (n=2,082)
(%) | 1990–1999 (n=835)
(%) | 2000–2009 (n=1,247)
(%) | P-value |
|
| Synchronous | 18 (0.9) | 4 (0.5) | 14 (1.1) | 0.19 |
| Metachronous | 212 (10.2) | 71 (8.5) | 141 (11.3) | 0.04 |
|
Non-resected/Resected | 123/89 | 35/36 | 88/53 | |
| Totala | 223 (10.7) | 74 (8.9) | 149 (11.9) | 0.03 |
To identify the time trends of lung tumors in CRC,
we divided the CRC patients who had undergone curative resection
into 2 calendar periods, 1990–1999 (first decade) and 2000–2009
(second decade), according to the time of colorectal surgery. The
mean follow-up periods were 2,733±2,042 and 1,550±951 days,
respectively (p<0.0001). The frequency of synchronous lung
metastases (3.7%) was significantly higher in 2000–2009 than in
1990–1999 (1.4%, p=0.001). The percentage of CRC patients whose
synchronous lung metastases were curatively resected tended to be
higher in 2000–2009 than in 1990–1999 (1.1 vs. 0.5%), although the
difference was not statistically significant (p=0.19). The
frequency of metachronous lung metastases (11.3%) in 2000–2009,
despite a shorter duration of follow-up, was significantly higher
than in 1990–1999 (8.5%, p=0.04). In these patients, the resection
rate was 51% for the first decade and 38% for the second. The total
frequency of lung metastases in CRC patients in their lifetime was
significantly higher in the second decade (11.9%) than in the first
(8.9%, p=0.03).
Factors affecting the appearance of lung
metastases in CRC patients undergoing curative surgery
To identify risk factors related to the appearance
of lung metastases, clinicopathological parameters of CRC cases
with metachronous lung metastases were compared with the other
cases. Univariate analysis revealed that age, location, size and
depth of primary CRC, regional lymph node metastasis, distant organ
metastasis and a family history of CRC were correlated with lung
metastases (Table III). These
parameters were further subjected to multivariate logistic
regression analysis. The results revealed that a tumor in the
rectum [odds ratio (OR)=2.450] and the presence of lymph node
metastasis (OR=2.967) and distant organ metastases (OR=4.185) were
independent predictive factors for metachronous lung tumors in CRC
(p<0.0001, Table IV).
 | Table III.Clinicopathological factors of the
curatively operated CRC patients with and without metachronous lung
metastases. |
Table III.
Clinicopathological factors of the
curatively operated CRC patients with and without metachronous lung
metastases.
| Parameter | Metastasis (−)
(n=1,870) | Metastasis (+)
(n=212) | P-value |
|---|
| Gender (male,
female) | 1,210:660 | 127:85 | 0.17 |
| Age (years; mean ±
SD) | 64.4±11.2 | 62.2±10.2 | 0.008 |
| ECOG performance
status (0–1, 2–4) | 1,825:45 | 211:1 | 0.12 |
| Location of primary
cancer (colon, rectum) | 1,128:742 | 80:132 | <0.0001 |
| Histological
typea (diff.,
undiff.) | 1,744:117 | 200:12 | 0.84 |
| Maximum tumor
diameter (mm; mean ± SD) | 41.4±23.6 | 48.1±21.0 | <0.0001 |
| Depth (T0–2,
T3–4) | 706:1,162 | 26:186 | <0.0001 |
| Regional lymph node
metastasisa (−,
+) | 1,250:584 | 75:136 | <0.0001 |
| Distant metastasis
(M0, M1) | 1,764:106 | 151:61 | <0.0001 |
| Family history of
CRC (−, +) | 1,738:124 | 182:30 | <0.0001 |
| Smoking habit (−,
+) | 826:1,037 | 97:115 | 0.69 |
 | Table IV.Multivariate logistic regression
analysis on parameters associated with metachronous lung
metastases |
Table IV.
Multivariate logistic regression
analysis on parameters associated with metachronous lung
metastases
| Parameter | Chi-square
value | OR | 95% CI | P-value |
|---|
| Age (<65 vs. ≥65
years) | 0.890 | 1.163 | 0.849–1.593 | 0.35 |
| Location of primary
cancer (rectum vs. colon) | 31.459 | 2.450 | 1.791–3.351 | <0.0001 |
| Maximum tumor
diameter (>40 vs. ≤40 mm) | 0.852 | 0.862 | 0.629–1.182 | 0.36 |
| Depth (T3–4 vs.
T0–2) | 0.771 | 1.168 | 0.825–1.654 | 0.38 |
| Regional lymph node
metastasis (present vs. absent) | 44.182 | 2.967 | 2.155–4.098 | <0.0001 |
| Distant metastasis
(M1 vs. M0) | 50.860 | 4.185 | 2.824–6.202 | <0.0001 |
| Family history of
CRC (present vs. absent) | 1.786 | 1.350 | 0.869–2.098 | 0.18 |
Comparison of clinicopathological
parameters of CRC patients between 1990–1999 and 2000–2009
To investigate the causes for the increased lung
metastases in CRC patients, the profiles of curative CRC cases were
compared between 1990–1999 and 2000–2009. As shown in Table V, gender and ECOG PS distributions
were similar between the groups. Surgery was performed on older
patients in 2000–2009 than in 1990–1999 (average ages: 65.5 and
62.2 years, respectively; p<0.0001). The size of primary CRC was
smaller in the second period (mean diameter: 40.9 mm) than in the
first (43.9 mm, p=0.005). Throughout the 2 decades, >90% of
histology was differentiated adenocarcinoma. The rectum was the
most common location (42%) in both decades, although there was a
difference in the locations of primary colon cancers between the 2
time periods. There were no significant changes in the distribution
of pathological T and N numbers; however, more stage IV CRC
patients (M1) underwent curative surgery in 2000–2009 than in
1990–1999 (9% vs. 6%). In 1990–1999, 5% of the patients had
first-degree relative(s) with CRC, whereas 9% had a positive family
history of CRC in 2000–2009 (p=0.01). There was no intergroup
difference in the percentage of current or ex-smokers (54% and 56%
for 1990–1999 and 2000–2009, respectively, p=0.76).
 | Table V.Clinicopathological profile of the
curatively resected CRC patients in the periods 1990–1999 and
2000–2009. |
Table V.
Clinicopathological profile of the
curatively resected CRC patients in the periods 1990–1999 and
2000–2009.
| Period
| |
|---|
| Variable | 1990–1999 (n=835)
(%) | 2000–2009 (n=1,247)
(%) | P-value |
|---|
| Gender | | | |
| Male | 555 (66) | 782 (63) | 0.08 |
| Female | 280 (34) | 465 (37) | |
| Age (years) | | | |
| Mean ± SD | 62.2±11.0 | 65.5±11.0 | <0.0001 |
| ECOG performance
status | | | |
| 0 | 765 (92) | 1,129 (91) | 0.98 |
| 1 | 54 (7) | 89 (7) | |
| 2 | 12 (1) | 19 (2) | |
| 3 | 2 (0) | 5 (0) | |
| 4 | 2 (0) | 5 (0) | |
| Location of primary
cancera | | | |
| Right-sided
colon | 164 (20) | 325 (26) | 0.0008 |
| Left-sided
colon | 319 (38) | 400 (32) | |
| Rectum | 352 (42) | 522 (42) | |
| Histological
type | | | |
|
Differentiated | 771 (92) | 1,173 (94) | 0.50 |
|
Undifferentiated | 55 (7) | 74 (6) | |
| Unknown | 9 (1) | 0 (0) | |
| Maximum tumor
diameter (mm)b | | | |
| Mean ± SD | 43.8±24.3 | 40.9±22.8 | 0.005 |
| Depth | | | |
| T0c | 35 (4) | 52 (4) | 0.06 |
| T1 | 104 (12) | 212 (17) | |
| T2 | 121 (14) | 208 (17) | |
| T3 | 528 (63) | 746 (60) | |
| T4 | 27 (3) | 49 (4) | |
| Regional lymph node
metastasis | | | |
| N0 | 519 (62) | 806 (65) | 0.19 |
| N1 | 210 (25) | 297 (24) | |
| N2 | 64 (8) | 112 (9) | |
| N3 | 20 (2) | 17 (1) | |
| Distant
metastasis | | | |
| M0 | 786 (94) | 1,129 (91) | 0.003 |
| M1 | 49 (6) | 118 (9) | |
| Initial UICC
stage | | | |
| 0c | 37 (4) | 48 (4) | 0.001 |
| I | 194 (23) | 348 (28) | |
| II | 300 (36) | 389 (31) | |
| III | 255 (31) | 344 (28) | |
| IV | 49 (6) | 118 (9) | |
| Family history of
CRC | | | |
| Absent | 782 (94) | 1,135 (91) | 0.01 |
| Present | 48 (5) | 109 (9) | |
| Unknown | 5 (1) | 4 (0) | |
| Smoking habit | | | |
| Never smoked | 373 (45) | 550 (44) | 0.73 |
| Current or
ex-smoker | 457 (54) | 695 (56) | |
| Unknown | 5 (1) | 3 (0) | |
Survival of CRC patients according to the
onset of lung metastases and time period
In synchronous lung metastases, the 5-year survival
rate of patients who underwent pulmonary metastasectomy (n=18) was
65%. There was no surgery-related mortality. On the contrary,
patients who had not undergone pulmonary metastasectomy (n=46)
succumbed to the disease in less than 4.2 years (p=0.0003, data not
shown).
With regard to metachronous lung metastases, the
5-year survival rate following diagnosis of lung metastases was 60%
for cases of metastasectomy (n=89), whereas it was only 15% for
other palliative cases (n=123) (p<0.0001, data not shown). In
order to reveal any chronological changes in survival of these
patients, they were further subdivided according to the calendar
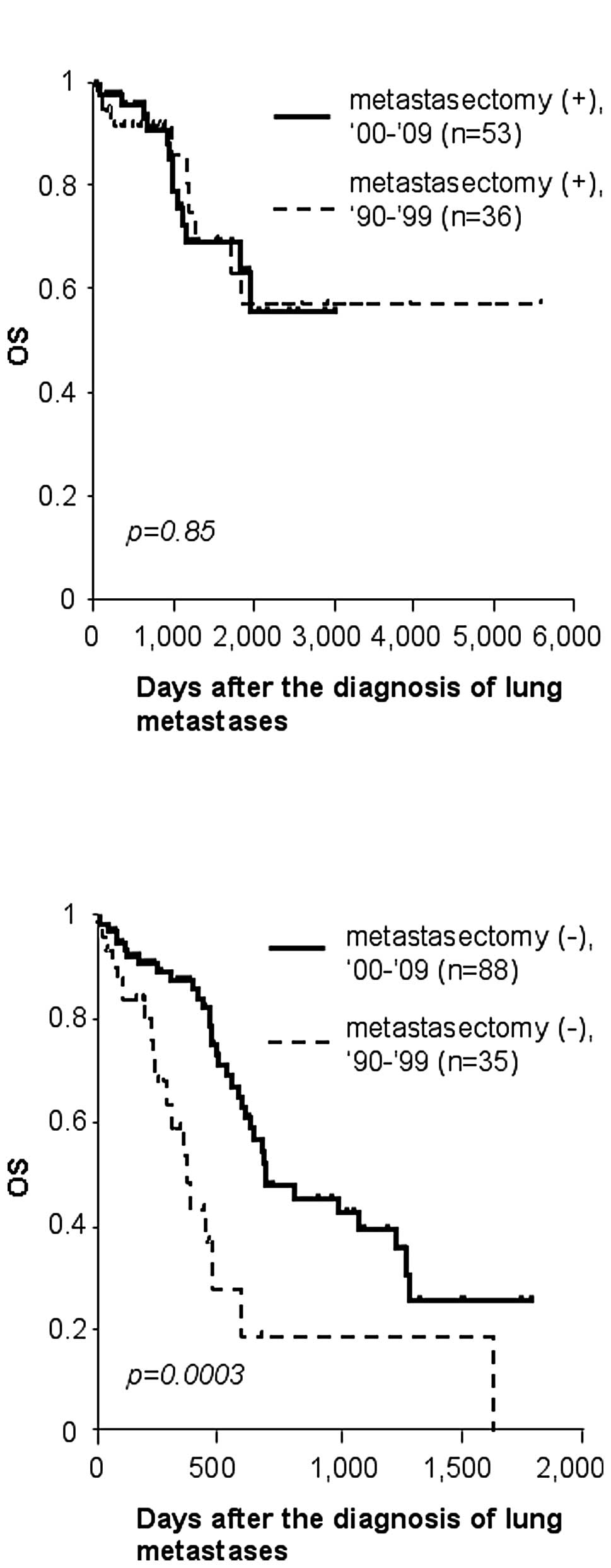
period. The survival curves of patients who received lung resection
for metastasized tumors were similar between the 2 time periods.
Their 5-year survival rate was 58% for the first decade, and 63%
for the second (p=0.85, Fig. 1A).
Notably, patients without metastasectomy survived significantly
longer in 2000–2009 than in 1990–1999 (p=0.003). Only 19% survived
2 years in the 1990–1999 group, whereas 48% survived for the same
duration in 2000–2009, due to non-surgical treatment for lung
metastases (Fig. 1B).
Features of non-resected metachronous
lung metastases between 1990–1999 and 2000–2009
Finally, we compared clinicopathological features of
metachronous lung metastases that were not resected between
1990–1999 and 2000–2009. As shown in Table VI, the frequencies of multiple,
bilateral metastases, elevated carcinoembryonic antigen (CEA)
levels in the serum and the presence of other organ metastases at
the time of diagnosis did not vary significantly between the time
periods. Disease-free interval (DFI) tended to be shorter in the
2000s, but the difference did not reach statistical significance.
Only the diameter of the largest lung deposit was significantly
smaller in patients in the 2000s (mean, 9.8±7.9 mm) than in the
1990s (mean, 17.7±15.0 mm; p=0.01).
 | Table VI.Characteristics of non-resected
metachronous lung metastases according to the time period. |
Table VI.
Characteristics of non-resected
metachronous lung metastases according to the time period.
| Variable | 1990–1999
(n=35) | 2000–2009
(n=88) | P-value |
|---|
| DFI (days) (mean ±
SD) | 776±676 | 533±465 | 0.06 |
| Number (single,
multiple) | 7:26 | 20:66 | 1.00 |
| Laterality
(ipsilateral, bilateral) | 8:25 | 35:53 | 0.17 |
| Maximum size (mm)
(mean ± SD) | 17.7±15.0 | 9.8±7.9 | 0.01 |
| Serum CEA level
(normal, elevatedb) | 5:26 | 23:60 | 0.30 |
| Other organ
metastases (absent, present) | 13:22 | 19:69 | 0.08 |
Discussion
Recent progress in multimodal treatments for CRC has
achieved a more favorable prognosis. An increasing number of
advanced CRC patients, even those with distant metastases, have the
opportunity to undergo curative surgery following state-of-the-art
neoadjuvant chemotherapy and radiation therapy, and consequently
are likely to survive for longer than they previously would have
(7). Given these trends, we
envisioned that doctors would encounter lung metastases more
frequently in the postoperative follow-up. Data collected over a
period of 20 years from our institution have clearly demonstrated
that the overall incidence of lung metastases in curative CRC cases
has risen from 8.9% in 1990–1999 to 11.9% in 2000–2009 (Table II). Due to a shorter follow-up
duration, the true frequency in the second decade may actually be
higher.
Our observation that lung metastases have increased
over time in CRC patients was partially consitent with a study by
Mitry et al based on their experience between 1976 and 2005
(8). They revealed that the risk
of synchronous metastases in the lung was 3.77 times higher in
1996–2005 than in 1976–1985. However, we also found a significant
increase in metachronous lung tumors in 2000–2009 as compared to
1990–1999, which differed from their results. The increasing
incidence of synchronous metastases may be attributable to the
improvement of diagnostic tools, such as CT scans (9); however, the high detection rate of
metachronous lung tumors is not explained simply by the improved
quality of imaging modalities, since these malignant lesions will
grow and be detected eventually. In order to investigate which
other factors caused lung deposits to be detected more often in
2000–2009 than in 1990–1999, we adopted two approaches. Firstly, we
aimed to identify parameters associated with the appearance of lung
tumors by univariate and multivariate analyses, and secondly, we
examined those parameters that changed over time. Synchronous lung
tumors were excluded in the first approach since the presence of
synchronous lung metastases itself is defined as M1 as an
explanatory variable. The analyses underscored parameters such as
rectal cancer and the presence of lymph node and distant organ
metastases at CRC surgery as predictive factors for late lung
metastases (Table IV). The second
approach elucidated a different set of parameters; the 2000–2009
cohort included older patients, smaller CRC tumors, more frequent
location in the right-sided colon, and higher percentages of
distant metastases and a positive family history of CRC compared to
the 1990–1999 cohort (Table V);
therefore, the presence of distant metastases was the only factor
commonly indicated by these comparison analyses. The number of
stage IV CRC patients was 1.6-fold higher in 2000–2009 than in
1990–1999 (Table V). It is well
accepted that hematogenous metastases from CRC generally occur
first in the liver, due to venous drainage via the portal system,
and then later appear systemically, including in the lung.
Consistent with this hypothesis, our results indicated that
extra-pulmonary [such as hepatic (representing 74% in our study)]
metastases were a risk factor for sequential lung metastases.
Surgical removal was beneficial to patients with
lung metastases in our series, as reported previously (10), although there was a selection bias
in the indications for metastasectomy in this retrospective study.
As shown in Fig. 1A, the overall
survival of CRC patients following metachronous pulmonary
metastasectomy was largely in concordance with the 5-year survival
rate of 24–68% reported previously (10–12).
It is noteworthy that CRC patients with inoperable lung metastases
exhibited markedly more favorable prognosis in the second decade
compared to the first decade (Fig.
1B). Few studies have exclusively investigated the trend in the
prognosis of unresectable lung metastases from CRC. A number of
investigators have been seeking prognostic factors for the
postoperative survival of patients with lung metastases using
univariate and multivariate evaluation. When summarizing the
results, incomplete removal of tumors, short DFI, multiple
metastases and elevated CEA levels in the serum were preferentially
documented as significant predictors of poor prognosis following
pulmonary metastasectomy (11–17).
With the exception of the first technical factor, these indices are
considered to indicate the more aggressive phenotype of metastatic
CRC cells. Provided that the same is universally true of lung
metastasis-positive CRC irrespective of pulmonary resection, these
markers would be benefit the evaluation of any chronological
changes in the grade of malignancy of non-resected lung metastases.
In this context, we compared clinicopathological factors of
unresected metachronous lung metastases including DFI, the
percentages of multiple metastases and CEA elevation between
1990–1999 and 2000–2009, but there existed no significant
differences in the majority of variables. Exceptionally, lung
deposits of smaller size were detected in 2000–2009 as compared to
1990–1999 (Table VI). The majority
of previous studies uniformly failed to define the size of the
largest metastasis as a predictive factor for survival of CRC
patients with pulmonary metastasectomy (11,12,14–17).
Taken together, we envision that earlier detection of lung deposits
as well as marked progress in multidisciplinary treatment, for
example, effective chemotherapeutic regimens in combination with
monoclonal antibodies (7), has
resulted in prolonged survival in patients with unresectable lung
metastases.
In conclusion, an increasing number of stage IV CRC
patients underwent curative resection between 2000 and 2009 in our
hospital, which resulted in a higher incidence of lung metastases
detected in the follow-up period. Diagnostic and non-surgical
therapeutic progress has been of particular benefit to CRC patients
with inoperable lung metastases; therefore, it will be of greater
importance to make accurate diagnosis of, and develop appropriate
treatment strategies for, lung tumors observed in CRC cases.
Acknowledgements
This study was supported by the
Ministry of Education, Culture, Sports, Science and Technology of
Japan, and the Ministry of Health, Labor and Welfare of Japan.
References
|
1.
|
J FerlayDM ParkinE
Steliarova-FoucherEstimates of cancer incidence and mortality in
Europe in 2008Eur J
Cancer46765781201010.1016/j.ejca.2009.12.01420116997
|
|
2.
|
A JemalR SiegelJ XuE WardCancer
statistics, 2010CA Cancer J Clin60277300201010.3322/caac.20073
|
|
3.
|
T MatsudaT MarugameK KamoCancer incidence
and incidence rates in Japan in 2005: based on data from 12
population-based cancer registries in the Monitoring of Cancer
Incidence in Japan (MCIJ) projectJpn J Clin
Oncol41139147201110.1093/jjco/hyq169
|
|
4.
|
S GalandiukHS WieandCG MoertelPatterns of
recurrence after curative resection of carcinoma of the colon and
rectumSurg Gynecol Obstet174273219921729745
|
|
5.
|
C PennaB NordlingerColorectal metastases
(liver and lung)Surg Clin N
Am8210751090200210.1016/S0039-6109(02)00051-812507210
|
|
6.
|
LH SobinMK GospodarowiczC WittekindTNM
Classification of Malignant Tumors7th editionJohn Wiley & Sons
IncNew Jersey2009
|
|
7.
|
D CunninghamW AtkinHJ LenzColorectal
cancerLancet37510301047201010.1016/S0140-6736(10)60353-4
|
|
8.
|
E MitryB GuiuS CosconeaV JoosteJ FaivreAM
BouvierEpidemiology, management and prognosis of colorectal cancer
with lung metastases: a 30-year population-based
studyGut5913831388201020732912
|
|
9.
|
R KirkeA RajeshR VermaMJ BanKartRectal
cancer: incidence of pulmonary metastases on thoracic CT and
correlation with T stagingJ Comput Assist
Tomogr31569571200710.1097/rct.0b013e318032e8c917882033
|
|
10.
|
J PfannschmidtH DienemannH
HoffmannSurgical resection of pulmonary metastases from colorectal
cancer: a systematic review of published seriesAnn Thorac
Surg84324338200710.1016/j.athoracsur.2007.02.093
|
|
11.
|
MW OnaitisRP PetersenJC HaneyPrognostic
factors for recurrence after pulmonary resection of colorectal
cancer metastasesAnn Thorac
Surg8716841689200910.1016/j.athoracsur.2009.03.03419463577
|
|
12.
|
K WatanabeK NagaiA KobayashiM SugitoN
SaitoFactors influencing survival after complete resection of
pulmonary metastases from colorectal cancerBr J
Surg9610581065200910.1002/bjs.6682
|
|
13.
|
O RenaC CasadioF VianoPulmonary resection
for metastases from colorectal cancer: factors influencing
prognosisTwenty-year experience Eur J Cardiothorac
Surg21906912200212062285
|
|
14.
|
I WatanabeT AraiM OnoPrognostic factors in
resection of pulmonary metastasis from colorectal cancerBr J
Surg9014361440200310.1002/bjs.433114598428
|
|
15.
|
T IizasaM SuzukiS YoshidaPrediction of
prognosis and surgical indications for pulmonary metastasectomy
from colorectal cancerAnn Thorac
Surg82254260200610.1016/j.athoracsur.2006.02.02716798225
|
|
16.
|
S YedibelaP KleinK FeuchterSurgical
management of pulmonary metastases from colorectal cancer in 153
patientsAnn Surg
Oncol1315381544200610.1245/s10434-006-9100-217009154
|
|
17.
|
N RamaA MonteiroJE BernardoL EugénioMJ
AntunesLung metastases from colorectal cancer: surgical resection
and prognostic factorsEur J Cardiothorac
Surg35444449200910.1016/j.ejcts.2008.10.04719136273
|