Introduction
Glutamine, a conditionally essential amino acid,
comprises approximately 60% of the free amino acids found in
skeletal muscle and is closely associated with the synthesis and
breakdown of muscle protein (1,2).
Chronic fatigue, underperformance and decreased plasma glutamine
concentrations are features of athletes with overtraining syndrome
(3). Therefore, glutamine is
supplemented in exercise and sports training (1).
As wheat gluten is a protein rich in glutamine, its
enzymatic hydrolysate is used for fortification of glutamine in
clinical enteral nutrition, especially in immunonutrition.
Recently, wheat gluten hydrolysate (WGH) was used for glutamine
supplementation in athletes as well. When consumed after a half
marathon, WGH suppressed increases in serum creatine kinase (CK)
(4,5), suggesting the mitigation of muscle
inflammation and promotion of recovery. Sawaki et al
investigated changes in biochemical parameters among male runners
with or without WGH intake after completing a half-marathon and a
45-km run. Plasma Gln was elevated after the ingestion,
representing that the glutamyl residue contained in WGH was
absorbed and contributed to the plasma level. The increase in serum
CK was suppressed at 90 min after WGH consumption, indicating that
WGH inhibits post-exercise muscle inflammation (4). Koikawa et al validated these
results through an additional investigation (5).
This anti-inflammatory effect of WGH has been
investigated in male distance runners, while its effect in females
has not been characterized. We, therefore, investigated the effects
of WGH consumption on distance running in female runners.
Materials and methods
Subjects
Six female distance runners belonging to a
university track team provided written, informed consent to
participate in this study. Their age, height, weight, body fat
ratio (%) and personal best for 5,000-m races (mean ± SD) were,
respectively, 19.8±1.6 years, 157.3±5.2 cm, 48.2±5.8 kg, 13.6±0.8%
and 16 min 34.61±19.31 sec. Their maximum oxygen uptake
(VO2 max) was estimated from their best time in a 12-min
run (Table I).
 | Table I.Baseline characteristics of the
subjects. |
Table I.
Baseline characteristics of the
subjects.
| Subject (n=6) | Age (years) | Height (cm) | Weight (kg) | Body fat (%) | Personal best at
5,000 m | VO2 max
(ml/kg/min) |
|---|
| A | 21.0 | 162.5 | 52.5 | 14.4 | 16 min 08.76 sec | 62.5 |
| B | 18.0 | 155.0 | 40.6 | 12.8 | 16 min 55.57 sec | 66.8 |
| C | 20.0 | 149.0 | 43.0 | 14.2 | 16 min 19.72 sec | 59.4 |
| D | 22.0 | 155.0 | 46.0 | 12.8 | 16 min 38.00 sec | 61.1 |
| E | 20.0 | 162.1 | 54.3 | 13.0 | 16 min 56.77 sec | 65.8 |
| F | 18.0 | 160.0 | 53.0 | 14.4 | 16 min 28.87 sec | 65.8 |
| Mean | 19.8 | 157.3 | 48.2 | 13.6 | 16 min 34.61 sec | 63.6 |
| SD | 1.6 | 5.2 | 5.8 | 0.8 | 19.31 sec | 3.0 |
Wheat gluten hydrolysate
One 4.5-g packet contained 3 g of WGH as a
granulated powder supplement, citric acid, sugars and thickeners.
The placebo supplement contained water-soluble dietary fiber
replaced for WGH. Nisshin Pharma (Tokyo, Japan) prepared and
provided the study supplements (Table
II).
 | Table II.Composition of the wheat gluten
hydrolysate (WGH) study supplement and placebo (per six-packet
dose). |
Table II.
Composition of the wheat gluten
hydrolysate (WGH) study supplement and placebo (per six-packet
dose).
| Study supplement | Composition | Content (g) |
|---|
| WGH | Wheat gluten
hydrolysate | 18.0 |
| Anhydrous
glucose | 6.0 |
| Anhydrous citric
acid | 2.7 |
| Sucralose (as
sweetener) | 0.048 |
| Lemon | 0.252 |
| Pullulan (as
thickener) | 0.405 |
| Placebo | Polydextrose | 18.0 |
| Anhydrous
glucose | 6.0 |
| Anhydrous citric
acid | 2.7 |
| Sucralose (as
sweetener) | 0.048 |
| Lemon | 0.252 |
| Pullulan (as
thickener) | 0.405 |
Study protocol
This randomized crossover, double-blind
investigation comprised two 3-day sessions at an interval of 2
weeks between them. The runners were divided into two groups, three
each, and performed a 2-h run at a pace of 190–200 m/min. One group
was given WGH, while the other group received the placebo at the
first session, and then the supplement was switched at the second
session.
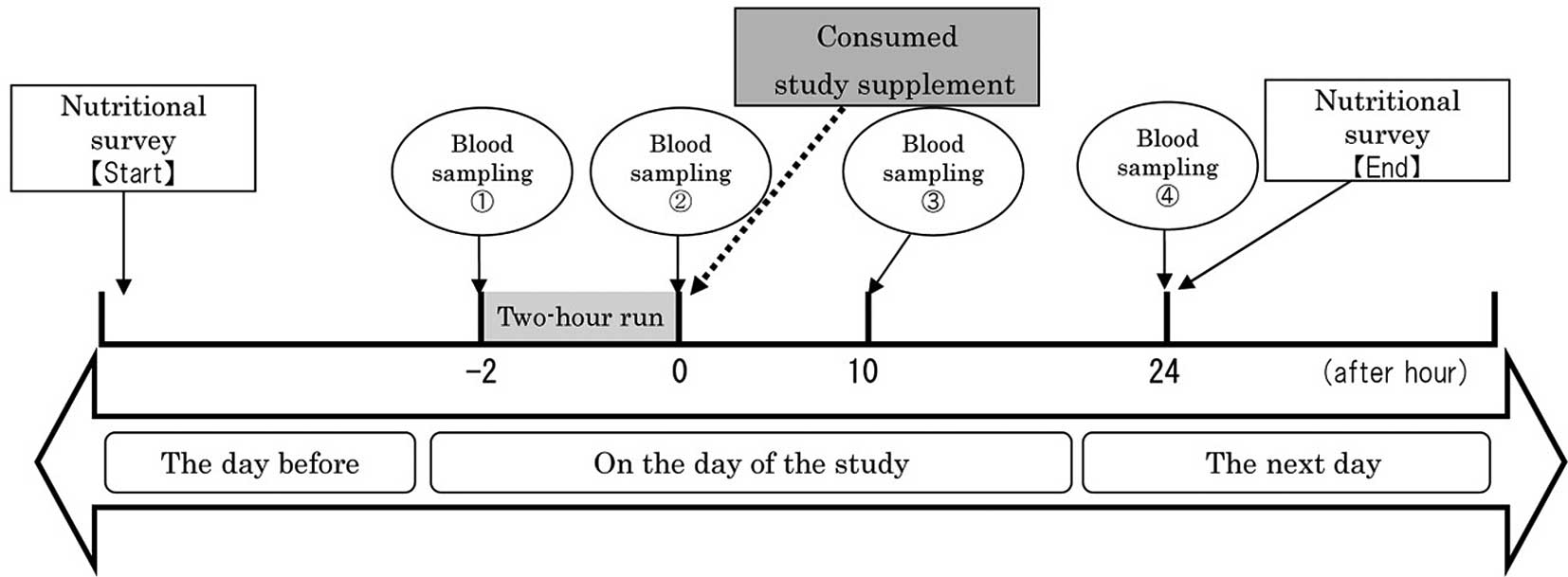
The runners refrained from training on the day
before the run. On the day of the experiment, a blood sample was
drawn from the median cubital vein before and immediately after the
2-h run. The runners then ingested six packets of WGH or placebo
supplements, and blood was collected at 10 and 24 h later.
White blood cell count (WBC) and serum CK activities
were determined in the blood samples, and plasma glutamine
concentrations were measured in the samples collected from before,
immediately and 10 h after the run. SRL (Tokyo, Japan) conducted
the analyses according to the methods standardized by the Japan
Society of Clinical Chemistry.
Nutritional intake of the runners was determined
based on the ‘Run! x Shoku-net Athlete Dietary Advice’ program
(Quality Life Service, Kanagawa, Japan). The program requires
detailed documentation of the type and amount of food that has been
consumed on a survey form and photographs of described meals before
and after consumption. Energy and nutrient intake were calculated
from the data on the survey forms and from the photographs. The
survey proceeded during the study from breakfast on the pre-run day
to breakfast on the day after the run.
All the runners participated in the same run and
were not permitted to participate in any strength conditioning or
other intense training. Fig. 1
shows the schedule and measurements taken during the study.
The protocol of this study was reviewed and approved
by the Ethics Committee of Juntendo University.
Statistical analysis
All data are expressed as the means ± SD. Serum CK
is expressed relative to the post-workout level, which was defined
as 100. Differences between the groups were assessed using a paired
t-test. The criterion for statistical significance was set at
p<0.05.
Results
The subjects ran ∼23 km at a mean speed of 191.7
m/min during the 2-h run. The workout intensity, estimated by their
maximal oxygen uptake of 63.6±3.0 ml/kg/min, was 63.8% of
VO2 max.
Serum CK increased, peaked at 10 h after the run,
and then declined (Table III).
When post-run activity was normalized at a value of 100 due to
large individual differences, the decline from 10 to 24 h after the
run was significant (p<0.05) in the WGH, but not in the placebo
group. However serum CK levels did not significantly differ between
the two groups at either 10 or 24 h after the run.
 | Table III.Transition of the biomarkers before
and after a 2-h run (mean ± SD). |
Table III.
Transition of the biomarkers before
and after a 2-h run (mean ± SD).
| Parameter (unit)
<normal range> | Group | Before | Immediately after
run | 10 h after run | 24 h after run |
|---|
| CK (U/l)
<45–210> | | | | | |
| WGH | 234±99.3 | 319±119.8 | 338±113.8 | 312±118.5 |
| Placebo | 199±55.3 | 295±76.3 | 330±139.1 | 306±120.7 |
| WBC
(103/μl) <3.5–9.1> | | | | | |
| WGH | 5.3±1.2 | 5.8±1.9 | 5.5±1.2 | 5.3±1.7 |
| Placebo | 5.7±1.9 | 5.9±1.5 | 6.7±2.2 | 5.5±2.1 |
| Gln (nmol/ml)
<478.3–658.5> | | | | | |
| WGH | 691±40.9 | 656±53.2 | 633±26.8 | - |
| Placebo | 702±73.3 | 648±83.9 | 629±74.4 | - |
The WBC counts in the WGH group increased from
5.3±1.2×103/μl before, to a peak of
5.8±1.9×103/μl immediately after the run, and then
gradually decreased to the level before the run. On the other hand
WBC counts in the placebo group were elevated by the 2-h run to a
peak at 10 h thereafter, and then declined. The decline
significantly differed between 10 and 24 h after the race
(p<0.05; Table III).
Mean plasma glutamine concentrations consistently
declined from before to 10 h after the run in both groups with no
significant differences between them (Table III).
No difference was observed in macronutrient intake
between the placebo and WGH groups (Table IV).
 | Table IV.Macronutrient intake during the study
(mean ± SD). |
Table IV.
Macronutrient intake during the study
(mean ± SD).
| Group | Energy
(kcal/day) | Protein (g/day) | Lipids (g/day) | Carbohydrates
(g/day) |
|---|
| WGH group | 1,976±467.4 | 75.1±12.1 | 63.5±14.7 | 272.6±74.1 |
| Placebo group | 1,678±336.8 | 65.8±16.7 | 53.5±9.0 | 239.4±51.8 |
Discussion
Sustained running, defined as running for 1–3 h at a
pace of 40–75% of VO2 max, is regarded as the
fundamental training for long distance runners to build aerobic
capacity. Glycogen in the skeletal muscles is exhausted within 2 h
of exercising at 60–70% of VO2 max (6). Therefore, the exercise load was set
as a 2-h sustained running at a pace of 190–200 m/min, and
intensity was estimated as 60–70% of VO2 max.
Extensive exercise results in delayed-onset muscle
soreness (DOMS), with an increase in serum CK activity and a
decrease in muscular strength (7).
Serum CK is thus considered an indicator of muscular damage
generated by training. Post-run levels of serum CK peak at 24 h
after completing a full marathon at 410±164 U/l (8) and a 100-km run at 7,012±2,262 U/l
(9). By contrast, mean serum CK in
the present study peaked at 10 h after the run and then declined.
These findings indicate that the run in this study was less
intensive than a full marathon or a 100-km run. Indeed, the pace
was moderate and the duration was shorter. Swaminathan et al
(10) identified a significant
correlation between lean body mass and serum CK activity.
Therefore, the absence of the expected increase in serum CK may be
attributed to the gender of the subjects, since female runners have
less lean body mass than male runners.
The serum CK activities significantly decreased from
10 to 24 h after the run in the WGH (p<0.05), but not in the
placebo group, when adjusted by the value obtained immediately
thereafter that was designated as 100. However, the difference
between the two groups was not significant. The rise in serum CK
activity up to 24 h after the run was approximately 10% of that
immediately after the run. The response of CK could be too small to
detect the anti-inflammatory effect of WGH in these female
runners.
The increase in the WBC count after an endurance
exercise is mainly due to the neutrophils mobilized into
circulation (11). The WBC counts
in the WGH group peaked immediately after the 2-h run and
subsequently declined to the pre-run level, whereas those in the
placebo group remained elevated for up to 10 h after the run. This
may reflect prolonged excitation in the placebo group, but the
changes were within the normal range.
Glutamine is the most abundant amino acid in the
body, and skeletal muscle is the primary source of plasma
glutamine. Plasma concentrations of glutamine fall after intensive
exercise, and also in athletes with overtraining syndrome. Since
the glutamine supply cannot meet its demand under physical stress
condition, it is considered to be a conditionally essential amino
acid (12).
Plasma glutamine concentrations markedly decline at
1 h after completing a 3-h workout at 55% of VO2 max
(13). Four hours of sustained
exercise at 50% of VO2 max cause a 60% decrease in
glutamine concentrations from baseline that recovers to 88% of
baseline after 5.5 h (14).
According to these data, we hypothesized that plasma glutamine
concentration should decrease after a 2-h run and then recover
within 10 h. Therefore plasma glutamine was monitored up to 10 h
after a 2-h run. However, it was even lower at 10 h than
immediately after the run. Since plasma glutamine is supplied by
skeletal muscle, the lower lean body mass in female runners may
have contributed to these findings.
This study aimed to confirm the anti-inflammatory
effect of WGH intake on changes in biomarkers associated with
running in female distance runners. However, we could not achieve
this goal because the kinetics of the biomarker were different from
that hypothesized based on the male runners; changes in serum CK
were insufficient and plasma glutamine remained decreased for up to
10 h after a 2-h run. The insignificant changes in serum CK may
have been due to the moderate intensity of the workout, although
changes in plasma glutamine levels seemed to contradict this notion
as they continued to decrease for up to 10 h after the run. As
skeletal muscle is the source of both CK and glutamine, the smaller
skeletal muscle mass in female runners may have affected the
results.
Since human trials with extensive exercise load tend
to be conducted with male subjects, studies investigating female
cases are limited. Thus, responses induced by intense physical
stress in females must often be estimated extrapolating from male
cases assuming the same kinetics. However, as described in this
study, there could be gender differences in the kinetics of
post-exercise biomarkers. It should be worthwhile to examine the
response to exercise in female subjects contrasting their male
counterparts.
Acknowledgements
This study was partly granted by
Nisshin Pharma Inc. (Tokyo, Japan), which was the manufacturer of
the wheat gluten hydrolysate used in this study.
References
|
1.
|
Gleeson M: Dosing and efficacy of
glutamine supplementation in human exercise and sport training. J
Nutr. 138:S2045–S2049. 2008.PubMed/NCBI
|
|
2.
|
Souba WW, Smith RJ and Willmore DW:
Glutamine metabolism by the intestinal tract. J Parenter Enteral
Nutr. 9:608–617. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rowbottom DG, Keast D, Goodman C and
Morton AR: The hematological, biochemical and immunological profile
of athletes suffering from the overtraining syndrome. Eur J Appl
Physiol. 70:502–509. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sawaki K, Takaoka I, Sakuraba K and Suzuki
Y: Effect of distance running and subsequent intake of glutamine
rich peptide on biomedical parameters of male Japanese athletes.
Nutr Res. 24:59–71. 2004. View Article : Google Scholar
|
|
5.
|
Koikawa N, Nakamura A, Nagaoka I, Aoki K,
Sawaki K and Suzuki Y: Delayed-onset muscle injury and its
modification by wheat gluten hydrolysate. Nutrition. 25:493–498.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Saltin B and Gollnick PD: Fuel for
muscular exercise: role of carbohydrate. Exercise Nutrition and
Energy Metabolism. Horton ES and Terjung RL: Macmillan; New York:
pp. 45–71. 1988
|
|
7.
|
Armstrong RB: Mechanisms of
exercise-induced delayed onset muscular soreness: a brief review.
Med Sci Sports Exerc. 16:529–538. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Suzuki K, Nakaji S, Yamada M, Liu Q,
Kurakake S, Okamura N, Kumae T, Umeda T and Sugawara K: Impact of a
competitive marathon race on systemic cytokine and neutrophil
responses. Med Sci Sports Exerc. 35:348–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gomez-Merino D, Drogou C, Guezennec CY,
Burnat P, Bourrilhon C, Tomaszewski A, Milhau S and Chennaoui M:
Comparison of systemic cytokine responses after a long distance
triathlon and a 100-km run: relationship to metabolic and
inflammatory processes. Eur Cytokine Netw. 17:117–124.
2006.PubMed/NCBI
|
|
10.
|
Swaminathan R, Ho CS and Donnan SPB: Body
composition and plasma creatine kinase activity. Ann Clin Biochem.
25:389–391. 1988. View Article : Google Scholar
|
|
11.
|
Suzuki K, Totsuka M, Nakaji S, Yamada M,
Kudoh S, Liu Q, Sugawara K, Yamaya K and Sato K: Endurance exercise
causes interaction among stress hormones, cytokines, neutrophil
dynamics, and muscle damage. J Appl Physiol. 87:1360–1367.
1999.PubMed/NCBI
|
|
12.
|
Calder PC and Yaqoob P: Glutamine and the
immune system. Amino Acids. 17:227–241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Robson PJ, Blannin AK, Walsh NP, Castell
LM and Gleeson M: Effects of exercise intensity, duration and
recovery on in vitro neutrophil function in male athletes. Int J
Sports Med. 20:128–135. 1999.PubMed/NCBI
|
|
14.
|
Rennie MJ, Edwards RH, Krywawych S, Davies
CT, Halliday D, Waterlow JC and Millward DJ: Effect of exercise on
protein turnover in man. Clin Sci (Lond). 61:627–639.
1981.PubMed/NCBI
|