Introduction
Both functional and non-functional pancreatic
neuroendocrine tumors (PNETs), including pancreatic neuroendocrine
carcinomas (PNECs), are hypervascular tumors and they are known to
express angiogenic molecules (1,2). For
these reasons, anti-angiogenic therapy is expected to be effective
against PNEC (3). Bevacizumab
(Avastin®; Genentech Inc., San Francisco, CA, USA) is a
recombinant human IgG1 monoclonal antibody against vascular
endothelial growth factor (VEGF) (4). We previously reported that
bevacizumab inhibited the induction of host angiogenesis, resulting
in significant tumor growth inhibition, but not in tumor cell
proliferation using QGP-1 which is a PNEC cell line, and expected a
further potent cytotoxic effect by various combinations with
anticancer drugs (5). On the basis
of the suggestion above, we compared an additional effect between
the combination of gemcitabine hydrochloride (Gemzar®,
Eli Lilly and Company, Indianapolis, IN, USA) (6) or oral S-1 (TS-1®, Taiho
Pharmaceutical Co. Ltd., Tokyo, Japan) (7) with bevacizumab and bevacizumab
alone.
Materials and methods
The QGP-1 PNEC cell line (8) was purchased from the Japanese
Collection of Research Bioresources (Osaka, Japan), and the BxPC-3
human pancreatic ductal carcinoma (DCC) cell lines were purchased
from the American Type Culture Collection (Manassas, VA, USA).
Cells were cultured at 37°C in RPMI-1640 (Gibco, Life Technologies
Japan Ltd., Tokyo, Japan) supplemented with 10% fetal calf serum
(FCS; Sigma, St. Louis, MO, USA) in a humidified atmosphere
containing 5% CO2.
Athymic female Balb/c-nu/nu nude mice (4–6 weeks
old) with a body weight (BW) of 20–22 g, obtained from Clea Japan
Inc. (Tokyo, Japan), were kept at the Animal Care and Use
Facilities at Tokyo Medical University under specific pathogen-free
condition. The cell suspension of each cell line with an adjusted
cell suspension of 2×107 cells/ml in RPMI-1640 (Gibco)
was mixed with Matrigel matrix (BD Biosciences, San Jose, CA, USA)
on ice at a 1:4 ratio. The mixture was implanted subcutaneously in
the back of mice. At predetermined time points during a 1-week
period after the cancer transplantation, 25 mice were randomly
divided into five groups and treated with bevacizumab and
gemcitabine or S-1 for 3 weeks. Bevacizumab (4 mg/kg) or human IgG
(Sigma) was administered intraperitoneally (i.p.) twice a week
(9). Gemcitabine (240 mg/kg) was
administered i.p. once a week (10). Hydroxypromethyl cellulose [0.2 ml
of 0.5% (w/v); Shin-Etsu Chemical Co., Ltd., Tokyo, Japan],
including dissolved powder-form S-1 (10 mg/kg) were orally
administered five times a week (11,12).
The treatment groups were as follows: BGS group, mice received
bevacizumab, gemcitabine and S-1; BG group, mice received
bevacizumab and gemcitabine; BS group, mice received bevacizumab
and S-1; B group, mice received bevacizumab alone; and IgG group,
mice received human IgG as non-treatment. Tumor volume was
calculated by the multiplication of π × longitudinal axis × minor
axis × minor axis; measurement was carried out using digital
calipers, once a week. The weight of the mice was measured once a
week. On the last day of the third week after start of the
therapies (on 28 day after cancer cell transplantation), each tumor
was removed and weighed. All experiments were approved by the
Animal Care and Ethics Committee of Tokyo Medical University.
Statistical analysis
Statistical analyses were performed using Stat View
(Abacus Concepts Inc., Berkely, CA, USA). The volume of the tumor
was compared using the Mann-Whitney U test. A two-side p-value of
<0.05 was considered to denote statistical significance.
Results
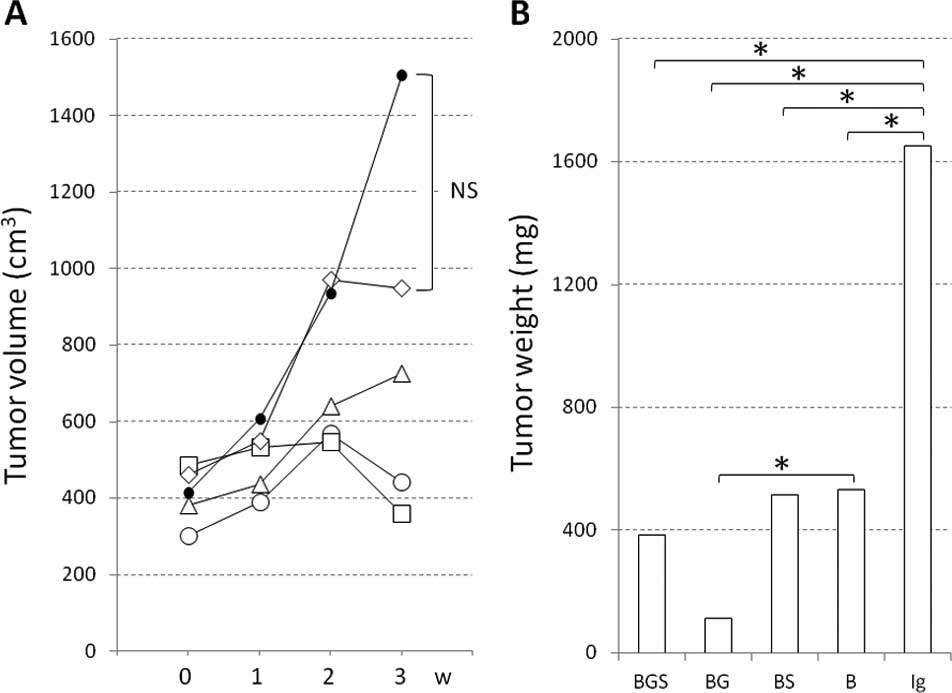
BxPC-3 cell tumors grew to approximately double that
of the QGP-1 cell tumors. The mean tumor volume (mm3) 1 week after
QGP-1 transplantation, and 1, 2 and 3 weeks after each treatment
was as follows: for the BGS group: 300.6, 389.8, 567.6 and 442.2,
respectively; for the BG group: 486.8, 531.8, 546.3 and 358.2,
respectively; for the BS group: 381.4, 436.1, 638.9 and 725.6,
respectively; for the B group: 462.0, 549.7, 970.4 and 949.9,
respectively; and for the IgG group: 414.2, 607.0, 935.4 and
1,504.2, respectively (Fig. 1A).
The BGS and the BG groups receiving gemcitabine showed marked tumor
growth inhibition from the 2nd week or later. By contrast, the BS
and the B groups not receiving gemcitabine showed tumor growth
inhibition in comparison to the IgG group; however, the tumor
increased after the 2nd week or later. The tumor volume of all
treatment groups apart from group B at the 3rd week was
significantly smaller than that of the IgG group (p<0.05). There
was no significant difference among the treatment groups. The mean
tumor weight in the BGS, BG, BS, B and IgG groups at the time of
tumor dissection was 382.9, 515.5, 114.7, 532.8 and 1,653.6 mg,
respectively. There was a significant difference between all
treatment groups and the IgG group (p<0.05), and between the BS
and the B group (p=0.03) (Fig.
1B).
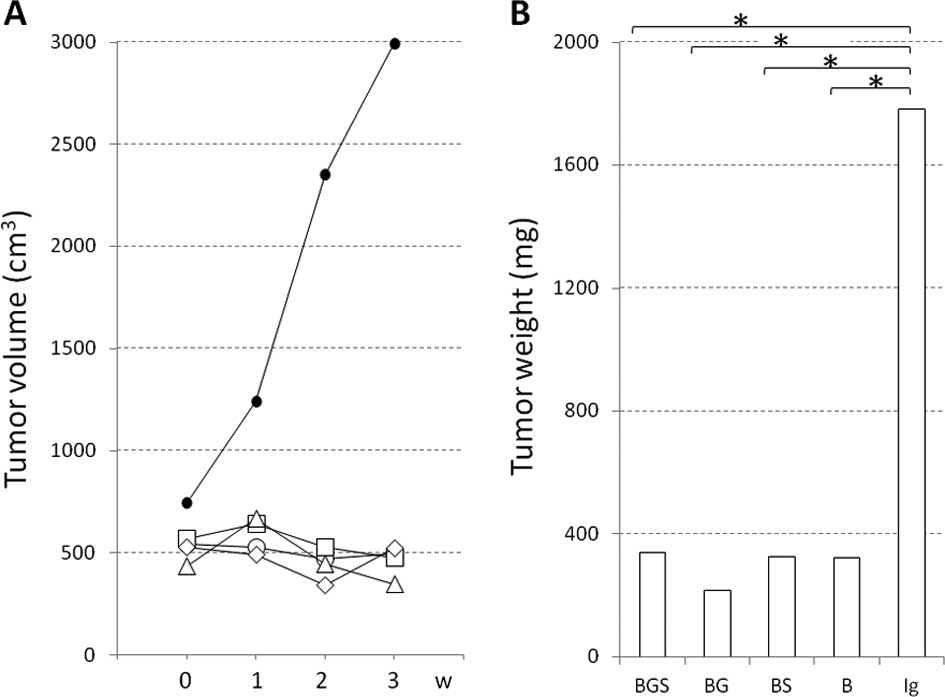
The mean tumor volume (mm3) of the BxPC-3
cell tumors was as follows: for the BGS group: 546.1, 527.5, 473.0
and 496.9, respectively; for the BG group: 567.4, 639.7, 528.8 and
475.8, respectively; for the BS group: 437.7, 665.5, 447.1 and
347.7, respectively; for the B group: 526.6, 493.6, 341.8 and
523.6, respectively; and for the IgG group: 743.7, 1,243.1, 2,350.8
and 2,991.2, respectively (Fig.
2A). The BGS, BG and BS groups showed slight tumor inhibition
from the 2nd week or later; however, only the B group exhibited
tumor growth. The tumor volume of all treatment groups at the 3rd
week was significantly smaller than that of the IgG group
(p<0.05), but not among the treatment groups. The mean tumor
weight in the BGS, BG, BS, B and IgG groups was 339.2, 325.7,
217.2, 322.8 and 1,782.7 mg, respectively. There was a significant
difference between all treatment groups and the IgG group
(p<0.05), but not among the treatment groups (Fig. 2B).
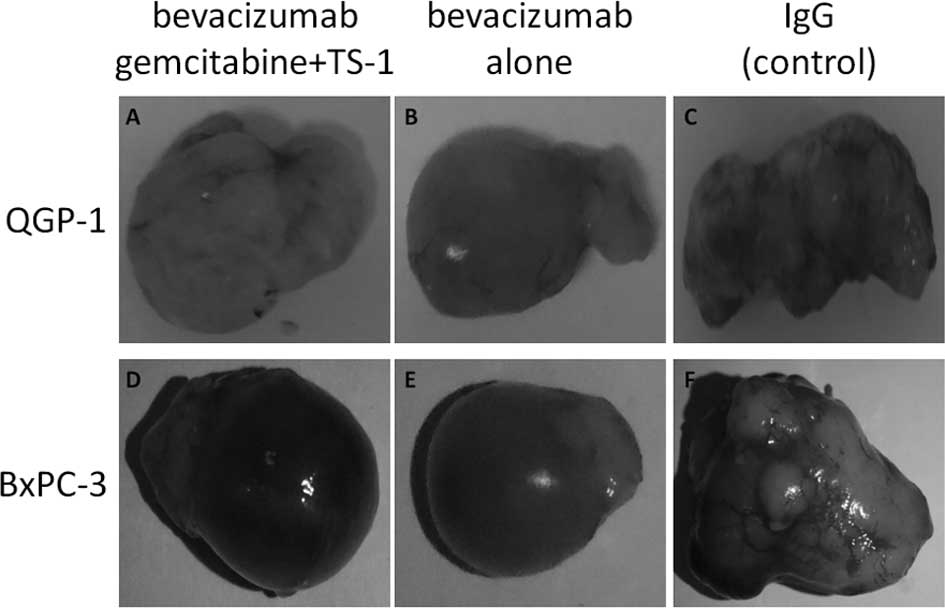
Macroscopic findings of the QGP-1 cell tumors showed
comparatively solid and little central necrosis and no marked
differences among the treatment groups (Fig. 3A–C). By contrast, the macroscopic
findings of the BxPC-3 cell tumors indicated intratumoral bleeding
and necrosis in all groups (Fig.
3D–F). Numerous subcutaneous blood vessels were overlying the
tumors in the IgG group bearing the QGP-1 and BxPC-3 cell tumors,
while few blood vessels were observed in the bevacizumab-treated
group.
As for the weight change in the QGP-1
cell-transplanted mice, all groups showed weight loss. In the BGS
and BG groups, the drug caused weight loss which was in particular
stronger than that in the IgG group. By contrast, weight loss was
not evident, but weight instead rather increased in the BxPC-3
cell-transplanted mice. We determined that the above results
reflected solely the characteristics of the cell lines.
Discussion
Inhibition of angiogenesis has become a target of
cancer therapy, and the anti-VEGF antibody/bevacizumab is
representative. Bevacizumab specifically binds to VEGF in the
bloodstream and inhibits the binding of VEGF to VEGF receptors in
vascular endothelial cells, thereby inhibiting angiogenesis. The
interstitial pressure around a tumor is usually increased,
inhibiting the delivery of anticancer drugs to tumor tissue.
Bevacizumab normalizes tumor blood vessels, reduces the
interstitial pressure and thereby improves the delivery of
anticancer drugs to tumor tissue (4). PNECs are also hypervascular tumors
and are known to express angiogenic molecules (1–3). For
these reasons, anti-angiogenic therapy is expected to be effective
against PNEC. In a randomized phase II trial of bevacizumab vs.
interferon-α for the treatment of patients (n=44) with unresectable
carcinoid tumors treated with octreotide, a somatostatin analogue,
the added effect of combining bevacizumab with the somatostatin
analogue, was reported (13). The
therapeutic response rates were 18 vs. 0%, and the 8-week
progression-free survival rates were 95 vs. 68%. We previously
reported that bevacizumab inhibited the induction of host
angiogenesis, resulting in significant tumor growth inhibition
(5).
In the selection of therapeutic agents, we focused
on the site of origin and growth of PNEC. PNECs are considered to
arise from Langerhans cells, endocrine acinar cells and multipotent
stem cells in the pancreatic ducts. By contrast, it has been
reported that pancreatic ductal cell carcinoma may arise from
pancreatic endocrine cells (14).
In addition, Langerhans cells or pancreatic endocrine cells are
reportedly involved in the growth of pancreatic ductal cell
carcinoma (14). In light of these
observations, we selected gemcitabine and S-1, which are
therapeutic agents for pancreatic ductal carcinoma, as candidate
therapeutic agents for PNEC, and confirmed a more beneficial effect
of gemcitabine/bevacizumab combination therapy over bevacizumab
monotherapy. Concerning the combination treatment of gemcitabine
and bevacizumab, a randomized controlled trial of gemcitabine +
placebo vs. gemcitabine + bevacizumab for the treatment of advanced
unresectable pancreatic cancer was conducted. However, no
significant differences were observed between the gemcitabine +
placebo and gemcitabine + bevacizumab groups in the therapeutic
response rates, median progression-free survival times and median
survival times. Thus, gemcitabine + bevacizumab therapy did not
prolong the survival time compared to gemcitabine therapy (15). On the contrary, a case report of
the utility of the combination therapy including bevacizumab and
gemcitabine for the progression of pancreatic cancer was reported
(16). Another candidate
therapeutic agent, S-1, was first developed in Japan (7,17).
Currently, gemcitabine and S-1 are the only drugs that contribute
to improving the prognosis of pancreatic cancer. Either gemcitabine
or S-1 is commonly used as a first-line treatment, but they are
sometimes used in combination with each other (18). Combination therapy with S-1,
irinotecan and bevacizumab has been reported to be useful in the
treatment of colorectal cancer with metastasis (19). In this study, we expected to obtain
better results using a combination therapy with bevacizumab,
gemcitabine and S-1, and confirmed a more beneficial effect of
bevacizumab/gemcitabine combination therapy over bevacizumab
monotherapy. However, the triple therapy was not superior to
bevacizumab/gemcitabine combination therapy in the QGP-1
cell-transplanted mice.
The effect of the mammalian target of rapamycin
(mTOR) inhibitor everolimus (Afinitor®) in patients with
advanced pancreatic neuroendocrine tumors has recently been
reported (20). In this clinical
trial, treatment with the mTOR inhibitor extended the median
survival time from 4.6 (in a placebo group) to 11 months (in the
treated group). It was also found that the mTOR inhibitor exerted
an angiogenesis-inhibitory effect through VEGF (21). Future research will be conducted to
investigate how to combine drugs for the treatment of pancreatic
neuroendocrine tumors.
In conclusion, we compared the effect of
bevacizumab/gemcitabine/S-1 combination therapy vs. bevacizumab
monotherapy on pancreatic neuroendocrine tumor cell lines.
Bevacizumab/gemcitabine combination therapy showed a strong
antitumor effect (a decrease from the maximum tumor volume) from 2
weeks after treatment initiation. By contrast, bevacizumab/S-1
combination therapy resulted in a slowdown of tumor growth, but not
in a decrease from the maximum tumor volume. Thus, we conclude that
gemcitabine is appropriate for use in combination with bevacizumab
for pancreatic neuroendocrine tumors.
Acknowledgements
The authors thank Mr. Hiroaki Tanaka
and Hiroshi Ohta, university students who belong to the Department
of Clinical Pharmacy of the Tokyo University of Pharmacy and Life
Sciences, for their valuable technical assistance.
References
|
1.
|
Eriksson B and Oberg K: Neuroendocrine
tumours of the pancreas. Br J Surg. 87:129–131. 2000. View Article : Google Scholar
|
|
2.
|
Takahashi Y, Akishima-Fukasawa Y,
Kobayashi N, et al: Prognostic value of tumor architecture,
tumor-associated vascular characteristics, and expression of
angiogenic molecules in pancreatic endocrine tumors. Clin Cancer
Res. 13:187–196. 2007. View Article : Google Scholar
|
|
3.
|
Miljković MD, Girotra M, Abraham RR and
Erlich RB: Novel medical therapies of recurrent and metastatic
gastroenteropancreatic neuroendocrine tumors. Dig Dis Sci. 57:9–18.
2011.PubMed/NCBI
|
|
4.
|
Jain RK, Duda DG, Clark JW and Loeffler
JS: Lessons from phase III clinical trials on anti-VEGF therapy for
cancer. Nat Clin Pract Oncol. 3:24–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kasuya K, Nagakawa Y, Suzuki M, Tanaka H,
Ohta H, Itoi T and Tsuchida A: Anti-vascular endothelial growth
factor antibody single therapy for pancreatic neuroendocrine
carcinoma exhibits a marked tumor growth-inhibitory effect. Exp
Ther Med. 2:1047–1052. 2011.
|
|
6.
|
Grindey GB, Hertel LW and Plunkett W:
Cytotoxicity and antitumor activity of 2′,2′-difluorodeoxycytidine
(gemcitabine). Cancer Invest. 8:313–318. 1990.
|
|
7.
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar
|
|
8.
|
Georgieva I, Koychev D, Wang Y, Holstein
J, Hopfenmüller W, Zeitz M and Grabowski P: ZM447439, a novel
promising aurora kinase inhibitor, provokes antiproliferative and
proapoptotic effects alone and in combination with bio- and
chemotherapeutic agents in gastroenteropancreatic neuroendocrine
tumor cell lines. Neuroendocrinology. 91:121–130. 2010. View Article : Google Scholar
|
|
9.
|
Shah DK, Veith J, Bernacki RJ and
Balthasar JP: Evaluation of combined bevacizumab and
intraperitoneal carboplatin or paclitaxel therapy in a mouse model
of ovarian cancer. Cancer Chemother Pharmacol. 68:951–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Braakhuis BJ, Ruiz van Haperen VW, Boven
E, Veerman G and Peters GJ: Schedule-dependent antitumor effect of
gemcitabine in in vivo model system. Semin Oncol. 4(Suppl 11):
42–46. 1995.PubMed/NCBI
|
|
11.
|
Fukushima M, Satake H, Uchida J, et al:
Preclinical antitumor efficacy of S-1: a new oral formulation of
5-fluorouracil on human tumor xenografts. Int J Oncol. 13:693–698.
1998.PubMed/NCBI
|
|
12.
|
Nakahira S, Nakamori S, Tsujie M, et al:
Pretreatment with S-1, an oral derivative of 5-fluorouracil,
enhances gemcitabine effects in pancreatic cancer xenografts.
Anticancer Res. 28:179–186. 2008.PubMed/NCBI
|
|
13.
|
Yao JC, Phan A, Hoff PM, et al: Targeting
vascular endothelial growth factor in advanced carcinoid tumor: a
random assignment phase ll study of depot octreotide with
bevacizumab and pegylated interferon alpha-2b. Clin Oncol.
26:1316–1323. 2008. View Article : Google Scholar
|
|
14.
|
Pour PM and Kazakoff K: Stimulation of
islet cell proliferation enhances pancreatic ductal carcinogenesis
in the hamster model. Am J Pathol. 149:1017–1025. 1996.PubMed/NCBI
|
|
15.
|
Kindler HL, Niedzwiecki D, Hollis D, et
al: Gemcitabine plus bevacizumab compared with gemcitabine plus
placebo in patients with advanced pancreatic cancer: phase III
trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin
Oncol. 28:3617–3622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Masellis AM, Sielaff TD and Bender GP:
Successful treatment of metastatic pancreatic adenocarcinoma with
combination chemotherapy regimens. Int J Clin Oncol. 14:478–481.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Nakai Y, Isayama H, Sasaki T, et al:
Impact of S-1 on the survival of patients with advanced pancreatic
cancer. Pancreas. 39:989–993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Murakami Y, Uemura K, Sudo T, Hayashidani
Y, Hashimoto Y, Ohge H and Sueda T: Impact of adjuvant gemcitabine
plus S-1 chemotherapy after surgical resection for adenocarcinoma
of the body or tail of the pancreas. J Gastrointest Surg. 13:85–92.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yamada Y, Yamaguchi T, Matsumoto H, et al:
Phase II study of oral S-1 with irinotecan and bevacizumab (SIRB)
as first-line therapy for patients with metastatic colorectal
cancer. Invest New Drugs. Sept 6–2011.(E-pub ahead of print).
|
|
20.
|
Oberg K, Akerström G, Rindi G and Jelic S;
ESMO Guidelines Working Group: Neuroendocrine
gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 21(Suppl 5):
v223–v227. 2010. View Article : Google Scholar
|
|
21.
|
Villaume K, Blanc M, Gouysse G, et al:
VEGF secretion by neuroendocrine tumor cells is inhibited by
octreotide and by inhibitors of the PI3K/AKT/mTOR pathway.
Neuroendocrinology. 91:268–278. 2010. View Article : Google Scholar : PubMed/NCBI
|