Spandidos Publications style
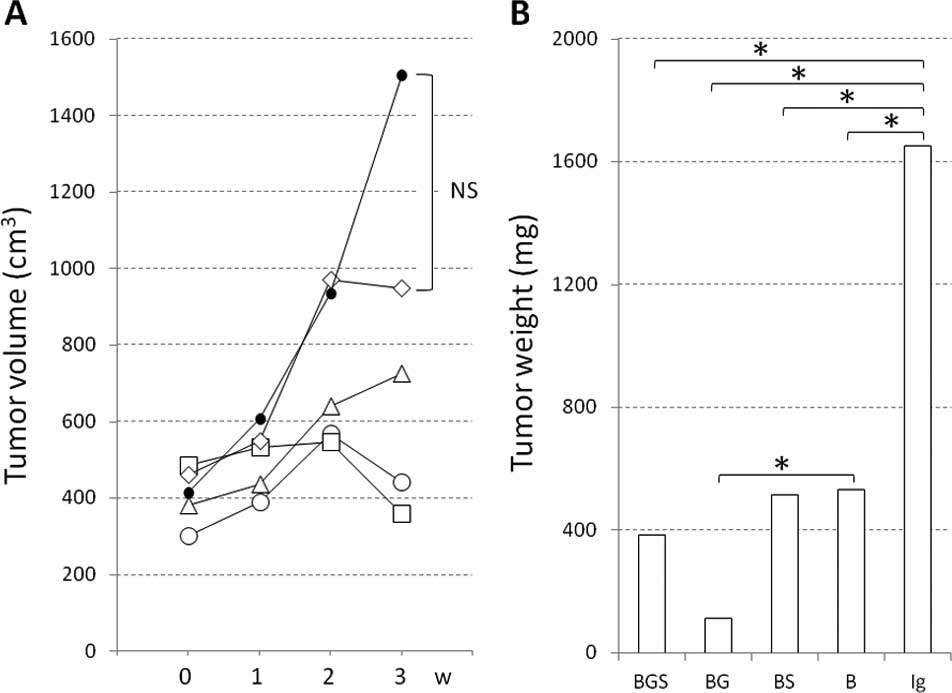
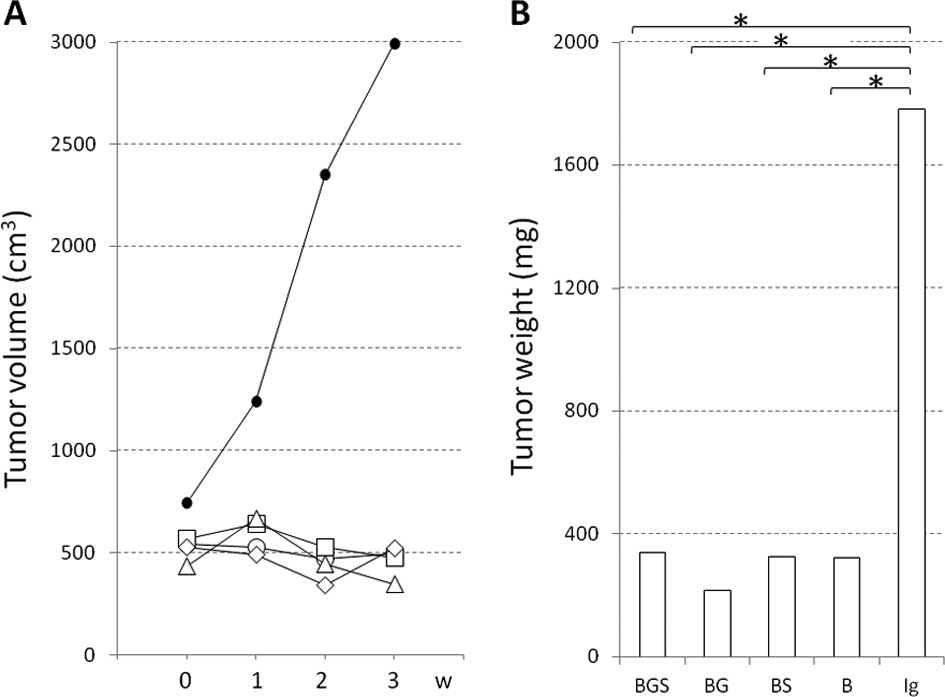
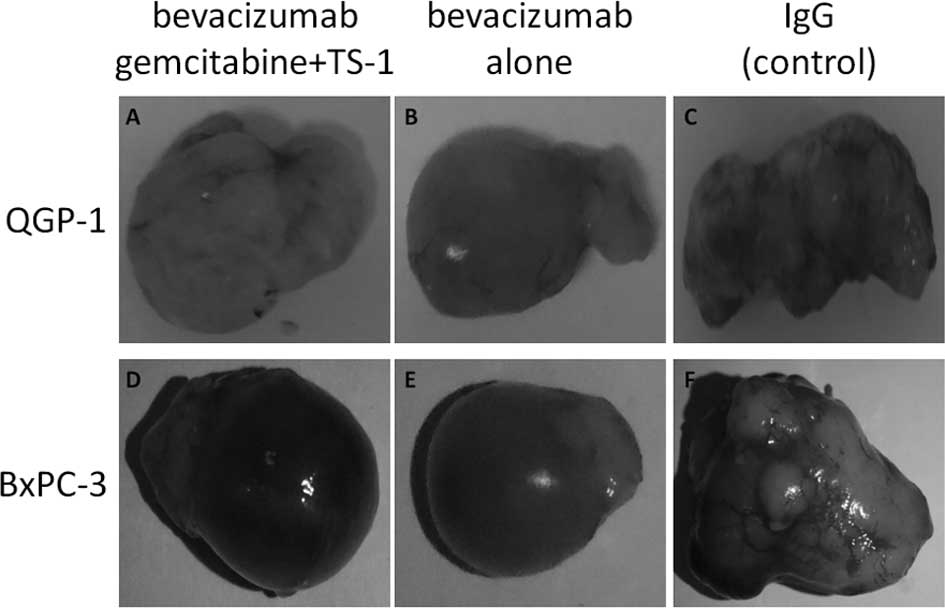
Kasuya K, Nagakawa Y, Suzuki M, Suzuki Y, Kyo B, Suzuki S, Matsudo T, Itoi T, Tsuchida A, Aoki T, Aoki T, et al: Combination therapy of gemcitabine or oral S-1 with the anti-VEGF monoclonal antibody bevacizumab for pancreatic neuroendocrine carcinoma. Exp Ther Med 3: 599-602, 2012.
APA
Kasuya, K., Nagakawa, Y., Suzuki, M., Suzuki, Y., Kyo, B., Suzuki, S. ... Aoki, T. (2012). Combination therapy of gemcitabine or oral S-1 with the anti-VEGF monoclonal antibody bevacizumab for pancreatic neuroendocrine carcinoma. Experimental and Therapeutic Medicine, 3, 599-602. https://doi.org/10.3892/etm.2012.456
MLA
Kasuya, K., Nagakawa, Y., Suzuki, M., Suzuki, Y., Kyo, B., Suzuki, S., Matsudo, T., Itoi, T., Tsuchida, A., Aoki, T."Combination therapy of gemcitabine or oral S-1 with the anti-VEGF monoclonal antibody bevacizumab for pancreatic neuroendocrine carcinoma". Experimental and Therapeutic Medicine 3.4 (2012): 599-602.
Chicago
Kasuya, K., Nagakawa, Y., Suzuki, M., Suzuki, Y., Kyo, B., Suzuki, S., Matsudo, T., Itoi, T., Tsuchida, A., Aoki, T."Combination therapy of gemcitabine or oral S-1 with the anti-VEGF monoclonal antibody bevacizumab for pancreatic neuroendocrine carcinoma". Experimental and Therapeutic Medicine 3, no. 4 (2012): 599-602. https://doi.org/10.3892/etm.2012.456